Introduction
In the dynamic field of medical technology, pilot clinical studies serve as a critical stepping stone toward innovation and patient care improvement. These preliminary investigations not only assess the feasibility and safety of new medical devices and procedures but also provide invaluable insights that can shape the trajectory of larger-scale clinical trials.
With recent advancements highlighted by companies such as eyeFlow, Inc. and PAVmed, the significance of these studies is underscored by their potential to identify challenges early on, ultimately enhancing the success rates of subsequent trials.
As researchers navigate the complexities of conducting pilot studies, understanding the regulatory landscape and assembling a multidisciplinary team becomes essential for fostering effective collaboration and ensuring ethical compliance.
This article delves into the intricacies of pilot clinical studies in medtech, outlining crucial steps, regulatory considerations, and strategies for optimizing outcomes in the evolving landscape of healthcare innovation.
Understanding Pilot Clinical Studies in Medtech
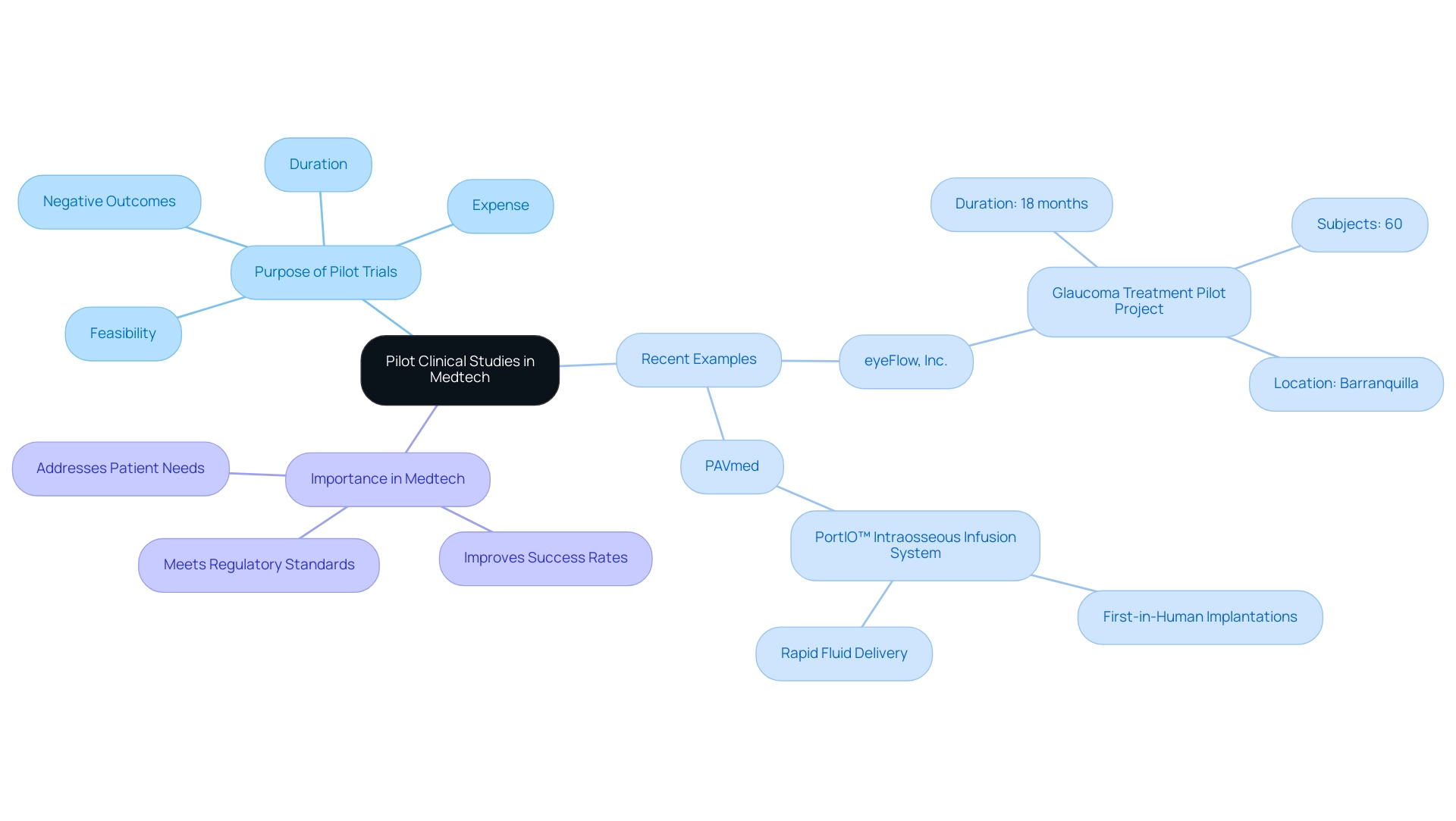
Pilot trials are initial investigations aimed at assessing the feasibility, duration, expense, and negative outcomes associated with a new medical technology or procedure. In the realm of medtech, these investigations are crucial as they assist researchers in recognizing potential problems prior to larger-scale trials. Recent examples from Colombia highlight their significance:
-
eyeFlow, Inc. has received approval from INVIMA to conduct an 18-month pilot project on an innovative glaucoma treatment aimed at reducing intraocular pressure, a key factor in managing glaucoma.
