Introduction
Navigating the regulatory landscape for Post-Market Clinical Follow-Up (PMCF) studies in Panama presents a complex yet essential challenge for organizations aiming to ensure the safety and efficacy of medical devices. With the oversight of the Panamanian Ministry of Health and evolving regulations, including recent changes that impose fines for non-compliance, understanding the intricate requirements is paramount.
This article delves into the critical steps necessary for implementing a robust PMCF strategy, emphasizing the importance of collaboration with local experts and adherence to ethical standards. By exploring the nuances of stakeholder engagement, data management, and compliance, organizations can better position themselves to thrive in this dynamic environment, ultimately enhancing patient outcomes and operational integrity.
Navigating the Regulatory Framework for PMCF in Panama
Carrying out assessments for Post-Market Clinical Follow-Up Panama requires a comprehensive understanding of the regulations set by the Panamanian Ministry of Health (MINSA) and the National Directorate of Medicines and Health Technologies. Engaging bioaccess®, with over 20 years of expertise in managing clinical trials, ensures that your post-market clinical follow-up plan aligns with crucial regulatory documents, such as the Medical Device Regulations and specific guidelines for post-market surveillance. It is essential to meticulously review these documents to guarantee compliance and obtain necessary approvals while adhering to established ethical standards.
Furthermore, leveraging the knowledge of local experts or legal advisors can provide invaluable insights into effectively navigating the complexities of the regulatory landscape. Recent regulatory changes, including the introduction of fines for late delivery of medicines—'In the case of late delivery of medicines or other products for human health, devices, and medical supplies, a fine will be applied that will never be less than 10% of the value of the portion not delivered'—highlight the importance of staying informed to maintain operational integrity and compliance. The Centralized Purchasing and Pricing System in Panama exemplifies these regulatory changes, aiming to emulate modern health systems in developed countries and reduce the time to acquire medications.
