Overview
The article provides a comprehensive guide on conducting pivotal studies for medical device approval in Brazil, emphasizing the importance of understanding the regulatory framework and following a systematic approach. It outlines key steps such as defining study objectives, obtaining ethical approvals, and ensuring compliance with ANVISA regulations, demonstrating that thorough preparation and expert guidance are essential for a successful approval process.
Introduction
Navigating the complex landscape of medical device approval in Brazil necessitates a comprehensive understanding of the regulatory framework established by ANVISA. This process is not merely a formality; it involves:
- A meticulous classification of devices
- Adherence to stringent guidelines
- A commitment to ethical research practices
With the increasing demand for innovative medical solutions, stakeholders must equip themselves with the knowledge and tools required to successfully:
- Design pivotal studies
- Implement Good Clinical Practices (GCP)
- Ensure compliance with regulatory standards
By examining the intricacies of the approval process and the essential steps to follow, this article aims to illuminate the path toward successful market entry for medical devices in Brazil, ultimately contributing to enhanced patient care and safety.
Understanding Brazil's Regulatory Framework for Medical Device Approval
Before starting pivotal studies for medical device approval in Brazil, a comprehensive grasp of the framework set by the health authority is essential. This framework includes a detailed classification system for medical devices, categorized from Class I (low risk) to Class IV (high risk), each demanding specific approval requirements. For instance, registration for food products with the health agency is valid for five years, highlighting the regulatory landscape's complexity.
Familiarity with the guidelines on Good Clinical Practices (GCP) and Good Manufacturing Practices (GMP) is crucial, as these are part of the pivotal studies for medical device approval Brazil that govern the conduct of clinical trials. Furthermore, compliance with Brazilian legislation, particularly Law No. 6,437/1977, which regulates health surveillance, must be ensured.
A case study on borderline products classification illustrates these complexities, as products like dermocosmetics often require registration with ANVISA to meet safety and efficacy standards. To navigate these intricacies effectively, it is advisable to engage with compliance consultants or legal advisors who specialize in pivotal studies for medical device approval Brazil. These experts can assist in various services, including:
