Overview
Designing clinical trials for medical devices in Mexico necessitates a structured approach that adheres to regulatory frameworks, ensures participant safety, and addresses recruitment challenges. This article underscores the significance of comprehensive planning, local stakeholder engagement, and efficient data management as critical components for successfully navigating the complexities of clinical trials in the region. Ultimately, these elements contribute to the advancement of medical technologies, reinforcing the need for collaboration and strategic action in this evolving landscape.
Introduction
In the intricate world of medical device development, clinical trials serve as a critical pillar, ensuring that innovations are safe and effective before they reach patients. These structured assessments unfold through various phases, each meticulously designed to gather essential data that regulatory bodies require for approval.
As the landscape of clinical research evolves—particularly in regions like Latin America—the role of decentralized trials and local stakeholder engagement becomes increasingly vital. Navigating regulatory frameworks and addressing recruitment challenges highlight the complexities of conducting clinical trials, necessitating a nuanced approach.
This article explores the multifaceted nature of clinical trials in medical device development, underscoring the significance of collaboration, data management, and post-market studies in advancing healthcare technology and improving patient outcomes.
Understanding the Role of Clinical Trials in Medical Device Development
Designing clinical trials for medical devices in Mexico is vital for the advancement of medical instruments, offering a systematic method to evaluate their safety and efficacy prior to market launch. These trials typically progress through distinct stages, beginning with initial feasibility research that assesses the tool's performance in a regulated environment. As the apparatus evolves, critical assessments are conducted to gather comprehensive data on its performance among a broader and more diverse population.
This detailed information is essential for regulatory submissions, particularly when designing clinical trials for medical devices in Mexico, as it substantiates that the device meets the safety and efficacy standards mandated by regulatory authorities such as COFEPRIS.
The significance of these studies extends beyond mere compliance; they are crucial for ensuring that medical devices deliver the intended benefits to patients. For instance, recent statistics indicate that decentralized studies can significantly enhance participant enrollment and diversity, particularly in regions where 70% of the population resides more than two hours from an academic medical center. This approach not only accelerates the testing process but also enriches the data collected, ultimately leading to more reliable outcomes.
Moreover, case studies underscore the critical importance of protecting proprietary information during research studies. Device manufacturers often express concerns that disclosing research data could jeopardize their competitive edge and innovation efforts. A notable example is ReGelTec's Early Feasibility Study on HYDRAFIL™ for treating chronic low back pain in Colombia, where procedures were effectively managed remotely, showcasing the efficacy of bioaccess®'s management services.
As a leading CRO and consulting partner for U.S. medical device firms in Colombia, bioaccess® emphasizes the necessity of a balanced approach to information transparency that safeguards innovation while ensuring patient safety. With over 20 years of experience in Medtech, bioaccess® supports a voluntary data bank to protect research and financial investments, which is crucial in mitigating the risk of mandatory disclosures that could prompt investigations abroad and adversely affect the medical technology landscape.
In conclusion, understanding the stages of studies and designing clinical trials for medical devices in Mexico, along with their implications for safety and effectiveness, is imperative for Medtech firms. By navigating these complexities adeptly, they can not only meet regulatory requirements but also contribute to the development of medical tools that enhance patient outcomes. bioaccess® plays a pivotal role in this process, leveraging its expertise and tailored approach to support research in Latin America, ultimately facilitating the expedited advancement of medical devices.
bioaccess® offers comprehensive study management services, including site selection, compliance evaluations, setup, and project oversight, ensuring a thorough and effective process.
Navigating the Regulatory Framework for Clinical Trials in Mexico
Designing clinical trials for medical devices in Mexico requires strict adherence to the regulatory framework established by COFEPRIS, the Mexican health authority. Sponsors must submit a comprehensive study protocol prior to study initiation, which is essential for outlining the design, objectives, and methodology of clinical trials for medical devices in Mexico. Additionally, obtaining ethical approval from an independent ethics committee is a prerequisite.
