Introduction
In the evolving landscape of medical technology, pilot clinical studies play a pivotal role in shaping the future of healthcare innovations. These preliminary investigations not only assess the feasibility and safety of new devices but also gather essential data that informs larger trials.
As seen in recent initiatives, such as eyeFlow, Inc.'s groundbreaking glaucoma treatment study in Colombia, pilot studies can yield significant insights that enhance the likelihood of success in subsequent phases.
With the complexities of regulatory requirements and the necessity for robust study protocols, understanding the intricacies of pilot clinical trials is crucial for stakeholders aiming to navigate this challenging yet rewarding terrain.
This article delves into the essential components of pilot clinical studies, including:
- Regulatory guidance
- Participant recruitment
It highlights the importance of strategic planning in advancing medical technology research.
Understanding the Purpose of Pilot Clinical Studies in Medtech
Pilot Clinical Studies for Medtech in clinical investigations function as preliminary assessments aimed at evaluating the feasibility, time, cost, and adverse events associated with larger clinical trials. Recent approvals, such as eyeFlow, Inc.'s 18-month trial in Barranquilla, Colombia—which aims to recruit 60 subjects for an innovative glaucoma treatment—highlight the successful outcomes possible in these efforts. Furthermore, PAVmed's first-in-human implantations of the PortIO™ Intraosseous Infusion System showcase the potential of preliminary trials to reduce risks and improve protocols.
These investigations are essential in identifying challenges and gathering preliminary data that inform decisions on research design, recruitment strategies, and necessary modifications to the device or intervention being tested as part of pilot clinical studies for Medtech in. Specifically, bioaccess® provides extensive research study management services, including:
- Feasibility assessments
- Site selection
- Compliance evaluations
- Project oversight
These services are crucial in supporting preliminary studies. Ultimately, this initial phase enhances the likelihood of success in subsequent stages of research by providing the necessary groundwork for informed decision-making.
Navigating Regulatory Guidance for Pilot Clinical Trials
When conducting Pilot Clinical Studies for Medtech in Latin America, it is vital to follow the regulatory guidelines established by authorities such as the FDA or EMA. This begins with:
- Determining the classification of the medical device.
- Understanding the requisite pre-market requirements.
- If necessary, preparing and submitting an Investigational Device Exemption (IDE) application, which permits the investigation of a device not yet approved for general use.
Adherence to Good Clinical Practice (GCP) guidelines is crucial for Pilot Clinical Studies for Medtech in order to meet ethical and scientific quality standards for designing, conducting, and reporting studies.
Engaging with regulatory bodies early in the process can streamline the navigation of these requirements, ultimately leading to a more efficient testing process. Utilizing the knowledge of professionals like Katherine Ruiz, a Regulatory Affairs specialist with over 20 years in Medtech, can further improve the management of Pilot Clinical Studies for Medtech in initial trials, ensuring:
- Thorough feasibility assessments
- Site selection
- Trial set-up
- Start-up and approval
- Compliance reviews
- Strong project management in addressing challenges such as financial limitations and recruitment issues encountered by medical device startups.
For more information and to discuss your project, BOOK A MEETING.
Developing a Robust Study Protocol
To create a strong research protocol for pilot clinical studies for Medtech in a trial, begin with a clear declaration of the project objectives and hypotheses. Outline the research design, including:
- Patient selection criteria
- Sample size
- Randomization methods
Detail the data collection methods, specifying timelines for assessments and follow-up.
Incorporate safety monitoring procedures and define endpoints that will be assessed. Additionally, ensure that the protocol adheres to regulatory requirements, such as those set by INVIMA, and is reviewed by an ethics committee to safeguard participant welfare. With more than 20 years of experience in overseeing medical device research, utilizing the knowledge of extensive research management services can improve the project's success.
These services encompass:
- Viability assessments
- Site selection
- Compliance reviews
- Testing setup
- Import permits
- Project management
A well-structured protocol is essential to pilot clinical studies for Medtech in guiding the research team and serving as a valuable tool for regulatory submissions and stakeholder communication.