- Trial set-up
- Obtaining import permits
- Ensuring compliance with reporting requirements
Experts like Katherine Ruiz, a recognized authority in compliance matters for medical devices and in vitro diagnostics in Colombia, and Ana Criado, Director of Compliance Affairs at Mahu Pharma, offer invaluable assistance in enabling a successful approval journey. According to a regulatory expert, 'Understanding the nuances of ANVISA regulations is critical for any successful medical device approval process,' emphasizing the importance of their expertise in this complex landscape.
Step-by-Step Guide to Designing Pivotal Studies for Medical Devices
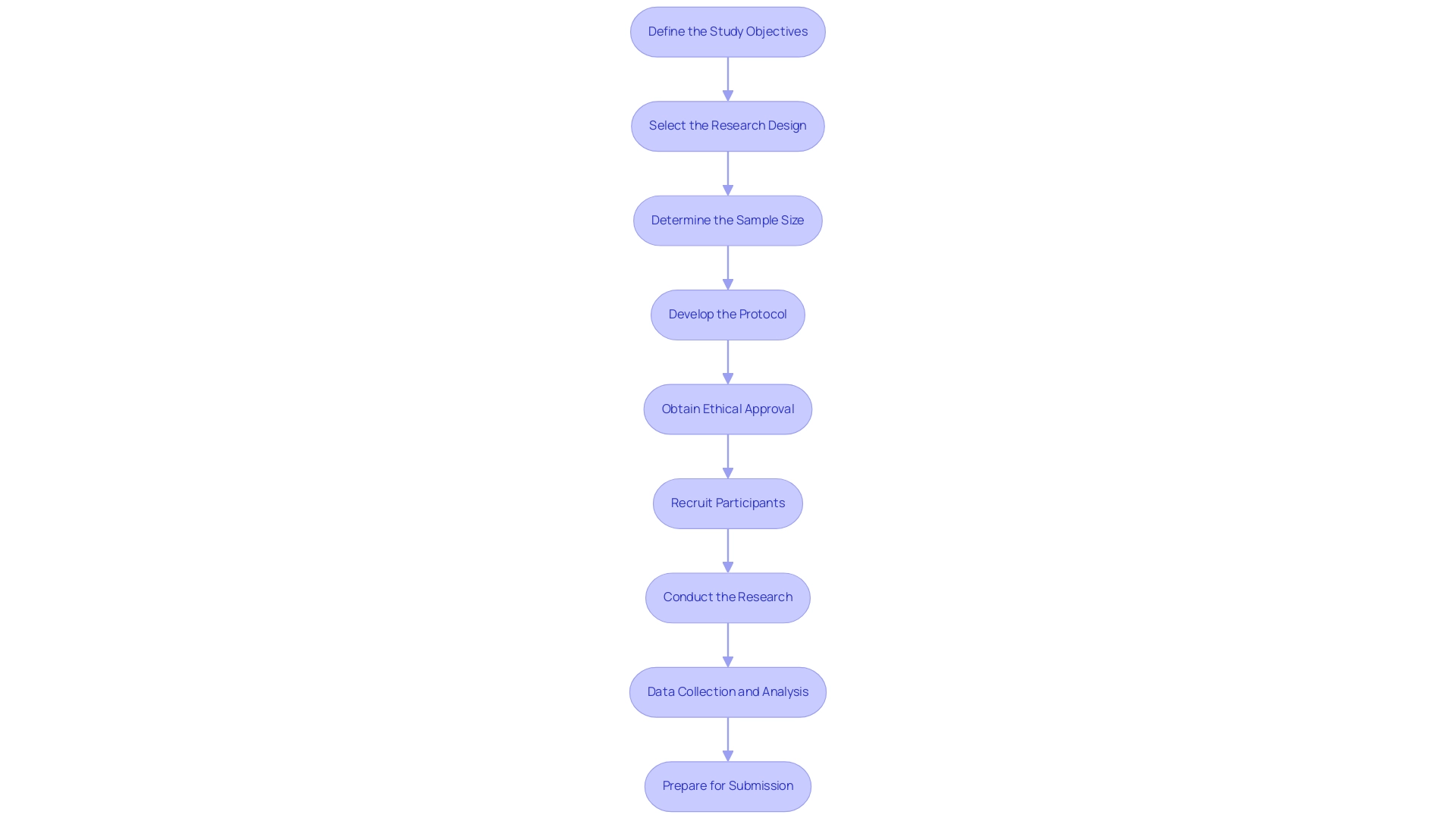
Designing pivotal studies for medical device approval in Brazil requires a systematic approach, and partnering with a trusted CRO like bioaccess® can enhance this process significantly. Here are the essential steps to follow:
- Define the Study Objectives: Clearly articulate both the primary and secondary objectives. This foundational step shapes the research's design and methodology, ensuring alignment with clinical goals.
- Select the Research Design: Choose an appropriate research design tailored to the objectives and the specific medical device under investigation. Common designs include randomized controlled trials and cohort studies, each serving distinct purposes.
- Determine the Sample Size: Calculate the necessary sample size to achieve statistical significance. For instance, Phase I trials typically necessitate 20-80 patients, while Phase II trials usually require 100-200 patients. It is crucial to factor in potential dropouts by increasing the sample size by 5-10% for enhanced reliability. As AV Gusev emphasizes, "Sample size calculation for clinical trials of medical decision support systems with binary outcome" is a critical aspect of clinical trial design that cannot be overlooked. Furthermore, a case analysis titled "Sample Size Calculation for Predictive Models" highlights the necessity of careful sample size planning, concluding that an increase of 5-10% is essential to ensure reliability, particularly when accounting for potential dropouts.
- Develop the Protocol: Draft a comprehensive research protocol that encompasses the design, methodology, inclusion/exclusion criteria, endpoints, and statistical analysis plan. Ensure that this protocol aligns with ANVISA guidelines, which influence various aspects of research design, including patient safety, ethical considerations, and data integrity to meet regulatory standards.