Given that discrepancies in documentation can significantly delay approval, meticulous preparation is essential. A thorough understanding of local regulations, including informed consent requirements and data protection laws, is critical for ensuring compliance in the design of clinical trials for medical devices in Mexico. Furthermore, engaging with local regulatory experts can greatly enhance the approval process, as they possess invaluable insights into navigating the complexities of COFEPRIS regulations. Recent data indicates that the average approval duration for research studies in Mexico has improved, reflecting a more efficient process.
However, the quality of the research protocol remains crucial for successful submissions in the context of designing clinical trials for medical devices in Mexico. Notably, Julio Martinez-Clark has supported over 100 Medtech opportunities in Latin America since 2010, underscoring the importance of local expertise in this field.
At bioaccess®, we offer a comprehensive range of clinical study management services, including:
- Feasibility assessments
- Site selection
- Compliance evaluations
- Study setup
- Import permits
- Project management
- Reporting
Our commitment to information security ensures that client issues are managed with integrity and transparency, fostering trust throughout the process. Case studies demonstrate the significance of self-regulation in the development of medical devices, highlighting how adherence to regulatory principles can enhance patient safety and build trust.
As emphasized by industry specialists, including Julio Martinez-Clark, Mexico's evolving reputation as a secure and informed location for research positions it as an attractive choice for Medtech firms designing clinical trials for medical devices in Mexico, focused on quality information and patient involvement. By leveraging his insights, we can assert that Mexico offers a favorable environment for companies conducting clinical trials.

Addressing Recruitment Challenges in Mexican Clinical Trials
Recruiting participants for medical studies in Colombia presents a landscape characterized by unique challenges, particularly regarding cultural perspectives on research and differing levels of health literacy among potential subjects. Many individuals may harbor misconceptions about research studies, often viewing them with skepticism or fear, including concerns about being judged or perceived as 'crazy.' Such apprehensions can significantly impede participation. To effectively navigate these challenges, it is essential to implement culturally sensitive recruitment strategies that resonate with the target population.
Engaging community leaders and healthcare providers in Colombia is a crucial step in fostering trust and promoting participation. These local figures serve as advocates, helping to clarify the research process and alleviate fears associated with involvement. Additionally, utilizing digital platforms for outreach can greatly expand the reach, enabling researchers to connect with diverse demographics and facilitate informed decision-making.
Socioeconomic factors also play a pivotal role in participant recruitment. Challenges such as transportation barriers and limited access to healthcare facilities can adversely affect participation rates. By proactively addressing these logistical issues, researchers can cultivate a more inclusive environment that encourages a diverse participant pool.
Successful case studies in Colombia illustrate the effectiveness of these culturally sensitive recruitment strategies. For example, initiatives that incorporate local customs and languages have demonstrated improved engagement and retention rates among participants. A pertinent case study titled "Cultural Competence in Mental Health Services" underscores the significance of cultural competence among professionals in delivering effective care to diverse populations, highlighting that enhancing cultural competence can strengthen the therapeutic relationship and improve treatment outcomes.
Moreover, the collaboration between GlobalCare Clinical Trials and bioaccess™ exemplifies how enhancing ambulatory services in Colombia can yield substantial outcomes, including a reduction in recruitment time by over 50% and a retention rate exceeding 95%. This underscores the necessity of adapting recruitment strategies to align with the linguistic and cultural needs of the target population. As noted by Sue & Sue, cultural competence emphasizes the acknowledgment of patients' cultures and the development of skills, knowledge, and policies to provide effective treatments.
By prioritizing cultural competence in recruitment efforts, researchers can enhance the therapeutic relationship and ultimately improve treatment outcomes for diverse populations. This approach is further validated by the statistic indicating that the study achieved information saturation after conducting interviews with 14 participants, underscoring the importance of thorough engagement in understanding participant perspectives.
