Introduction
Navigating the intricacies of Institutional Review Board (IRB) proposals is a critical endeavor for researchers committed to ethical and compliant study practices. As the landscape of clinical research continues to evolve, understanding the essential components and guidelines for crafting a robust IRB proposal becomes paramount.
From comprehending institutional requirements to conducting thorough risk assessments, each step plays a vital role in ensuring the safety and rights of research participants. This article delves into the fundamental aspects of developing an effective IRB proposal, highlighting best practices that not only streamline the submission process but also enhance the likelihood of approval.
By adhering to these detailed guidelines, researchers can lay a solid foundation for their studies, ultimately contributing to the integrity and advancement of scientific inquiry.
Essential Guidelines for Crafting an IRB Proposal
To develop a robust IRB proposal, it is essential to adhere to the following guidelines:
- Understand Institutional Requirements: It is critical to familiarize yourself with the specific guidelines established by your institution's IRB. Each institution may implement unique requirements concerning format, submission procedures, and required documentation, which must be carefully followed to avoid delays in the review process.
- Conduct a Thorough Literature Review: Before drafting your document, perform a comprehensive literature review to identify existing research relevant to your study. This review not only justifies the significance of your research but also demonstrates familiarity with the current state of knowledge in your field, a factor that can significantly enhance the strength of your submission.
- Engage Stakeholders Early: Involve key stakeholders, such as co-investigators, community representatives, and patient advocacy groups, early in the planning process. This engagement facilitates the gathering of diverse perspectives, ensuring that the proposal addresses relevant concerns and ethical considerations from the outset.
- Outline Research Objectives Clearly: Articulate the objectives of your research with precision. Ensure that these objectives are specific, measurable, achievable, relevant, and time-bound (SMART), which will provide clarity and focus throughout the research.
- Plan for Compliance: Detail how your study will adhere to ethical standards and regulatory requirements. This includes comprehensive plans for participant recruitment, informed consent processes, and data management practices, all of which are critical for maintaining compliance and integrity in research.
By diligently following these guidelines, researchers can establish a solid foundation for their IRB submissions, thereby increasing the likelihood of approval and facilitating smoother progress through the IRB review process. Patrick Flume, Associate Provost for Research Compliance and Regulatory Affairs, emphasizes this by stating, "The RKS staff tracks all IRB applications deemed to be NRR to ensure that they have reached out to offer support and guidance as well as to track the effectiveness of the RKS program," highlighting the importance of tracking and supporting IRB applications as part of an effective research compliance framework. Furthermore, it is significant that the NIH National Center for Advancing Translational Sciences (NCATS) supports projects through grant funding, under Grant Number UL1 TR001450, highlighting the importance of well-prepared submissions in obtaining necessary resources.
Furthermore, a case analysis titled "Need for Further Research on IRB Processes" indicates a gap in understanding the IRB approval process and the role of regulatory support services, illustrating the critical need for these guidelines to enhance application outcomes.
Key Components of an Effective IRB Proposal
A thorough evaluation and adherence to ethical standards are ensured by incorporating several critical components in an effective IRB research proposal.
-
Study Title and Abstract:
Begin with a concise title followed by a brief abstract that encapsulates the research's purpose, methodology, and significance.
-
Research Design and Methodology:
Clearly delineate the study's design—such as a randomized controlled trial or observational study—along with the methodology, including subject selection criteria, intervention specifics, and data collection techniques.
-
Risk-Benefit Analysis:
A comprehensive discussion on the potential risks to individuals is essential, alongside strategies for mitigating these risks. Furthermore, it is essential to express the expected advantages of the research for both individuals involved and the wider community.
-
Informed Consent Process:
Detail the procedures for obtaining informed consent, ensuring that participants receive all necessary information and that their autonomy is fully respected throughout the process.
-
Data Management Plan:
Outline the framework for data collection, storage, and analysis, emphasizing confidentiality and compliance with applicable data protection regulations.
-
Funding and Conflict of Interest Disclosure:
Clarity about funding sources and any possible conflicts of interest is essential for upholding integrity in the research process.
Incorporating these components not only improves the quality and thoroughness of the IRB research proposal submissions but also aligns with ethical guidelines, thus enabling a smoother review process. As emphasized in recent discussions, effective suggestions are integral to reducing average IRB review times, which continue to be a focus for improvement according to findings from a 2011 Milbank Quarterly meta-analysis. Additionally, the Federal Register published rules and regulations for the protection of human subjects on January 19, 2017, emphasizing the importance of these components.