This investigation will recruit 60 subjects at a research center in Barranquilla, a location noted for its growing role in clinical research. -
Additionally, PAVmed successfully completed first-in-human implantations of its PortIO™ Intraosseous Infusion System, which is designed for rapid fluid delivery in emergency situations.
Such research offers essential insights into device performance and safety, enabling adjustments and refinements based on real-world data.
In the Dominican Republic, where the medtech landscape is rapidly changing, conducting pilot clinical studies for medtech can significantly improve the success rate of subsequent trials by ensuring that the technologies meet regulatory standards and effectively address patient needs.
Step-by-Step Process for Conducting Pilot Clinical Studies
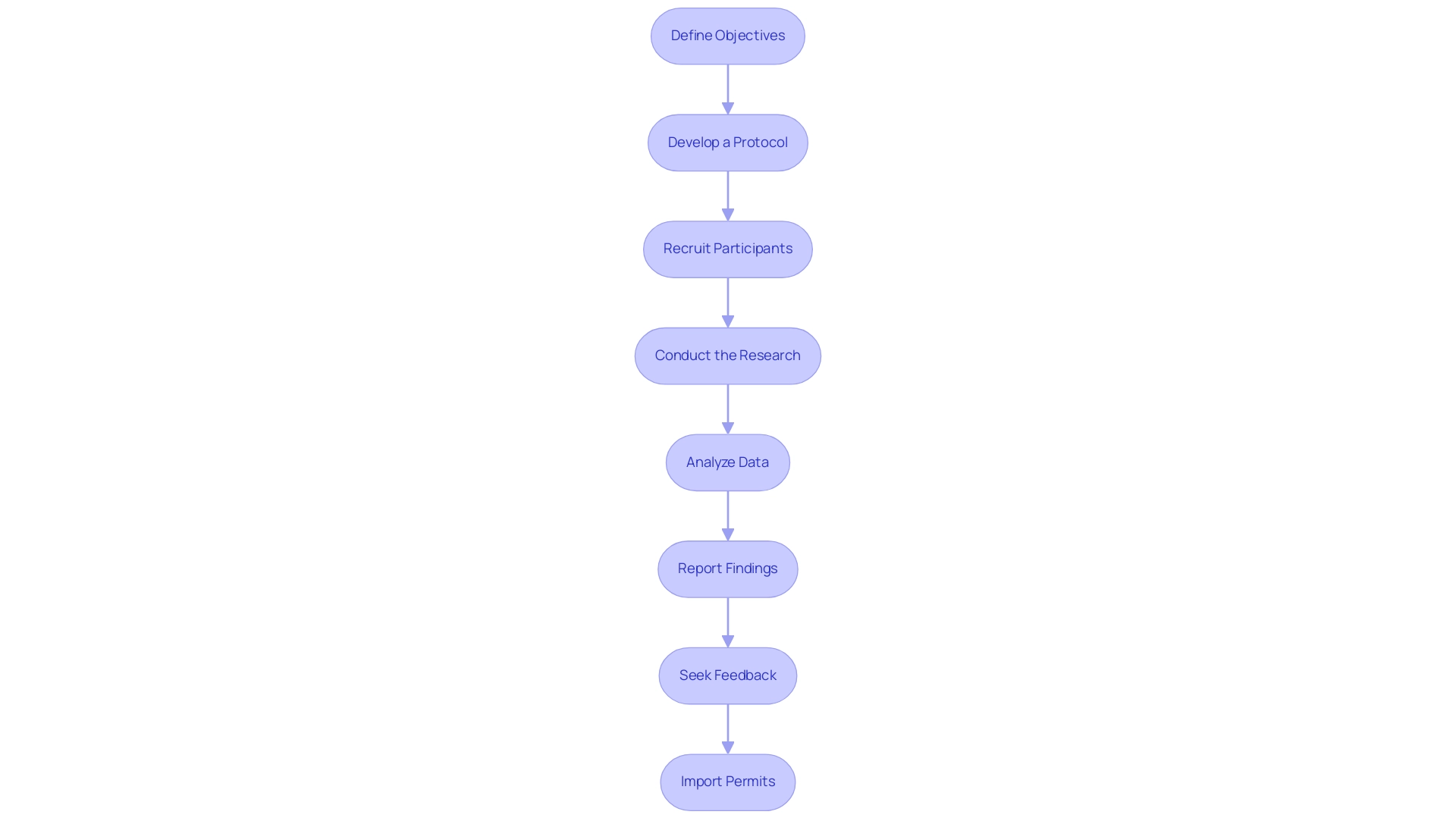
To carry out a preliminary clinical trial in medtech, follow these steps:
-
Define Objectives: Clearly outline the goals of the pilot project, including what you aim to validate or explore regarding the medical technology.
-
Develop a Protocol: Create a detailed research protocol that includes the methodology, participant criteria, data collection methods, and analysis plans. Ensure compliance with local regulations, particularly those set by INVIMA and other relevant authorities, in order to successfully pilot clinical studies for Medtech in the Dominican Republic.
-
Obtain Ethical Approval: Submit the research protocol to an ethics committee for review and approval. This step is crucial for ensuring participant safety and adherence to ethical standards.
-
Recruit Participants: Identify and recruit a suitable number of participants who meet the inclusion criteria. Engage with local healthcare providers to facilitate recruitment and ensure effective site selection, a service expertly offered by bioaccess®, which has over 20 years of experience in Medtech.
-
Conduct the Research: Implement the research according to the protocol, ensuring that all data is collected accurately and consistently. Monitor participant safety throughout the study.
-
Analyze Data: Once the research is complete, analyze the collected data to assess the technology's performance and identify any issues that arose during the investigation.
-
Report Findings: Prepare a comprehensive report outlining the results of the research, including any suggestions for future experiments or adjustments to the technology. This includes thorough project management and reporting services provided by bioaccess®.
-
Seek Feedback: Present the findings to stakeholders, including regulatory bodies and potential investors, to gather feedback and refine the approach for larger studies.
-
Import Permits: Ensure that all necessary import permits are acquired from Colombia's Ministry of Industry and Commerce (MinCIT) prior to shipping investigational devices to your location in Colombia.
By adhering to these steps and utilizing the extensive management services offered, including feasibility evaluations and project oversight, researchers can efficiently carry out preliminary research, such as Pilot Clinical Studies for Medtech in the Dominican Republic, that provides valuable insights into the advancement and application of medtech solutions in Latin America.