Selecting the Right Sites for Clinical Trials: Key Considerations
Selecting appropriate locations for clinical studies is a multifaceted process that demands a comprehensive evaluation of several critical factors. A meticulous assessment of the site's infrastructure is vital; this encompasses the availability of essential medical equipment and facilities that facilitate robust data collection. The qualifications and experience of site personnel, particularly principal investigators and research coordinators, are instrumental to the study's success.
Understanding the site's patient demographics is crucial to ensure alignment with the target population for the study. Furthermore, collaborating with local healthcare providers can significantly enhance participant recruitment and strengthen the site's capabilities. A recent analysis revealed that inadequate site selection can result in financial losses averaging $50,000 per site, highlighting the necessity for careful planning. Additionally, a study by Carlisle et al. indicated that 48,027 patients participated in studies concluding in 2011 that failed to adequately address the primary research question, further emphasizing the significance of effective site selection. Conducting feasibility studies prior to site selection can yield valuable insights into the site's potential for successful execution.
In Latin America, particularly in Colombia, bioaccess® has emerged as a trustworthy CRO and consulting partner for U.S. medical device companies, enhancing the research landscape. With over 20 years of experience in Medtech, bioaccess specializes in managing a variety of studies, including:
- Early-Feasibility Studies (EFS)
- First-In-Human Studies (FIH)
- Pilot Studies
- Pivotal Studies
- Post-Market Clinical Follow-Up Studies (PMCF)
The partnership between bioaccess and Caribbean Health Group aims to position Barranquilla as a premier location for medical studies, supported by Colombia's Minister of Health.
This initiative not only bolsters the infrastructure for research studies but also stimulates local economies through job creation and advancements in healthcare.
In Mexico, the infrastructure for clinical studies has shown promising progress. Statistics suggest that a significant percentage of research sites are equipped with the necessary facilities; however, there remains potential for improvement in certain areas. For instance, a case study involving Boltzmann Labs' ClinBolt tool demonstrates how advanced algorithms and historical data analysis can optimize the site selection process.
ClinBolt empowers sponsors to make informed decisions, enhancing recruitment rates and reducing timelines, thereby improving the overall efficacy of research studies.
As Tim Fitzpatrick noted, "Clinical Studies - PT01: Landscape: Purpose, People…" this underscores the critical importance of understanding the environment in which studies operate. Ultimately, the criteria for selecting research locations should encompass not only the physical infrastructure but also the expertise of the personnel and the site's ability to effectively engage with the local patient community. By prioritizing these factors, researchers can refine their study designs and achieve better outcomes in the Medtech sector.

Ensuring Effective Data Management and Analysis in Clinical Trials
Efficient information management is vital for the success of clinical studies, particularly within the medical device industry. It encompasses systematic processes for collecting, storing, and analyzing information, ensuring both accuracy and reliability. By implementing robust Clinical Data Management Systems (CDMS), researchers facilitate real-time monitoring of trial progress and participant safety, which is crucial for maintaining compliance with regulatory requirements.
Establishing clear protocols for information entry and validation minimizes errors and enhances integrity. Regular audits and quality checks are essential components of the information management process, ensuring that any discrepancies are swiftly addressed. The database is secured following information cleansing, preventing additional modifications and ensuring stability for precise evaluation.
Moreover, utilizing advanced statistical analysis software significantly enhances the evaluation process, allowing for more refined interpretations of the gathered information. As industry trends indicate, 73% of pharmaceutical firms now employ cloud-based management systems for information, a substantial increase from only 45% five years ago. This shift illustrates a transition toward more effective handling practices. When selecting a CDMS, it is crucial to assess functionality and features, adherence to regulatory standards, user interface and usability, and integration capabilities.