As Amy Schwarzhoff, MBA, Director of the Children’s Hospital of Philadelphia IRB, noted, 'The authors appreciate the importance of a well-structured plan in ensuring ethical compliance and efficient review.' Additionally, the case analysis titled 'Conclusion on IRB Review Times' offers insights into the factors influencing IRB review durations, emphasizing the importance of thorough submissions. By adhering to these guidelines, researchers can better position their IRB research proposal for success.
Developing a Comprehensive Informed Consent Process
Developing a comprehensive informed consent process is critical for an ethical IRB research proposal in clinical research. Researchers should consider the following steps to ensure clarity and understanding:
-
Create Clear and Understandable Consent Documents: Draft consent forms using plain language that clearly outlines the project's purpose, procedures, risks, benefits, and individuals' rights.
Avoiding jargon and complex terms is essential for effective communication.
-
Provide Adequate Time for Consideration: It's imperative to allow potential individuals sufficient time to read the consent form and ask questions prior to agreeing to take part. This time is crucial for ensuring that individuals make informed decisions about their involvement.
-
Conduct Consent Discussions: Engaging individuals in discussions about the study fosters trust and ensures that their concerns are adequately addressed. Personal interactions enhance understanding and can significantly improve the informed consent experience.
-
Document Consent: Consent must be documented properly, with individuals signing the consent form before any study-related activities commence.
It is important to keep signed forms secure and confidential, thereby respecting the privacy of individuals.
-
Offer Ongoing Consent: Recognizing that consent is an ongoing process is vital. Researchers should keep participants informed of any new information that may influence their willingness to continue in the research.
Participants should always feel empowered to withdraw from the research at any time without penalty.
Insights from various investigations, including the research by Itoh K et al., highlight the motivations, comprehension, and expectations of patients in Phase I trials in Japan, emphasizing the importance of clear communication in informed consent. Additionally, a notable research conducted by Harrison et al. (1995) involving 175 adult patients regarding an HIV vaccine demonstrated high levels of understanding regarding confidentiality and side effects, with comprehension rates ranging from 83% to 100%.
However, it is crucial to note that 18% of individuals in other studies admitted they had not fully read the study information letter, as noted by Pope et al., underscoring the need for clear and accessible consent forms. Furthermore, having no active withdrawals in the intervention group suggests that a well-structured informed consent process may contribute positively to retention of individuals. By following these best practices in their IRB research proposal, researchers can maintain ethical standards and better safeguard the rights of individuals throughout the research process.
Conducting a Risk Assessment for Your Research
Conducting a thorough risk evaluation is essential for guaranteeing the safety of individuals in research projects, especially within the context of extensive clinical trial management services. Here are the essential steps to follow:
- Identify Potential Risks: Start by creating an extensive list of possible physical, psychological, and social hazards that individuals may encounter during the research. It's important to consider both immediate and long-term effects, as well as the varied experiences of those involved. For instance, a research involving 402 adults with community-acquired pneumonia emphasized the necessity for a comprehensive evaluation of individual wellbeing throughout the research process. According to statistics, approximately 20% of individuals in clinical trials report experiencing adverse effects, underscoring the importance of risk assessment. Utilizing feasibility assessments can help in identifying these risks early in the planning phase.
- Evaluate the Likelihood and Severity of Risks: For each identified risk, assess the probability of occurrence and the severity of its potential impact on individuals. Utilizing a risk matrix can help categorize these risks as low, moderate, or high, allowing for a more structured evaluation. Recent discussions in the field have emphasized the use of confidence intervals for relative risk and odds ratios as valuable tools in evaluating these risks. Project management services can facilitate this evaluation by providing necessary data and analysis.
- Develop Mitigation Strategies: Outline specific strategies for minimizing or eliminating each identified risk. This may involve modifying study protocols to enhance safety, providing additional assistance to individuals, or implementing stringent safety protocols. A well-structured risk management plan is critical for addressing any concerns raised by the Institutional Review Board (IRB) in an IRB research proposal and for ensuring compliance with INVIMA's regulations. As mentioned by ethics expert Matthew P Smeltzer, "A proactive risk assessment not only safeguards individuals but also enhances the integrity of the research process." Compliance reviews can play a key role in this process by ensuring that all protocols meet regulatory standards.
- Incorporate Risk Management in the IRB Research Proposal: It is essential to clearly articulate the identified risks and corresponding mitigation strategies within the proposal. This not only demonstrates a thoughtful approach to participant safety but also enhances the credibility of the IRB research proposal in the eyes of the IRB, which plays a pivotal role in overseeing ethical research practices. A comprehensive understanding of INVIMA's oversight functions will further enhance the compliance of the plan. Including specific references to the trial setup process can also reinforce the proposal's robustness.