Key Regulatory Considerations for Pilot Studies
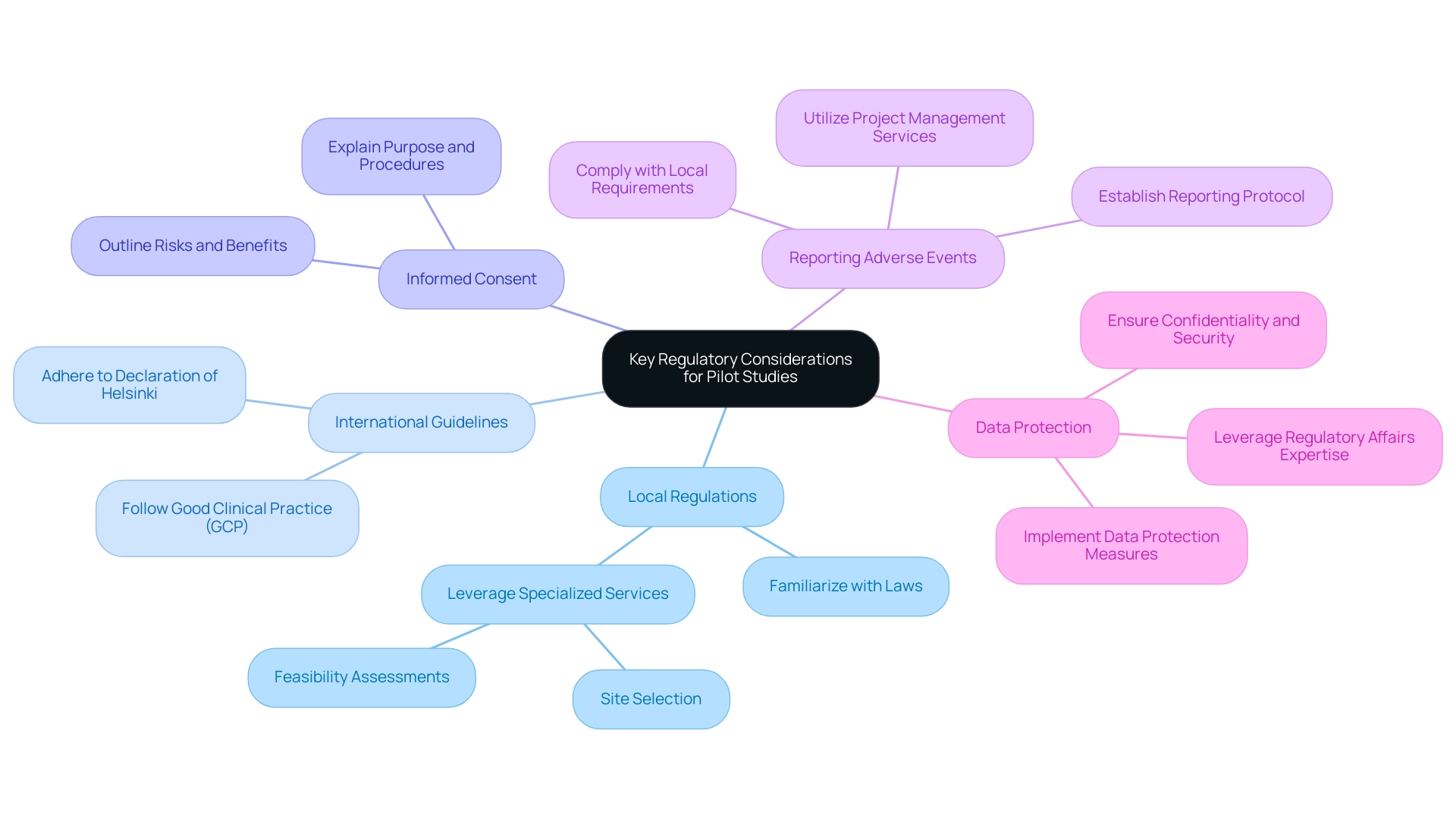
When conducting pilot medical trials in the Dominican Republic, researchers must be aware of the following regulatory considerations:
-
Local Regulations: Familiarize yourself with the laws and regulations governing clinical research in the Dominican Republic, including those set forth by the Ministry of Public Health. Adhering to these regulations is essential, and leveraging the knowledge of a specialized service provider like bioaccess®, which provides feasibility assessments and site selection, can simplify this process.
-
International Guidelines: Adhere to international guidelines such as the Declaration of Helsinki and Good Clinical Practice (GCP) to ensure ethical conduct and participant safety.
-
Informed Consent: Ensure that informed consent is obtained from all participants, clearly explaining the purpose, procedures, risks, and benefits of the research.
-
Reporting Adverse Events: Establish a protocol for reporting any adverse events or complications that arise during the research, complying with local reporting requirements. bioaccess® provides extensive project management and oversight services, including study setup and approval processes, to aid in this area effectively.
-
Data Protection: Implement measures to protect participant data in accordance with data protection laws, ensuring confidentiality and security. Utilizing bioaccess®'s expertise in Regulatory Affairs can improve adherence to these laws.