This system is expected to decrease medicine prices by 20% to 30%, allowing health authorities to prioritize quality and effectiveness over cost alone. The law took over two years to reach approval, underscoring the complexity and duration of the regulatory process that must be navigated. bioaccess® focuses on various kinds of research, including:
- Early-Feasibility Assessments (EFS)
- First-In-Human Trials (FIH)
- Pilot Evaluations
- Pivotal Investigations
- Post-Market Clinical Follow-Up Panama
Our service capabilities encompass:
- Feasibility and selection of research sites and principal investigators
- Comprehensive trial setup
- Project management
- Rigorous monitoring and reporting
This ensures a thorough approach to Post-Market Clinical Follow-Up Panama studies.

Step-by-Step Guide to Implementing Post-Market Clinical Follow-Up Activities
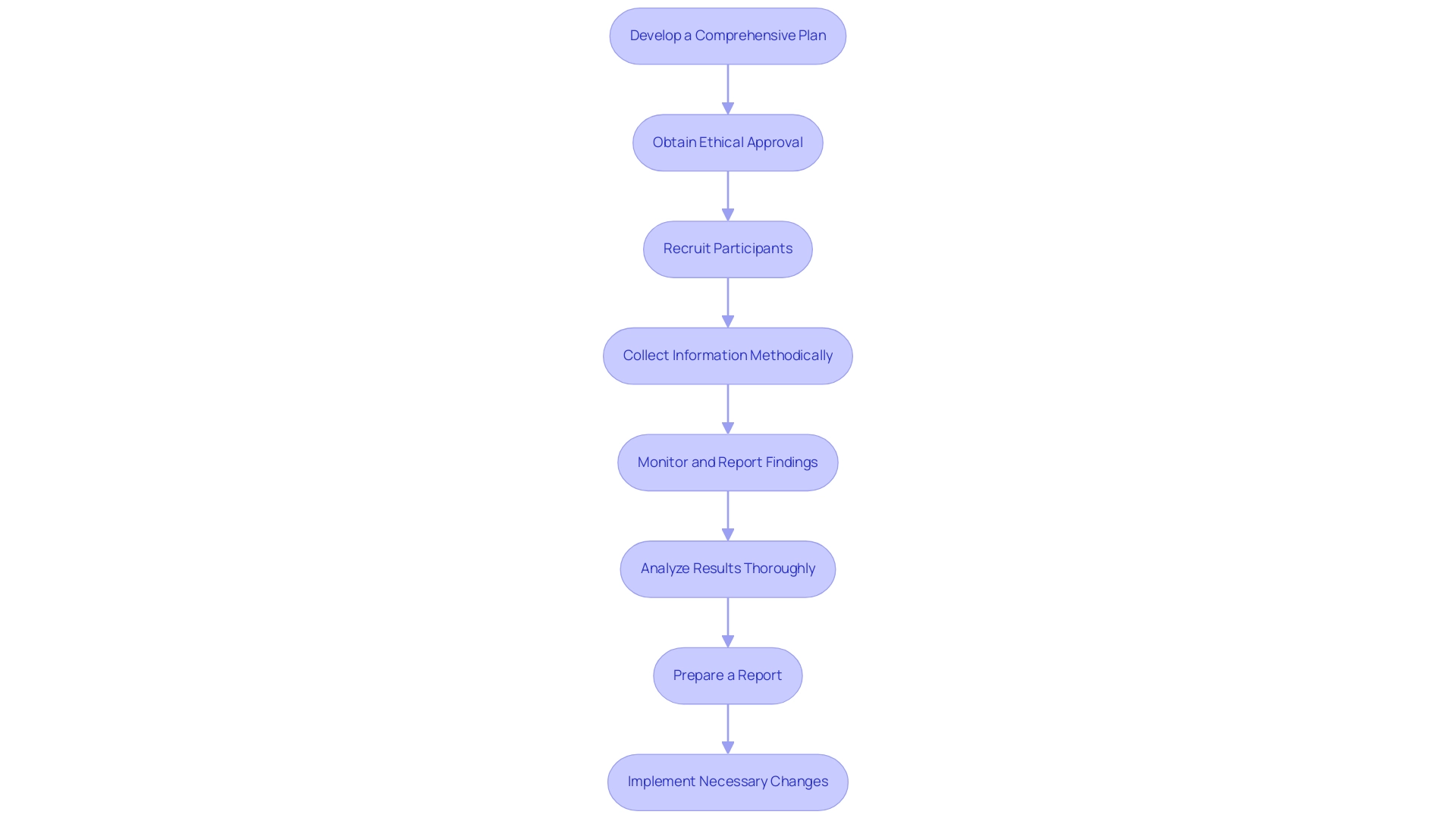
- Develop a comprehensive plan for Post-Market Clinical Follow-Up Panama by formulating a detailed plan that clearly outlines the objectives, methodologies, and timelines. This strategic document should align with regulatory requirements, including a thorough risk management strategy to address potential challenges associated with the medical device. Notably, both Class IIb implantable and Class III devices require a conformity assessment by a notified body, which should be a key consideration in your planning. Utilizing the knowledge of a trial management team, such as bioaccess®, which possesses over 20 years of experience in Medtech, can guarantee that your post-market follow-up plan is strong and in accordance.
- Obtain Ethical Approval: It is imperative to submit your Post-Market Clinical Follow-Up Panama plan to the relevant ethics committee for approval. This process is essential to ensure adherence to ethical standards and the protection of participant rights, which is a fundamental aspect of conducting clinical research.
- Recruit Participants: Identify and enlist participants for the Post-Market Clinical Follow-Up Panama activities carefully. It is crucial to obtain informed consent that aligns with ethical guidelines, thereby upholding the integrity of the study.
- Collect Information Methodically: Execute the information gathering process as delineated in your Post-Market Clinical Follow-Up Panama plan. Employ standardized forms and methodologies to guarantee consistency and reliability in the information gathered, which is vital for meaningful analysis.