- Review and Update Regularly: Risk management is an ongoing process. Researchers should continuously monitor and review risks throughout the project's duration. If new risks emerge or existing ones evolve, it is imperative to promptly communicate these changes in the IRB research proposal to ensure that participant safety remains the top priority. Regular updates to research documents, facilitated by clinical trial management services, can help maintain an accurate risk profile.
By thoroughly conducting a risk assessment with the support of comprehensive clinical trial management services, including feasibility studies, project management, and compliance reviews, researchers can significantly enhance the safety of their studies and uphold ethical standards in research practices. This proactive approach fosters trust and confidence among participants, ultimately leading to more robust and ethical research outcomes.
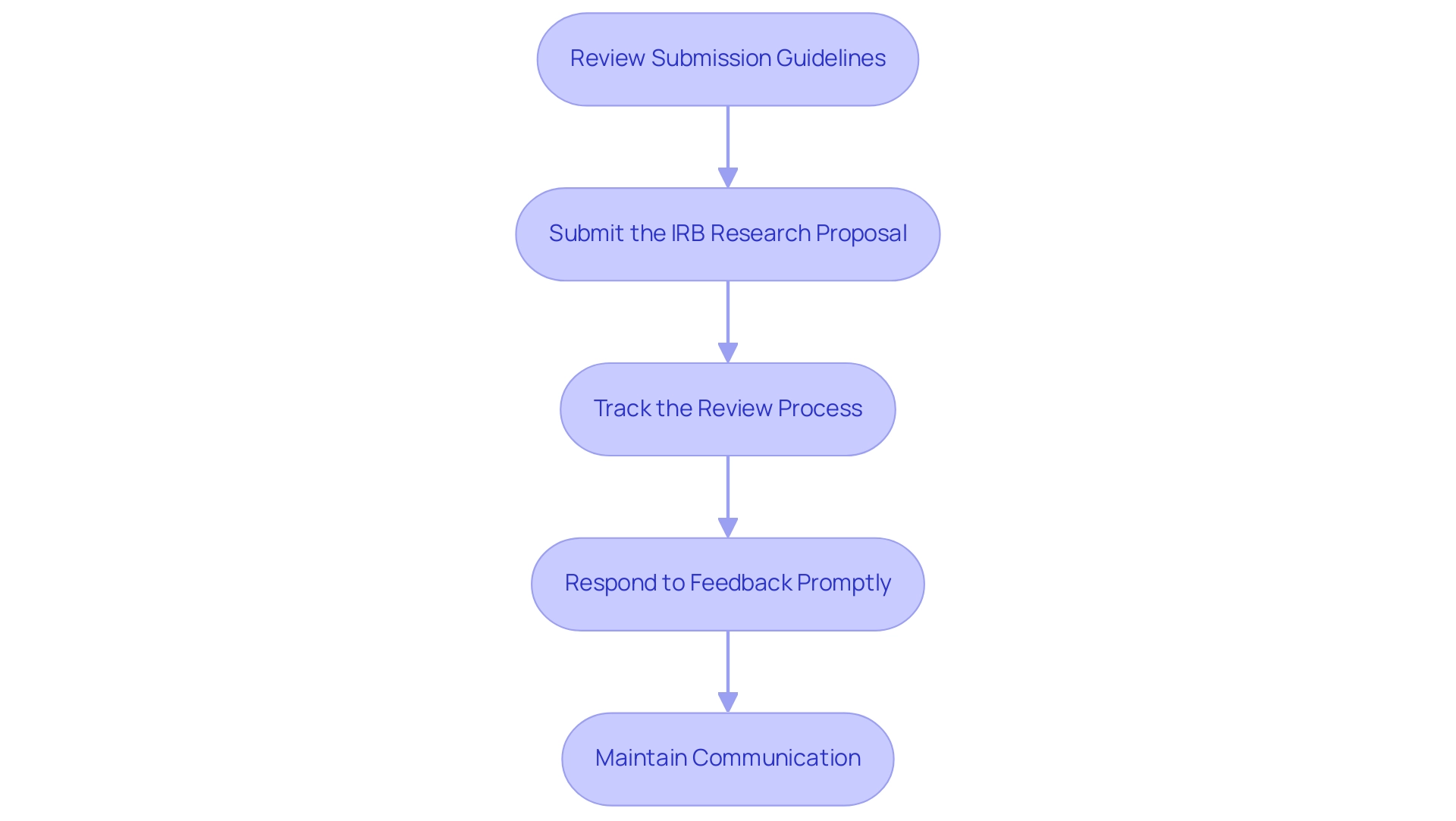
Submitting the IRB Proposal and Follow-Up
Successfully submitting your IRB proposal and managing follow-up actions requires a systematic approach:
- Review Submission Guidelines: Begin by thoroughly reviewing the IRB’s submission guidelines to ensure your submission adheres to the required formatting and documentation standards. Compliance at this stage is critical to avoiding unnecessary delays.