Recruiting and Training Study Participants
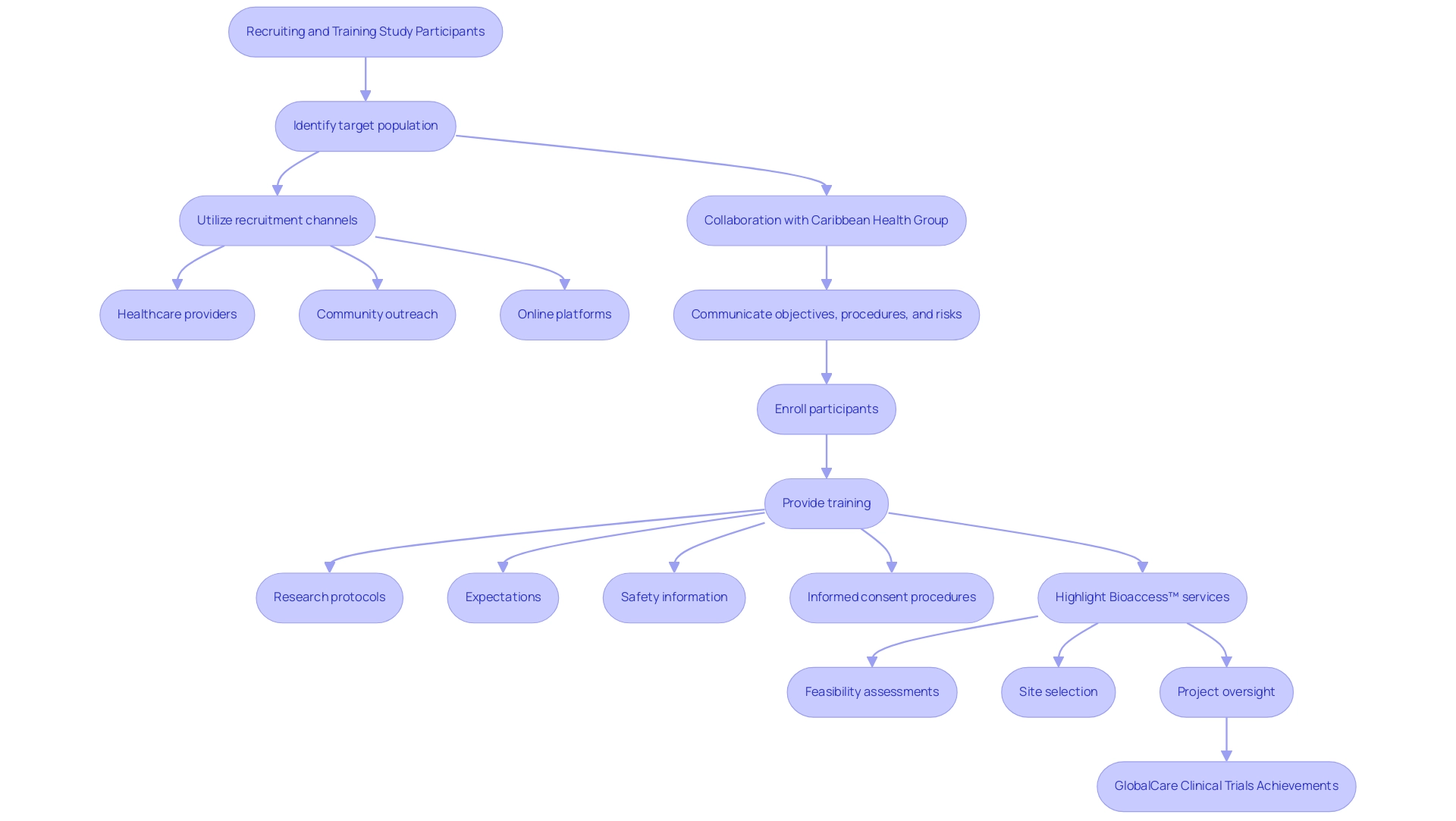
Recruiting participants for pilot clinical studies for Medtech in Latin America requires a strategic approach, especially where collaboration is key. Start by identifying the target population and utilizing various recruitment channels such as:
- Healthcare providers
- Community outreach
- Online platforms
Bioaccess™, in collaboration with the Caribbean Health Group, is striving to pilot clinical studies for Medtech in Barranquilla, which can improve recruitment efforts in the region.
This initiative is further supported by Colombia's Minister of Health, emphasizing the credibility of the efforts to pilot clinical studies for Medtech in attracting clinical research projects. Clearly convey the objective, procedures, and potential risks of the research to prospective participants. Once participants are enrolled, provide comprehensive training that covers:
- Research protocols
- Expectations
- Safety information
This training should also include informed consent procedures, emphasizing the participants' rights and the significance of their role in the research. Notably, GlobalCare Clinical Trials has achieved over a 50% reduction in recruitment time and a 95% retention rate within Colombia, demonstrating the effectiveness of such strategic approaches. Bioaccess™ provides a variety of trial management services, including:
- Feasibility assessments
- Site selection
- Project oversight
A well-informed participant is more likely to follow research protocols, leading to more reliable results.