- Monitor and Report Findings: In the context of Post-Market Clinical Follow-Up Panama, continuously oversee the collection process, ensuring timely reporting of any adverse events or significant findings to the appropriate regulatory authorities, as mandated by compliance protocols. Collaborating with a partner like bioaccess® can streamline this process, given their deep experience in project management and monitoring.
- Analyze Results Thoroughly: Upon completion of information collection, conduct a comprehensive analysis of the results to evaluate the safety and performance of the medical device during the Post-Market Clinical Follow-Up Panama. This step is essential to understanding the device's therapeutic benefits and ensuring compliance with INVIMA regulations.
- Prepare a report: Compile your findings into a structured report that summarizes the data collected, the analyses performed, and the conclusions drawn. This document should be submitted to regulatory authorities as part of your compliance obligations, ensuring that it meets the standards set by INVIMA and other relevant bodies.
- Implement Necessary Changes: If the findings reveal any issues with the medical device, collaborate with your team to develop and implement corrective actions aimed at enhancing safety and effectiveness. This proactive approach not only addresses immediate concerns but also fosters continuous improvement in device performance and patient outcomes.
Alongside bioaccess®, the company also focuses on overseeing Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, and Pivotal Studies, offering a thorough range of trial management services. As Dr. Amie Smirthwaite, Senior Vice President of Intelligence and Innovation at RQM+, states, "This collaborative workflow helps bridge any product gaps that may be identified and build in-house skills and capabilities to deliver transformative solutions." Furthermore, referring to the RQM+ case analysis, their approach to compliance and proactive PMS plans illustrates how effective strategies can lead to successful outcomes and enhanced device performance.
Engaging Stakeholders in PMCF
To effectively engage stakeholders during post-market clinical follow-up (PMCF) studies in Latin America, particularly under the oversight of INVIMA, it is essential to implement comprehensive strategies that foster collaboration and enhance outcomes:
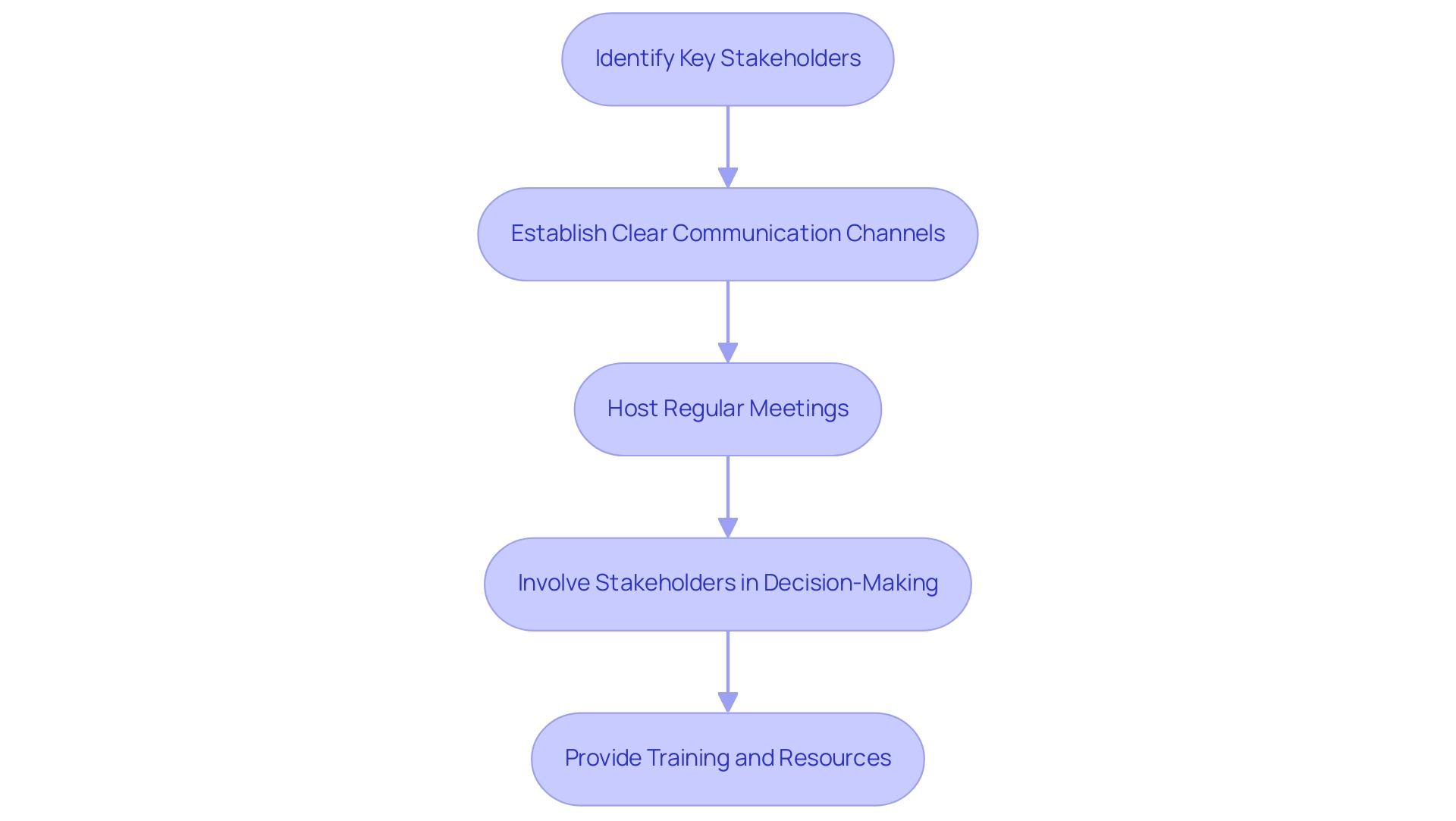