By addressing these regulatory factors and utilizing the management services offered by bioaccess®, including Early-Feasibility Assessments and First-In-Human Evaluations, researchers can conduct pilot clinical studies for medtech in the Dominican Republic that are not only effective but also ethical and compliant with legal standards.
Building a Multidisciplinary Research Team
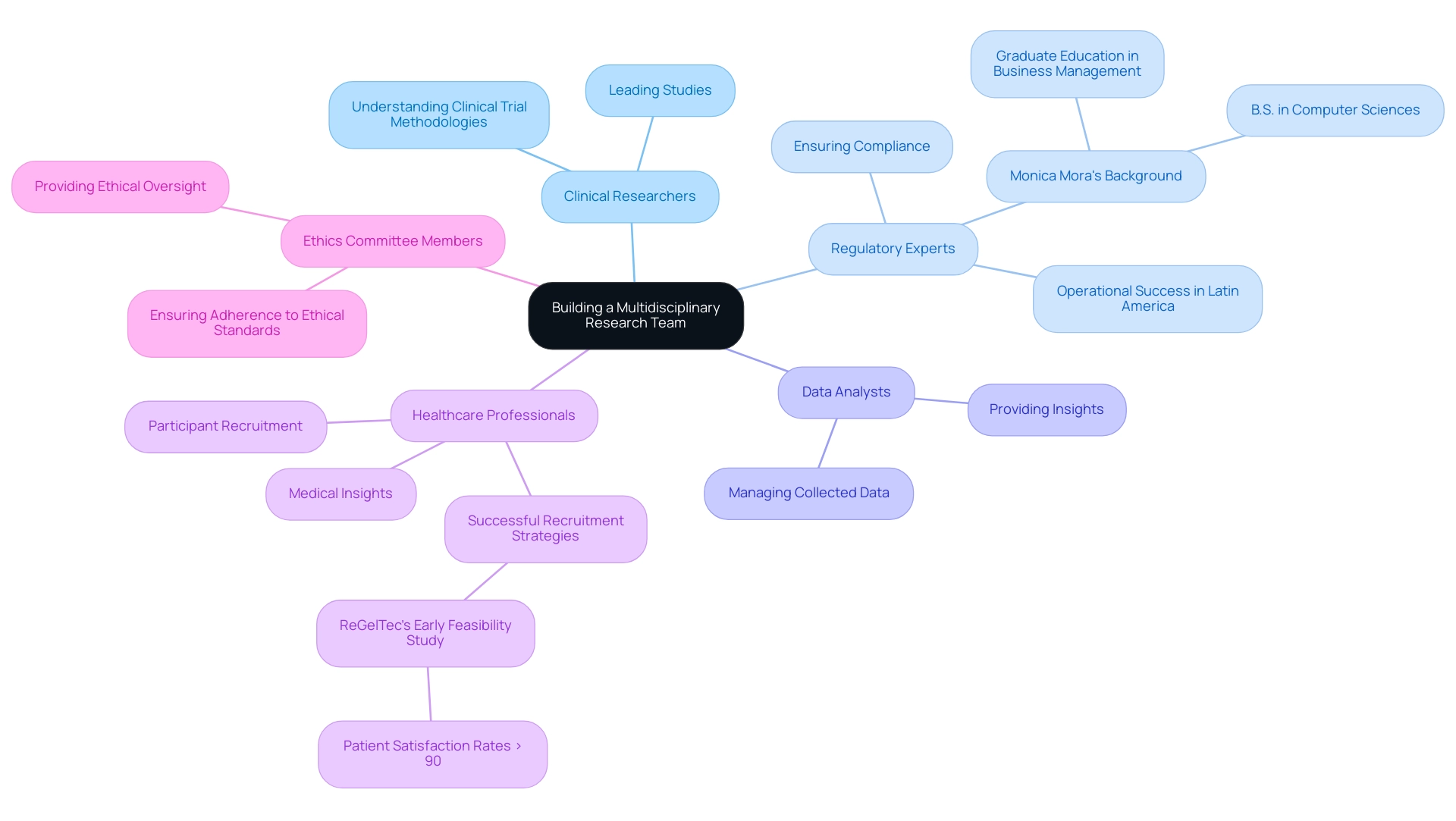
To maximize the success of pilot medical trials, it is essential to create a multidisciplinary research team that includes:
- Clinical Researchers: Experienced researchers who understand clinical trial methodologies and can lead the study.
