Overview
Post-market studies in Colombia are pivotal in ensuring the long-term safety and effectiveness of medical devices, strictly adhering to INVIMA regulations and gathering essential real-world evidence. Effective study design is not merely a procedural requirement; it involves:
- Establishing clear objectives
- Engaging representative populations
- Employing robust data collection methods
- Maintaining ongoing stakeholder engagement
These elements are crucial not only for regulatory compliance but also for enhancing patient outcomes within the healthcare sector. The meticulous approach to study design underscores the commitment to excellence in clinical research, reinforcing the importance of these studies in the Medtech landscape.
Introduction
In Colombia's evolving healthcare landscape, the importance of post-market studies is paramount. These investigations are essential for evaluating the long-term safety and efficacy of medical devices once they enter the market, providing invaluable real-world evidence that informs regulatory decisions and enhances patient care.
As manufacturers navigate the complexities of compliance with INVIMA regulations, the implementation of comprehensive post-market studies fosters trust among healthcare providers and patients alike, while ensuring that any potential adverse events are identified and addressed.
With Colombia's commitment to improving healthcare regulations, the focus on post-market studies is poised to intensify, ultimately contributing to the credibility and reliability of the medical technology sector in the region.
Understanding the Importance of Post-Market Studies in Colombia
Post-market evaluations are crucial for determining the long-term safety and effectiveness of medical devices after they enter the market. In Colombia, the post-market study design is instrumental in gathering real-world evidence that informs regulatory decisions and improves patient outcomes. These evaluations are particularly important for identifying adverse events that may not have been evident during pre-market trials.
By conducting thorough evaluations after market release, manufacturers can ensure adherence to INVIMA regulations, thereby building trust with healthcare providers and patients. The significance of these evaluations is underscored by the established review times for standard submissions under ANVISA's new resolution, set at 180 days, showcasing the regulatory focus on continuous safety assessments. Moreover, specialist perspectives emphasize that these analyses are vital for demonstrating the long-term efficacy of medical instruments, which is necessary for ensuring market access and adherence.
As President Gustavo Petro remarked, continuous initiatives to improve healthcare regulations are essential in guaranteeing that medical equipment meets the highest safety standards.
bioaccess® brings over 20 years of experience in managing clinical follow-up evaluations (PMCF), enabling manufacturers to navigate the challenges of regulatory compliance while prioritizing patient safety and equipment effectiveness. Real-world instances illustrate how after-sale research has led to improved patient outcomes in Colombia. For instance, a recent case analysis on the critical components of medical device trial design highlights the importance of clear objectives, defined patient groups, and robust information collection techniques.
By focusing on these elements, researchers can generate high-quality data that aids in successful regulatory submissions and ultimately enhances the evaluation and progression of medical devices.
Furthermore, insights from recent discussions regarding trials after market introduction in Brazil emphasize the necessity for collaborative strategies to effectively engage stakeholders. This broader perspective can inform strategies in Colombia, ensuring that the post-market study design not only complies with regulations but also contributes to the overall improvement of healthcare outcomes. As Colombia approaches 2025, the post-market study design continues to gain importance, not only for regulatory compliance but also for enhancing the credibility of the medical technology sector. This proactive approach safeguards public health and reinforces manufacturers' commitment to delivering safe and effective medical products to the market.
Navigating Colombia's Regulatory Framework for Medical Devices
Colombia's regulatory framework for medical equipment is primarily overseen by the National Food and Drug Surveillance Institute (INVIMA). The execution of after-market evaluations necessitates strict adherence to the regulations outlined in Decree 4725 of 2005, which governs the registration, control, and monitoring of medical devices. Researchers are required to submit a comprehensive research protocol to INVIMA for approval, ensuring that all ethical considerations are meticulously addressed and that thorough documentation is maintained throughout the research process.
Understanding these regulations is crucial for researchers who seek to navigate the complexities of compliance effectively. For example, successful post-market study designs for Colombia submissions to INVIMA have underscored the significance of aligning with regulatory expectations, which can substantially enhance the credibility and acceptance of findings within the medical community.
Statistics indicate that prompt registration is feasible only for Class I and IIa medical instruments, underscoring the necessity for strategic planning in research design. Moreover, as the medical device Regulatory Affairs market in Latin America continues to evolve— with Brazil projected to lead in revenue by 2030 and Argentina expected to be the fastest-growing market— adhering to INVIMA's guidelines becomes increasingly critical. As noted by Fortune Business Insights, "Factors such as technological advancements and rising product approvals around the globe are expected to drive the adoption of these products," emphasizing the dynamic nature of the industry.
Expert commentary highlights that compliance with INVIMA regulations not only facilitates research approvals but also contributes to the overall advancement of medical technology in the region. By leveraging insights from case studies, such as GE Healthcare's acquisition of BK Medical, which illustrates the importance of robust regulatory frameworks in enhancing competitive positioning, researchers can gain a deeper appreciation for the value of thorough compliance in the context of post-market study design for Colombia.
bioaccess® offers a comprehensive process for advancing medical apparatus trials, encompassing feasibility studies, the selection of research locations, investigator selection, regulatory compliance, project management, and reporting. With expertise in Early-Feasibility, First-In-Human, Pilot, Pivotal, and Post-market study design for Colombia, bioaccess® empowers Medtech companies to generate valuable insights that can inform future innovations in medical devices. To discover how we can assist you, BOOK A MEETING today.