- Identify Key Stakeholders: Recognize and map out key stakeholders, including regulatory bodies like INVIMA, ethics committees, healthcare professionals, and patient advocacy groups. Understanding their roles and perspectives is crucial for successful engagement, especially given INVIMA's role in ensuring the safety and efficacy of medical devices.
- Establish Clear Communication Channels: Develop and maintain open lines of communication to facilitate ongoing information sharing and feedback. This ensures that all stakeholders are kept informed and can voice their concerns or suggestions throughout the process, promoting transparency in line with INVIMA's regulatory functions.
- Host Regular Meetings: Organize regular meetings or updates to keep stakeholders apprised of PMCF activities. These sessions offer chances to discuss progress, tackle any emerging concerns, and strengthen stakeholder involvement in the ongoing research, ensuring alignment with bioaccess®’s commitment to managing clinical trials effectively, including Early-Feasibility Trials (EFS), First-In-Human Trials (FIH), Pilot Trials, and Pivotal Trials.
- Involve Stakeholders in Decision-Making: Actively encourage stakeholder input in decision-making processes, particularly in areas such as research design and participant recruitment strategies. This approach enhances buy-in and strengthens the collective commitment to the success of the research. As noted by Bombard et al., "the main enablers of engagement were related to techniques to improve the design, recruitment, involvement and leadership action, and to create a receptive context."
- Provide Training and Resources: Offer targeted training sessions and resources to stakeholders, equipping them with the knowledge required to understand the process and their specific roles within it. Such initiatives significantly improve engagement and the effectiveness of communication strategies.
These strategies align with findings from a systematic review by Bombard et al., which identified that successful patient engagement hinges on techniques to improve design, recruitment, involvement, and leadership action. Moreover, insights from the case analysis titled 'Patient Engagement in Quality of Care' highlight that while most patient experiences were positive, some felt their involvement was tokenistic, emphasizing the need for genuine engagement. Additionally, the analysis of 32 cases of corporate social innovations (CSI) underscores the impact of effective engagement strategies in reducing inequality and enhancing sustainability within PMCF.