- Obtain Ethical Approval: Submit the protocol for review and approval by an Institutional Review Board (IRB) or Ethics Committee. This critical step safeguards participant welfare and upholds ethical standards in clinical research.
- Recruit Participants: Implement a targeted recruitment strategy aimed at the appropriate patient demographic, ensuring that informed consent is thoroughly obtained from all participants. Leveraging bioaccess®'s extensive network and expertise can streamline this process, enhancing recruitment efficiency.
- Conduct the Research: Execute the research in strict accordance with the approved protocol, maintaining adherence to Good Clinical Practice (GCP) guidelines throughout the research process.
- Data Collection and Analysis: Collect data meticulously and analyze it in accordance with the statistical plan outlined in the protocol. Emphasizing data integrity is paramount to ensure the validity of the research results.
- Prepare for Submission: Compile the findings and prepare the requisite documentation for submission to ANVISA. This encompasses the medical research report (CSR) and any supplementary materials needed for review by authorities.
By following these steps and working with bioaccess®, which has over 20 years of experience in the Medtech sector, you will be well-equipped to create pivotal studies for medical device approval in Brazil that not only fulfill compliance requirements but also positively impact the successful approval of your medical device. Additionally, bioaccess® offers comprehensive services including compliance reviews, project management, and ongoing support, ensuring a streamlined clinical trial process.
Implementing Good Clinical Practices (GCP) in Your Study
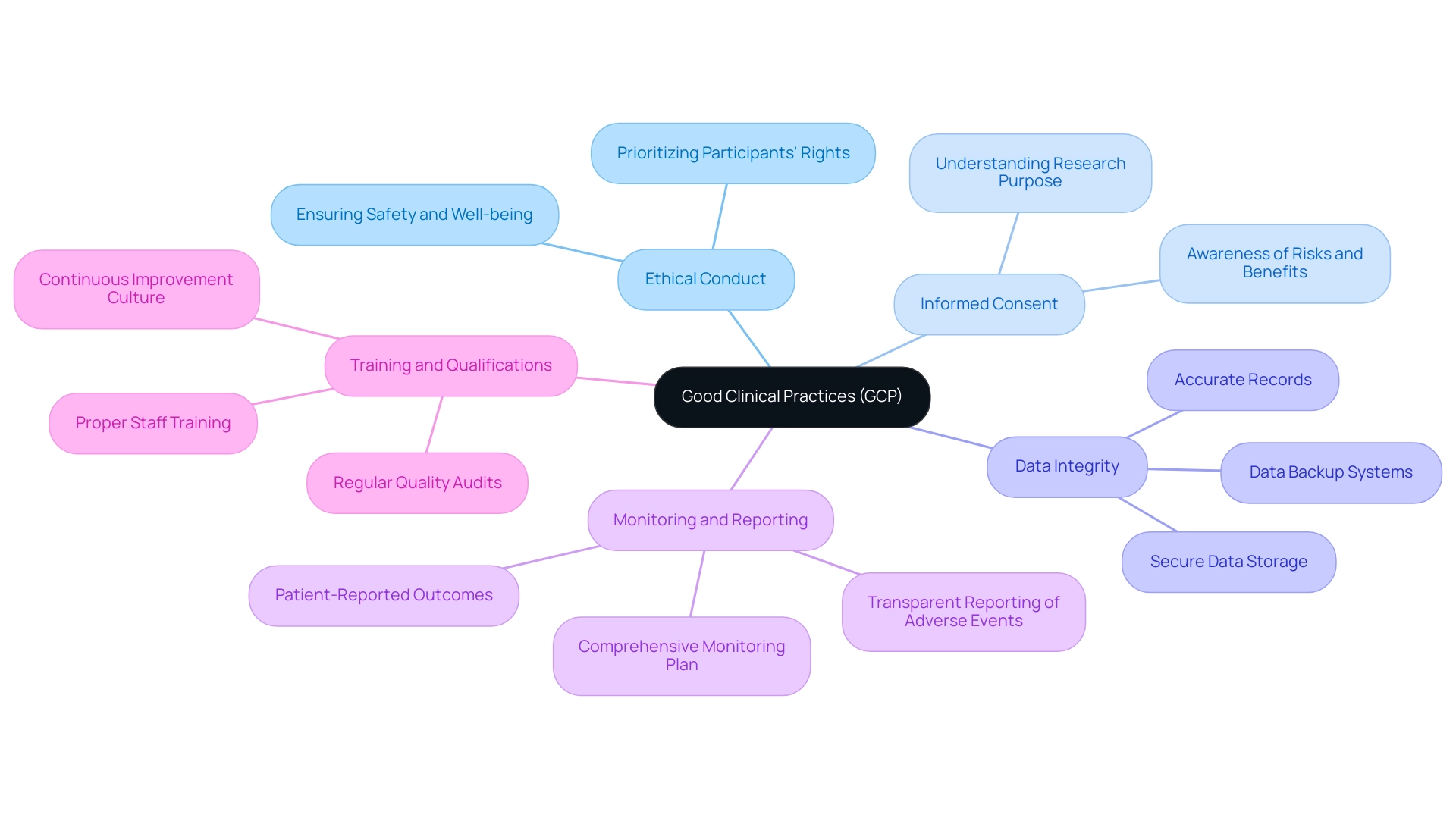
Implementing Good Clinical Practices (GCP) in crucial trials is essential for ensuring ethical standards and regulatory compliance. Here are several key principles to consider:
- Ethical Conduct: Designing and carrying out research with an unwavering commitment to ethics is paramount. This entails prioritizing participants’ rights, safety, and overall well-being throughout the research process.
- Informed Consent: Securing informed consent from all participants is critical. Participants must fully understand the research's purpose, procedures, potential risks, and benefits prior to enrollment, as their informed choice significantly impacts trial outcomes.