By prioritizing efficient information management, researchers can bolster the credibility of their findings, ensuring they withstand rigorous regulatory examination. This commitment to information precision and dependability not only elevates the overall standard of medical studies but also streamlines the design of clinical trials for medical devices in Mexico, expediting the pathway to successful marketing of innovative healthcare instruments. For instance, bioaccess® employs a range of tools for medical data management, enhancing the precision, efficiency, and adherence of data handling processes, thereby ensuring data integrity throughout the research lifecycle.
Additionally, bioaccess® offers comprehensive trial management services, including feasibility studies, site selection, compliance evaluations, trial preparation, import permits, project oversight, and reporting. These services are tailored for designing clinical trials for medical devices in Mexico, addressing the specific challenges of conducting medical equipment studies in Latin America. With over 20 years of experience in Medtech, bioaccess® specializes in Early-Feasibility Studies, First-In-Human Studies, Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies, reinforcing its commitment to regulatory excellence and innovation in the region.
The Importance of Post-Market Clinical Follow-Up Studies
Post-market clinical follow-up studies (PMCF) are essential for the continuous evaluation of the safety and effectiveness of medical products once they enter the market. These studies play a vital role in identifying unexpected negative effects and ensuring that products consistently meet established safety standards. Regulatory authorities require PMCF as a crucial element of the post-market monitoring structure, demonstrating the industry's dedication to patient safety and product reliability.
At bioaccess®, we specialize in managing PMCF studies with a focus on innovation and regulatory excellence. Creating a thorough PMCF strategy is crucial, and our knowledge guarantees that this plan distinctly outlines the study's goals, methodologies, and schedules for information gathering. Interacting with stakeholders—including healthcare professionals and patients—offers invaluable insights into the real-world performance of the product, enhancing the study's relevance and impact.
In 2025, the importance of PMCF studies is underscored by the latest regulatory expectations. Notified bodies now require PMCF plans to conform with the MDCG 2020-7 guidance document, which details strategies for gathering and evaluating real-world data on usage, performance, therapeutic advantages, and adverse events. This alignment not only guarantees compliance but also enhances the credibility of the instrument among healthcare providers and patients.
Moreover, it is essential for the notified body to confirm the sufficiency of the PMCF plan and evaluate the coherence of evidence with the plan.
Expert insights highlight that effective PMCF strategies are crucial for monitoring long-term safety. Jasper, who has worked in the MedTech sector for 20 years, stresses that manufacturers must show the continuous safety and performance of their products throughout their lifecycle. PMCF serves as a systematic approach to gather clinical data that updates clinical evaluations and identifies new risks, ensuring that any emerging risks are promptly addressed.
Statistics indicate that a well-organized PMCF can greatly improve the overall safety and effectiveness of medical products in real-world environments. Successful case studies, such as the one titled "Regulatory Requirements for PMCF," illustrate how proactive PMCF strategies have led to improved patient outcomes and increased trust in medical technologies. By prioritizing PMCF, organizations can not only comply with regulatory requirements but also contribute to the advancement of medical device safety and efficacy.