By cultivating a receptive environment and emphasizing authentic stakeholder involvement, organizations can improve the quality of care and ensure safety and effectiveness in their Post-Market Clinical Follow-Up Panama evaluations. With over 20 years of experience in Medtech, bioaccess® brings the expertise necessary to navigate these complex processes successfully.
Data Management and Compliance in PMCF
-
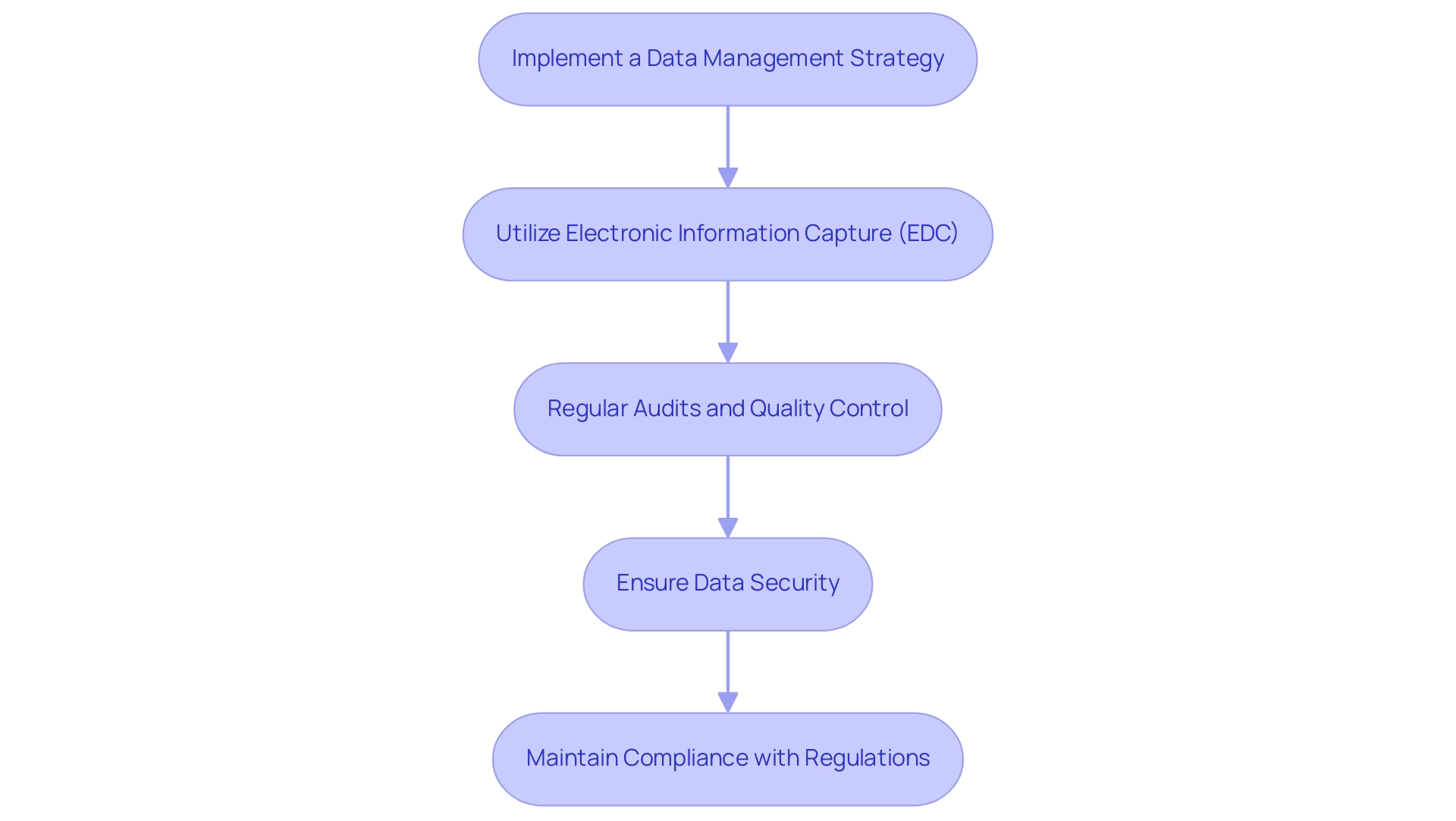
Implement a Data Management Strategy: Establish a comprehensive information management plan that clearly outlines the processes for collecting, storing, and analyzing information. This plan should outline stringent procedures to preserve information integrity and confidentiality, essential elements in sustaining trust and adherence in medical research, especially in the context of Post-Market Clinical Follow-Up Panama evaluations managed by bioaccess®, which has over 20 years of experience in Medtech.