- Regulatory Experts: Professionals well-versed in regulatory requirements, such as those represented by our Chief Operating Officer, Monica Mora. With a B.S. Degree in computer sciences and graduate education in business management, Monica has led regulatory strategies for various medical device companies in Latin America, ensuring compliance and operational success.
- Data Analysts: Specialists who can manage and analyze the data collected during the research, providing insights into the technology's performance.
- Healthcare Professionals: Physicians and other healthcare providers who can assist in participant recruitment and offer medical insights, paralleling the successful recruitment strategies seen in ReGelTec’s Early Feasibility Study for HYDRAFIL™ in Colombia, where patient satisfaction rates exceeded 90%.
- Ethics Committee Members: Individuals who can provide ethical oversight and ensure that the research adheres to ethical standards.
By fostering collaboration among these professionals, researchers can enhance the quality of their initial investigations, particularly in the context of pilot clinical studies for Medtech in the Dominican Republic. This collaborative approach is evident in GlobalCare Clinical Trials' partnership with bioaccess™, which has achieved over a 50% reduction in recruitment time and a retention rate exceeding 95%. Such strategies lead to more reliable results and effective medical technologies.
Evaluating Outcomes and Planning for Future Research
Following the conclusion of a preliminary medical trial, researchers should:
-
Assess Results: Analyze the data to determine if the pilot project met its objectives and identify any significant findings or challenges. For instance, ReGelTec's Early Feasibility Study on HYDRAFIL™ demonstrated effective treatment for chronic low back pain in eleven patients, showcasing the potential for similar studies.
-
Gather Feedback: Collect input from participants and team members to gain insights into the execution of the project and areas for enhancement, as experienced in Flow-FX's first-in-human examination of the Flow-Screw device, which aims to improve antibiotic delivery.
-
Make Recommendations: Based on the findings, provide suggestions for changes to the technology or research design for future experiments, ensuring alignment with best practices in medical research.
-
Plan Next Steps: If the preliminary research is successful, outline a strategy for large-scale medical experiments, including timelines, funding needs, and additional regulatory considerations. Global Care Clinical Trials exemplifies this by achieving over a 50% reduction in recruitment time and a 95% retention rate through their partnership with bioaccess™. This partnership emphasizes bioaccess®'s extensive clinical research management services, which encompass feasibility assessments, site selection, compliance reviews, setup, import permits, project management, and reporting.
-
Disseminate Findings: Share the results with stakeholders, including regulatory bodies, potential investors, and the medical community, to foster collaboration and support for future research.
By thoroughly evaluating outcomes and strategically planning for future research, researchers can ensure that Pilot Clinical Studies for Medtech in Dominican Republic contribute meaningfully to the advancement of medical technologies in Latin America. Additionally, understanding the regulatory landscape and compliance requirements is crucial for successful implementation in subsequent trials.
Conclusion
Pilot clinical studies are a vital component of the medical technology landscape, offering essential insights that pave the way for successful larger-scale trials. By understanding the step-by-step process of conducting these studies, researchers can effectively navigate the complexities associated with feasibility, safety, and regulatory compliance. The examples highlighted, such as eyeFlow, Inc. and PAVmed, illustrate the significant impact that pilot studies can have in identifying challenges early and refining technologies based on real-world data.
Moreover, assembling a multidisciplinary research team is crucial for maximizing the potential of pilot studies. Collaboration among clinical researchers, regulatory experts, data analysts, and healthcare professionals fosters an environment conducive to innovation and efficiency. The success stories from various studies underscore the importance of effective recruitment strategies and ethical oversight in achieving reliable results.
Ultimately, the insights gained from pilot clinical studies not only enhance the success rates of subsequent trials but also contribute to the continuous improvement of patient care. As the medtech landscape evolves, prioritizing these preliminary investigations will be instrumental in addressing patient needs and adhering to regulatory standards. By leveraging the knowledge and strategies outlined, researchers can significantly influence the future of medical technology and its impact on healthcare.