- Data Integrity: Upholding data integrity is vital for credible research. This involves maintaining accurate and complete records of all study-related activities. Implement robust systems for data collection, management, and analysis to safeguard this integrity. Additionally, secure data storage and backup systems are crucial to protect participant rights and privacy.
- Monitoring and Reporting: A comprehensive monitoring plan is essential for overseeing progress and ensuring adherence to the protocol. For example, patient-reported outcomes were suspiciously consistent across all participants at one site, highlighting the importance of diligent monitoring and data integrity in clinical trials. Prompt and transparent reporting of any adverse events is crucial to maintain trust and compliance.
- Training and Qualifications: It is imperative that all staff involved in the research are properly trained and qualified for their specific roles. Frequent updates and training sessions not only improve adherence to GCP standards but also promote a culture of ongoing enhancement, as emphasized in examples of GCP compliance. As Kate from Pharma Focus Europe states, "With a passion for translating complex pharmaceutical concepts, we contribute to the mission of delivering up-to-date and impactful information to the global Pharmaceutical community."
In the context of Colombia, it is also important to consider the role of INVIMA (Instituto Nacional de Vigilancia de Medicamentos y Alimentos) in overseeing adherence to standards for research trials. Juan Cuya, MD, a Clinical Trial Associate with knowledge in compliance matters, underscores the importance of aligning practices with INVIMA's guidelines to ensure successful research outcomes.
By following these principles of GCP and incorporating the regulatory framework established by INVIMA, you not only fulfill regulatory obligations but also improve the credibility and reliability of your research outcomes, ultimately aiding in the progression of ethical standards in medical research.
Navigating the Approval Process with ANVISA
Successfully navigating the regulatory approval process requires a strategic approach, particularly when supported by extensive trial management services such as those provided by bioaccess®, which has over 20 years of experience in Medtech. Here are the essential steps to follow:
- Prepare Documentation: Begin by ensuring that all required documentation is meticulously complete and accurate. This includes the clinical study protocol, investigator brochure, informed consent forms, and any additional supporting documents essential for your submission. bioaccess® can assist in compiling and reviewing these documents to ensure compliance with regulatory standards.