-
Utilize Electronic Information Capture (EDC): Leverage electronic information capture systems to enhance the efficiency and accuracy of information collection. It is essential to select an EDC system that adheres to regulatory standards, thereby ensuring compliance and fostering a streamlined workflow in clinical follow-up studies. Notably, advancements in EDC technology continue to evolve, promising better usability and integration in 2024.
-
Regular Audits and Quality Control: Implement systematic audits of information collection processes to monitor compliance with established protocols. Establishing strong quality control measures will facilitate the early identification and rectification of discrepancies, ensuring the reliability of the information collected in accordance with bioaccess®'s rigorous standards.
-
Ensure Data Security: Safeguard sensitive information by instituting robust security measures, including encryption and stringent access controls. These actions are essential in preventing unauthorized access and ensuring the safeguarding of patient information throughout the study, particularly during the Post-Market Clinical Follow-Up Panama phases.
-
Maintain Compliance with Regulations: Continuously assess and ensure compliance with pertinent regulations and guidelines. As emphasized by MEDDEV 7.2-1 rev 4, "In accordance with the Directives, the assessment and the assessment report must be actively refreshed with information obtained from post-market." This method is essential for synchronizing information management practices with both local and international standards, thus maintaining the integrity of the research process. Furthermore, manufacturers are accountable for deciding the quantity and kind of PMS information included in their Clinical Evaluation Reports (CERs) to ensure a precise benefit-risk analysis, emphasizing the significance of adherence in the Post-Market Clinical Follow-Up Panama evaluations. For instance, the Study Report (CSR) finalization process exemplifies effective data management practices, as it compiles analysis and conclusions drawn from statistical results, ensuring transparency and adherence to regulatory requirements. Furthermore, bioaccess® offers tailored services such as feasibility studies and site selection to ensure the success of clinical trials.
Conclusion
Navigating the regulatory framework for Post-Market Clinical Follow-Up (PMCF) studies in Panama is essential for organizations committed to ensuring the safety and efficacy of medical devices. A comprehensive understanding of the requirements set forth by the Panamanian Ministry of Health, alongside the recent regulatory changes, is critical. This article has outlined the key steps necessary for implementing an effective PMCF strategy, from developing a robust plan and securing ethical approvals to engaging stakeholders and managing data compliance.
- Collaboration with local experts, such as bioaccess®, plays a pivotal role in successfully navigating the complexities of the regulatory landscape. Their extensive experience in clinical trial management not only enhances compliance but also ensures that organizations can effectively address challenges and leverage opportunities within this dynamic environment. Moreover, engaging stakeholders meaningfully fosters transparency and collective commitment, ultimately improving patient outcomes and operational integrity.
In summary, a strategic approach to PMCF, characterized by meticulous planning, rigorous data management, and proactive stakeholder engagement, is essential for achieving compliance and enhancing the performance of medical devices. By prioritizing these elements, organizations can contribute to improved healthcare delivery in Panama while reinforcing their commitment to patient safety and regulatory adherence.