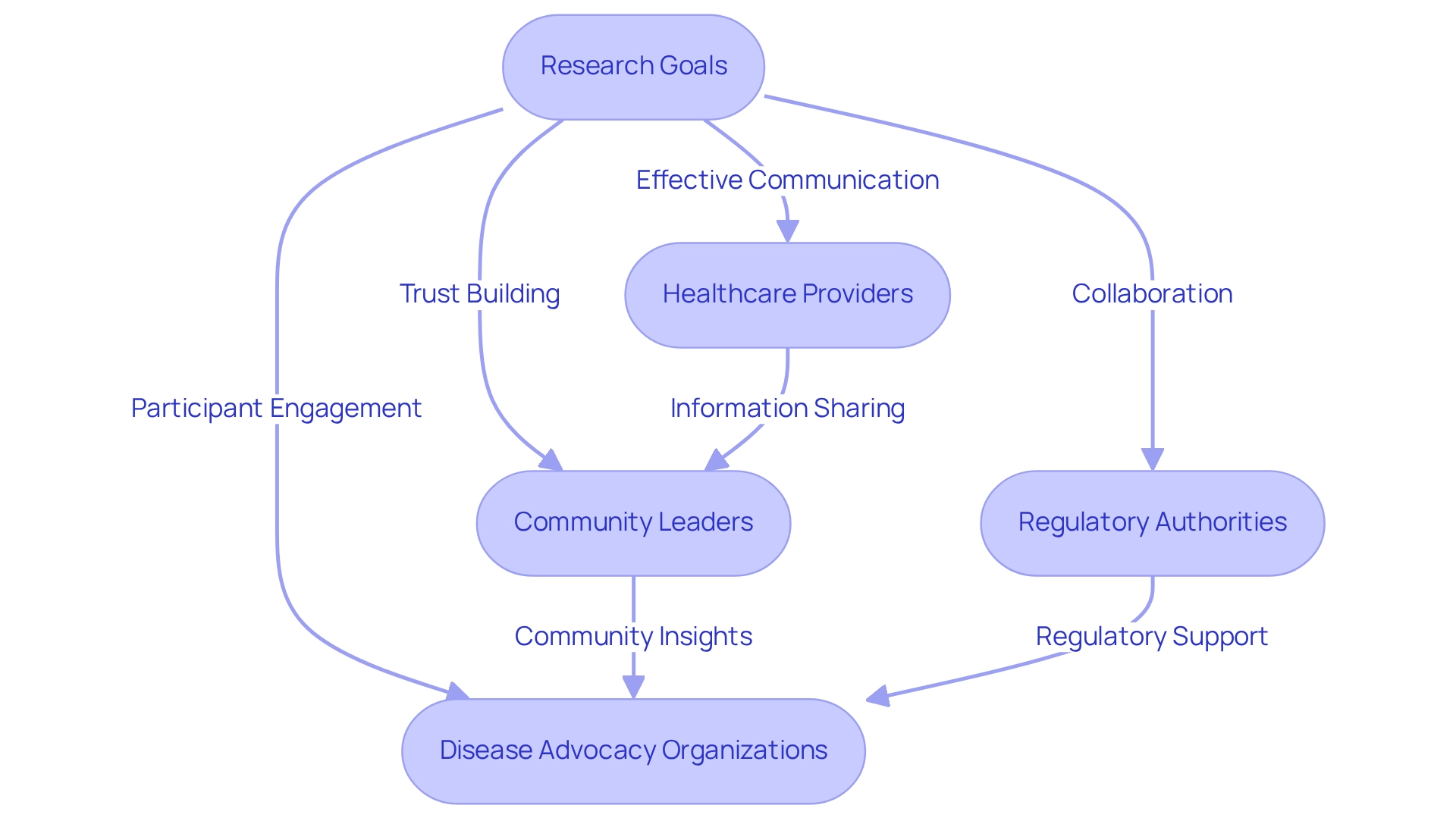
Collaborating with Local Stakeholders for Successful Trials
Cooperation with local stakeholders is vital for the success of research studies in Latin America, particularly in Barranquilla, where bioaccess™ and Caribbean Health Group are collaborating to position the city as a leading site for medical research. Involving healthcare providers, community leaders, and regulatory authorities not only boosts the credibility of the study but also significantly enhances participant recruitment efforts. This initiative, backed by Colombia's Minister of Health, aims to attract more research projects to the area, fostering an environment conducive to innovation and development.
Effective communication is essential; researchers must clearly articulate the study's goals and methods to build trust and ensure alignment with community needs. This requires maintaining open channels of communication and providing frequent updates, which promotes transparency and supports continuous stakeholder involvement throughout the process. Furthermore, community participation is crucial in improving recruitment and retention rates, especially among diverse populations.
Statistics reveal that individuals identifying as Black/African-American or Hispanic/Latinx are the most represented group in service occupations, highlighting the importance of workforce diversity in recruitment efforts. By actively engaging local stakeholders, researchers can gain deeper insights into and respond to the specific issues and preferences of the community, ultimately resulting in more successful study outcomes.