Collecting and Analyzing Data
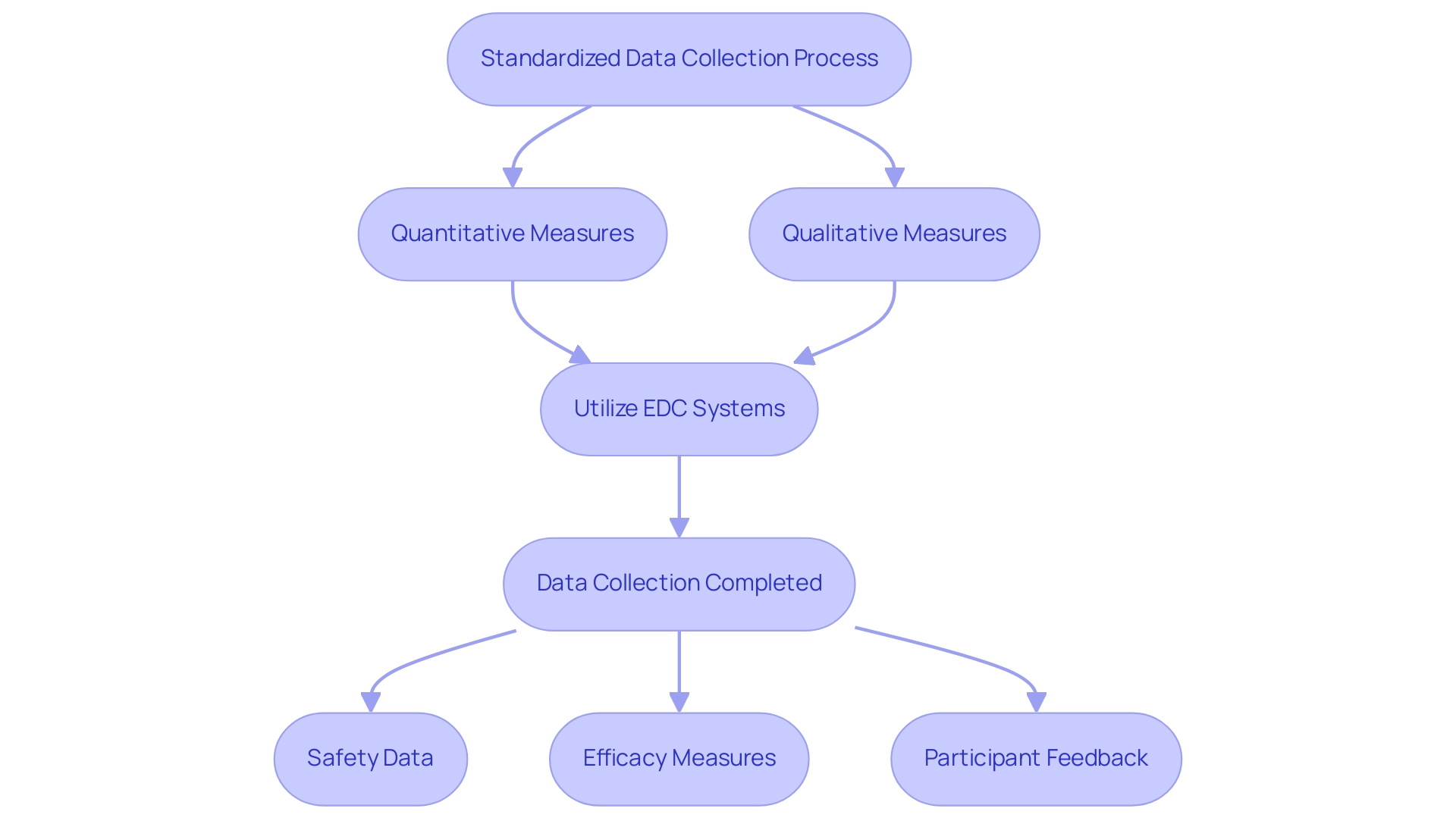
Gathering and examining information in preliminary clinical trials conducted by bioaccess® involves several key steps. Begin by implementing a standardized data collection process that includes both quantitative and qualitative measures, ensuring alignment with the research protocol to maintain consistency. Utilize electronic data capture (EDC) systems to streamline data entry and minimize errors, thereby enhancing compliance and efficiency.
bioaccess® provides comprehensive services such as:
- Feasibility studies
- Site selection
- Compliance reviews
to support pilot clinical studies for Medtech in the study process. Once data is collected, conduct preliminary analyses to identify trends and patterns that inform the feasibility of the full-scale trial. This analysis should encompass:
- Safety data
- Efficacy measures
- Participant feedback
enabling researchers to make informed decisions about the next steps in the research process.
With over 20 years of expertise in Medtech, bioaccess® leverages its extensive experience to conduct pilot clinical studies for Medtech in Latin America, ensuring a tailored approach that meets the unique needs of clinical research endeavors.
Conclusion
Pilot clinical studies are instrumental in the advancement of medical technology, serving as a vital first step in evaluating the feasibility, safety, and potential of new treatments and devices. Through strategic planning and adherence to regulatory guidelines, these studies provide essential insights that shape the design of larger trials. The examples of eyeFlow, Inc. and PAVmed illustrate how successful pilot studies can minimize risks and refine protocols, ultimately enhancing the likelihood of success in subsequent phases.
Navigating the complexities of regulatory requirements and developing robust study protocols are crucial for the success of pilot studies. Engaging with regulatory experts and employing comprehensive clinical trial management services ensures that studies comply with necessary guidelines while addressing challenges such as participant recruitment and data collection. The emphasis on thorough training and clear communication with study participants further contributes to the reliability of the results obtained.
In conclusion, the importance of pilot clinical studies cannot be overstated. They lay the groundwork for informed decision-making and strategic advancements in medical technology, paving the way for innovations that can significantly improve patient care. As the healthcare landscape continues to evolve, stakeholders must prioritize these preliminary investigations, recognizing their critical role in the successful development of new medical interventions.