- Submit the Application: Utilize the electronic system for application submission, making certain that all documents are uploaded correctly to avoid delays. bioaccess® provides guidance on the submission process, ensuring that all requirements are met.
- Respond to Queries: It is crucial to be prepared for any queries or requests for additional information from the regulatory agency during the review process. Prompt and thorough responses can significantly expedite the approval timeline, especially given the recent news indicating an anticipated 30% reduction in analysis time due to the optimized analysis procedure. This emphasizes the importance of timely communication, a service that bioaccess® prioritizes to ensure a successful submission.
- Monitor Application Status: Regularly check the status of your application through the online portal. Staying proactive in monitoring allows you to identify and address any potential issues before they escalate. bioaccess® offers support in tracking application progress and addressing any concerns that may arise.
- Prepare for Audits: The regulatory agency may conduct audits or inspections as part of their approval process. Ensure that all study-related activities and documentation are well-organized and readily accessible to facilitate a smooth audit process. bioaccess® can help in preparing for these audits by ensuring that all documentation is in order and that all team members are informed of the audit procedures.
By following these steps and maintaining a proactive approach, you can effectively navigate the ANVISA approval process for pivotal studies for medical device approval in Brazil, thereby increasing the likelihood of a successful result for your research. As noted by regulatory consultants, a well-prepared submission can significantly enhance the efficiency of the approval journey. With bioaccess®'s expertise in early-feasibility, first-in-human, pilot, pivotal, and post-market follow-up studies, you’ll have the support needed to streamline compliance and project management.
As Mario Henrique Furlanetto Miranda states, "Conceptualization, Investigation, Writing – review & editing" are critical components that contribute to a successful submission. Furthermore, the complexity of biological product registrations often presents challenges that highlight the necessity of meticulous documentation and proactive communication.
Post-Approval Activities and Market Launch Considerations