- Submit the IRB research proposal: Utilize the designated platform or process established by your institution’s IRB for submitting your request. Confirm that all necessary documents are included to facilitate a complete review.
- Track the Review Process: Once your submission is submitted, actively monitor its status throughout the IRB review process. Being proactive in checking for updates or any requests for additional information can help preempt potential delays.
- Respond to Feedback Promptly: In the event of requests for revisions or additional information from the IRB, respond swiftly and comprehensively. Address all feedback thoroughly to aid in a smooth and efficient review process. Notably, case reports are often exempt from IRB oversight, which may simplify certain submissions. As noted, "Of note, case reports are typically exempted for IRB oversight."
- Maintain Communication: It is crucial to establish and maintain open lines of communication with the IRB. Should delays or issues arise, do not hesitate to reach out for updates on the status of your proposal. Principal Investigators and faculty advisors may attend Full Committee meetings if necessary, but remember that they cannot participate in the final discussion or vote.
By comprehensively understanding the submission process and effectively managing follow-up actions, researchers can significantly improve their chances of obtaining an IRB research proposal approval in a timely manner. Statistics indicate that an additional month is often required for hospital approval following IRB approval, making these steps even more vital for expediting research timelines. Upon completion of the IRB review process, Investigators receive notification of the Committee's action, which may include requests for additional information, and the approval period for protocols is limited to a maximum of 12 months, depending on perceived risks and timely submission of revisions.
Conclusion
Navigating the complexities of Institutional Review Board (IRB) proposals is essential for researchers dedicated to ethical research practices. This article outlines the critical components and best practices necessary for crafting a robust IRB proposal, from understanding institutional requirements to conducting thorough risk assessments and developing comprehensive informed consent processes.
Key takeaways include:
- The importance of engaging stakeholders early
- Clearly outlining research objectives
- Ensuring compliance with ethical standards
Additionally, a well-structured informed consent process and a meticulous risk assessment are vital to safeguarding participant rights and wellbeing. By integrating these elements into the proposal, researchers can significantly enhance both the quality and the likelihood of approval from the IRB.
Ultimately, adhering to these guidelines not only streamlines the submission process but also reinforces the integrity of scientific inquiry. As the landscape of clinical research evolves, a commitment to ethical considerations remains paramount, ensuring that studies contribute positively to the advancement of knowledge while prioritizing the safety and rights of participants.
Frequently Asked Questions
What are the essential guidelines for developing a robust IRB proposal?
The essential guidelines include understanding institutional requirements, conducting a thorough literature review, engaging stakeholders early, outlining research objectives clearly, and planning for compliance with ethical standards and regulatory requirements.
Why is it important to understand institutional requirements for an IRB proposal?
Understanding institutional requirements is crucial because each institution may have unique guidelines regarding format, submission procedures, and required documentation, which must be followed to avoid delays in the review process.
How can conducting a literature review enhance an IRB proposal?
A thorough literature review helps identify existing research relevant to the study, justifying its significance and demonstrating familiarity with the current state of knowledge, thus strengthening the proposal.
Who should be engaged early in the IRB proposal planning process?
Key stakeholders such as co-investigators, community representatives, and patient advocacy groups should be involved early to gather diverse perspectives and address relevant concerns and ethical considerations.
What does it mean to outline research objectives clearly in an IRB proposal?
Outlining research objectives clearly means articulating them in a specific, measurable, achievable, relevant, and time-bound (SMART) manner, providing clarity and focus throughout the research.
What should a researcher include to plan for compliance in their IRB proposal?
Researchers should detail how their study will adhere to ethical standards and regulatory requirements, including plans for participant recruitment, informed consent processes, and data management practices.
What are the critical components of an effective IRB research proposal?
The critical components include a study title and abstract, research design and methodology, risk-benefit analysis, informed consent process, data management plan, and funding and conflict of interest disclosure.
Why is a risk-benefit analysis important in an IRB proposal?
A risk-benefit analysis is essential to discuss potential risks to individuals and strategies for mitigating these risks, while also expressing the expected advantages of the research for participants and the wider community.
What is the significance of the informed consent process in an IRB proposal?
The informed consent process is vital to ensure that participants receive all necessary information and that their autonomy is respected throughout the research.
How does clarity about funding sources and conflicts of interest contribute to an IRB proposal?
Clarity regarding funding sources and potential conflicts of interest upholds integrity in the research process, ensuring transparency and trustworthiness.
What role does effective proposal preparation play in the IRB review process?
Well-prepared submissions improve the quality and thoroughness of IRB proposals, which can lead to smoother review processes and potentially reduce review times.