The role of disease advocacy organizations is equally important; they enhance communication and collaboration in research studies, bridging the gap between participants and researchers. Their involvement can improve participant engagement and address frustrations related to information sharing, a vital element of trust in the experimental process. The ongoing debate about whether approval for data sharing should be a prerequisite for participation in studies further underscores the necessity for effective communication with stakeholders.
The impact of these local collaborations on research success rates is profound, as they foster a cooperative atmosphere that encourages participant engagement and enhances the overall efficacy of research initiatives. Additionally, the comprehensive trial management services provided by bioaccess™, including feasibility studies, site selection, compliance reviews, trial setup, import permits, project management, and reporting, are crucial to the success of these trials, ultimately benefiting the local economy through job creation and healthcare enhancement. This collaboration was announced on March 29, 2019, marking a significant step towards advancing clinical research in Barranquilla.
Conclusion
In conclusion, navigating the complexities of clinical trials in medical device development requires a comprehensive approach that prioritizes collaboration, cultural competence, and meticulous data management. The success of these trials is not merely in meeting regulatory requirements but in fostering innovations that significantly enhance patient outcomes. By engaging local stakeholders, researchers can build trust and improve participant recruitment, addressing unique challenges faced in regions like Latin America. Furthermore, effective data management practices ensure the integrity and reliability of trial outcomes, while post-market clinical follow-up studies are essential for long-term device safety and effectiveness.
Ultimately, Medtech companies that embrace these principles will shape the future of healthcare technology. Their commitment to collaboration and cultural sensitivity will foster a safer and more effective landscape for patients worldwide. The call to action is clear: by navigating these complexities effectively, we can advance medical device technology and improve the lives of countless individuals.
Frequently Asked Questions
Why are clinical trials important for medical devices in Mexico?
Clinical trials are vital for evaluating the safety and efficacy of medical devices before market launch, ensuring they meet regulatory standards and deliver intended benefits to patients.
What are the stages of clinical trials for medical devices in Mexico?
Clinical trials typically begin with feasibility research to assess performance in a regulated environment, followed by critical assessments to gather comprehensive data on device performance among a broader population.
What role does COFEPRIS play in clinical trials for medical devices in Mexico?
COFEPRIS is the Mexican health authority that establishes the regulatory framework for clinical trials, requiring sponsors to submit a comprehensive study protocol and obtain ethical approval from an independent ethics committee before initiation.
How can the design of clinical trials impact participant enrollment and diversity?
Decentralized studies can significantly enhance participant enrollment and diversity, especially in regions where many individuals live far from academic medical centers, leading to more reliable outcomes.
What concerns do manufacturers have regarding proprietary information during clinical trials?
Manufacturers are concerned that disclosing research data could jeopardize their competitive edge and innovation efforts, which is critical for their business.
How does bioaccess® support clinical trials for medical devices in Mexico?
Bioaccess® offers comprehensive study management services, including feasibility assessments, site selection, compliance evaluations, study setup, import permits, project management, and reporting, while ensuring information security and transparency.
What is the significance of local expertise in the regulatory approval process for clinical trials?
Engaging with local regulatory experts enhances the approval process by providing insights into navigating COFEPRIS regulations, which is crucial for successful submissions.
What recent trends have been observed regarding the approval duration for research studies in Mexico?
Recent data indicates that the average approval duration for research studies in Mexico has improved, reflecting a more efficient process.
How does bioaccess® ensure the integrity and transparency of the clinical trial process?
Bioaccess® is committed to information security, managing client issues with integrity and transparency, which fosters trust throughout the clinical trial process.
Why is Mexico considered an attractive location for conducting clinical trials for medical devices?
Mexico is viewed as a secure and informed location for research, with a reputation for quality information and patient involvement, making it appealing for Medtech firms.