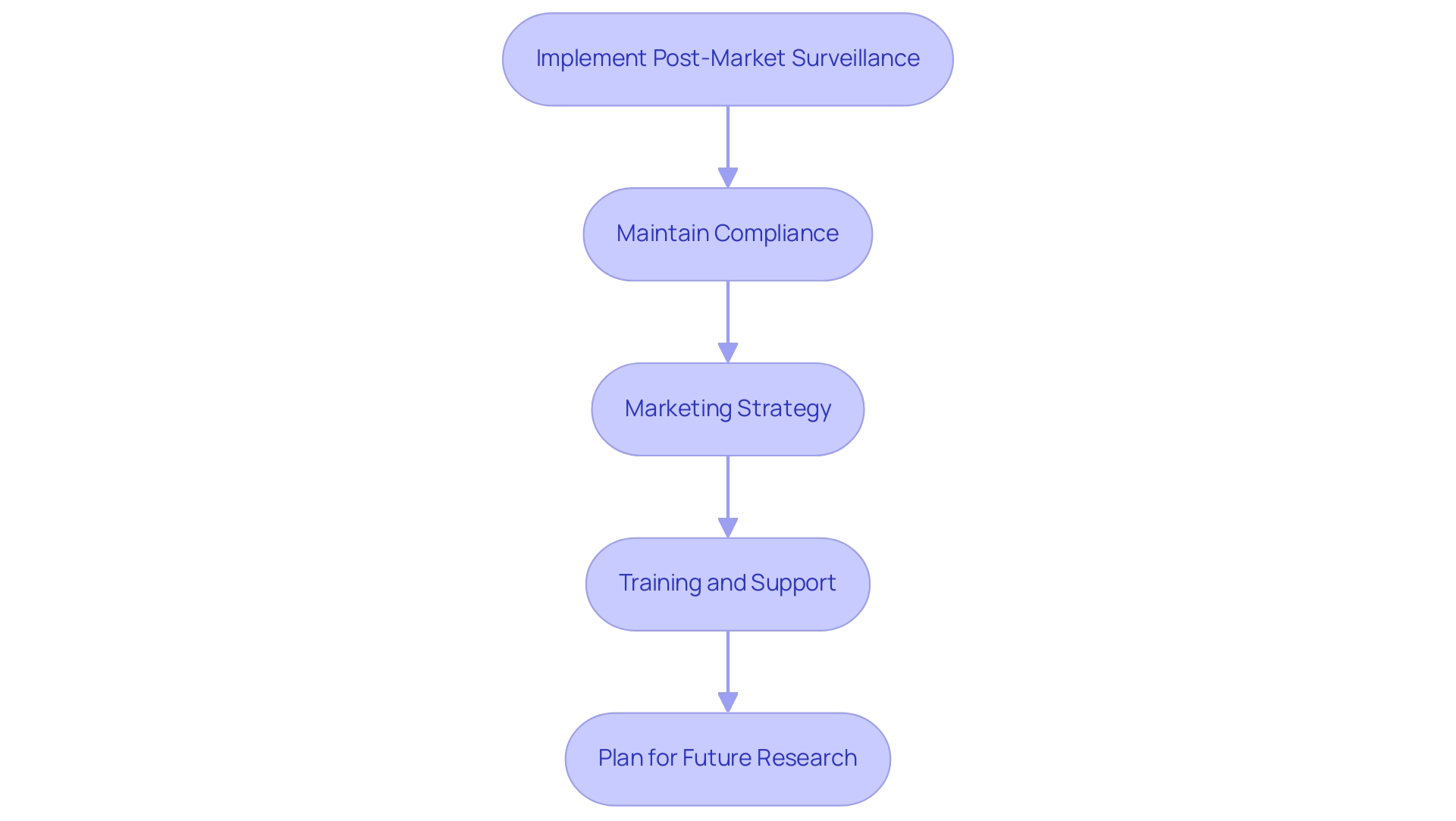
Following authorization from the regulatory agency, it is crucial to engage in a series of post-approval activities that will facilitate a successful market launch:
- Implement Post-Market Surveillance: Establish a robust post-market surveillance plan to continuously monitor the device's performance and safety in clinical use. This plan should encompass the systematic collection of data related to adverse events as well as feedback from users, ensuring that any emerging safety concerns are promptly addressed. bioaccess® can assist in designing and executing this surveillance plan, leveraging its expertise in managing Post-Market Clinical Follow-Up Studies (PMCF).
- Maintain Compliance: Ongoing compliance with ANVISA regulations is imperative. Routine audits should be performed to ensure compliance with any new or evolving legal requirements. It is essential to have documented procedures in place for serious adverse events, including the requirement to report to SNVS within the required timeframe. Updating your quality management system as necessary will also support sustained compliance, an area where bioaccess®'s experience in Early-Feasibility Studies (EFS) and First-In-Human Studies (FIH) can be invaluable.
- Marketing Strategy: Craft a comprehensive marketing strategy that emphasizes the benefits and unique features of your medical device. Actively engage with healthcare professionals and relevant stakeholders at major healthcare trade shows in Brazil, such as the Sao Paulo International Dental Congress, Hospitalar, and HIS, to foster awareness and encourage adoption, ensuring your product stands out in a competitive market.
- Training and Support: Deliver thorough training and support to healthcare providers and users to ensure they are well-informed about the proper use of the device. This initiative not only enhances user confidence but also improves overall satisfaction with the product.
- Plan for Future Research: Strategically arrange further evaluations, such as Early-Feasibility Assessments (EFA) or First-In-Human Trials (FIH), to further assess the device's performance or to explore new indications for use. Such research can provide valuable insights that enhance future marketing efforts and compliance submissions. For instance, understanding adverse event reporting conditions can clarify regulatory obligations and potential exemptions, ensuring informed decision-making.
By concentrating on these essential post-approval activities, alongside leveraging the expertise offered by bioaccess® in managing pivotal studies for medical device approval in Brazil, you can significantly enhance the likelihood of your medical device's success in the Brazilian market, ultimately contributing to improved patient outcomes. For more information, please reach out to Jefferson Oliveira, Healthcare, Life Sciences, and Biotech Sectors Commercial Specialist, at Jefferson.Oliveira@trade.gov.
Conclusion
Navigating the medical device approval landscape in Brazil requires a deep understanding of the regulatory framework established by ANVISA. The classification of devices, adherence to stringent guidelines, and a commitment to ethical research practices are paramount to ensuring compliance and successful market entry. Stakeholders must prioritize the design of pivotal studies, implement Good Clinical Practices (GCP), and remain vigilant in their adherence to regulatory standards.
A systematic approach to designing pivotal studies, including:
- Defining objectives
- Selecting appropriate study designs
- Ensuring robust data integrity
is essential for meeting regulatory expectations. Furthermore, the importance of maintaining ethical principles, such as informed consent and participant safety, cannot be overstated. These elements collectively enhance the credibility and reliability of clinical research, which is critical for gaining approval.
Post-approval activities play a crucial role in the successful launch and sustained market presence of medical devices. Implementing effective post-market surveillance, maintaining ongoing compliance, and developing comprehensive marketing strategies are vital steps in this process. By actively engaging with healthcare providers and continuously evaluating device performance, stakeholders can ensure that their products not only meet regulatory requirements but also contribute positively to patient care and safety.
Ultimately, the journey through the medical device approval process in Brazil is intricate yet rewarding. By leveraging the right expertise, adhering to established guidelines, and committing to ethical practices, stakeholders can navigate these complexities effectively, paving the way for innovative medical solutions that enhance healthcare outcomes.
Frequently Asked Questions
What is the classification system for medical devices in Brazil?
The classification system categorizes medical devices from Class I (low risk) to Class IV (high risk), with each class requiring specific approval requirements.
How long is the registration for food products valid in Brazil?
Registration for food products with the health agency is valid for five years.
Why is familiarity with Good Clinical Practices (GCP) and Good Manufacturing Practices (GMP) important?
Familiarity with GCP and GMP is crucial as they govern the conduct of clinical trials, which are part of the pivotal studies for medical device approval in Brazil.
What legislation must be complied with for health surveillance in Brazil?
Compliance with Brazilian legislation, specifically Law No. 6,437/1977, which regulates health surveillance, must be ensured.
What is a case study example mentioned in the article?
A case study on borderline products classification shows that products like dermocosmetics often require registration with ANVISA to meet safety and efficacy standards.
Who can assist with the complexities of medical device approval in Brazil?
Engaging with compliance consultants or legal advisors who specialize in pivotal studies for medical device approval can help navigate these complexities.
What services can compliance experts provide?
Compliance experts can assist with trial set-up, obtaining import permits, and ensuring compliance with reporting requirements.
What are the essential steps for designing pivotal studies for medical device approval in Brazil?
The essential steps include defining study objectives, selecting research design, determining sample size, developing the protocol, obtaining ethical approval, recruiting participants, conducting the research, data collection and analysis, and preparing for submission.
How many patients are typically needed for Phase I and Phase II trials?
Phase I trials typically require 20-80 patients, while Phase II trials usually require 100-200 patients.
What should be included in the research protocol?
The research protocol should encompass the design, methodology, inclusion/exclusion criteria, endpoints, and statistical analysis plan, aligning with ANVISA guidelines.
What is the role of the Institutional Review Board (IRB) or Ethics Committee?
The IRB or Ethics Committee reviews and approves the research protocol to safeguard participant welfare and uphold ethical standards in clinical research.
What is emphasized during data collection and analysis?
Emphasizing data integrity is paramount to ensure the validity of the research results during data collection and analysis.
What documentation is required for submission to ANVISA?
The submission to ANVISA includes the medical research report (CSR) and any supplementary materials needed for review by authorities.