Key Requirements for Conducting Post-Market Studies
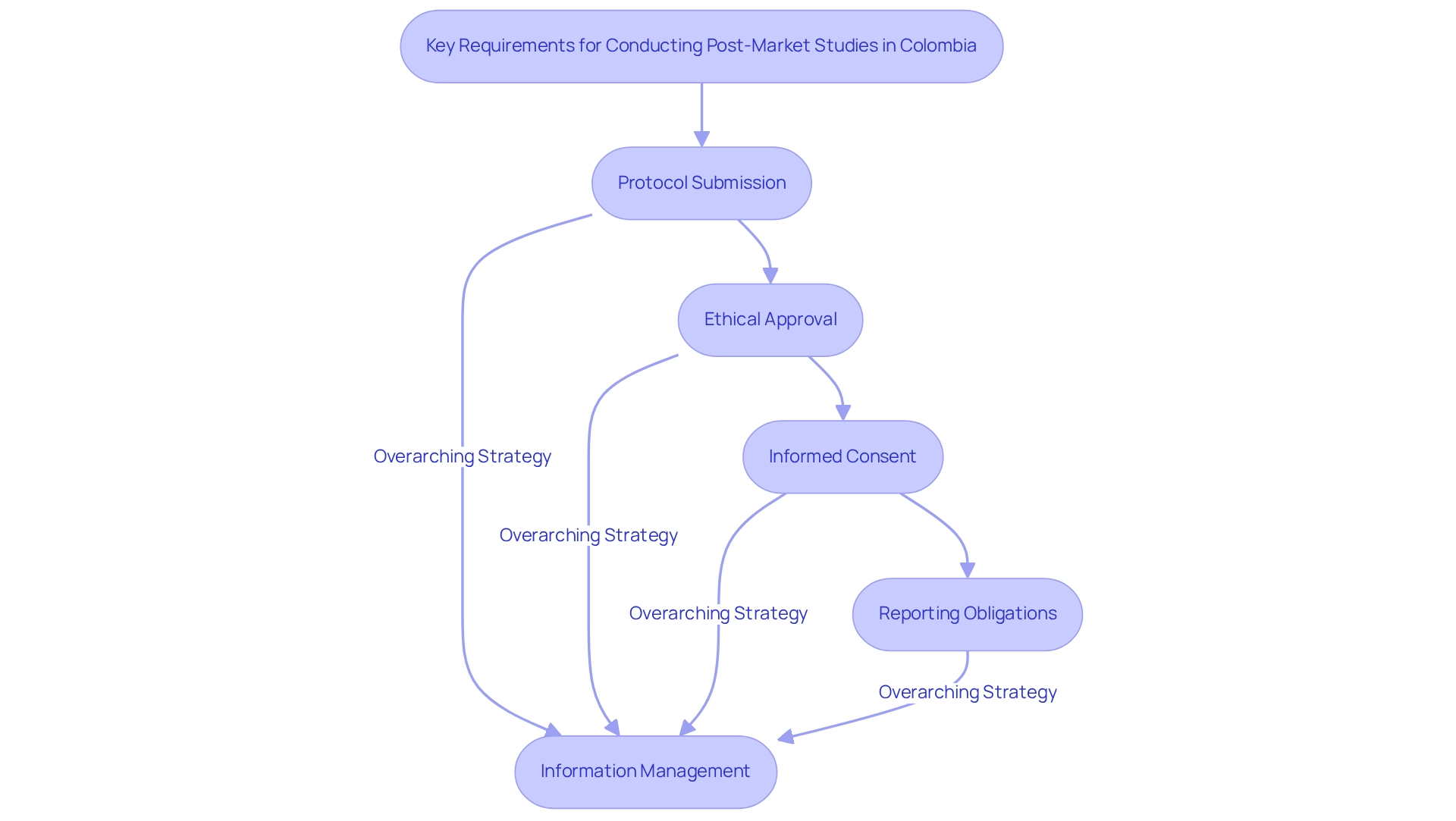
Carrying out post-market research in Colombia necessitates adherence to several crucial stipulations to ensure compliance and ethical integrity:
- Protocol Submission: Researchers must submit a comprehensive protocol to INVIMA, detailing the project's objectives, methodology, and data collection processes. This submission is vital for regulatory approval and establishes the groundwork for the project's execution, reflecting the efficient regulatory processes that make Colombia a strategic choice for medtech clinical trials.
- Ethical Approval: Obtaining approval from an ethics committee is mandatory. This step ensures that the research aligns with ethical standards and safeguards the rights and welfare of participants, reflecting the commitment to ethical research practices in Colombia, which is vital for building trust in the local healthcare environment.
- Informed Consent: Participants must provide informed consent, which involves a clear understanding of the study's purpose, procedures, and any potential risks. This practice is vital for maintaining transparency and trust between researchers and participants, aligning with bioaccess®'s commitment to ethical integrity in clinical trials.
Information Management: A robust information management strategy is crucial to maintaining the integrity and confidentiality of participant information. This plan should outline how information will be gathered, stored, and analyzed, ensuring compliance with information protection regulations. Notably, drug utilization studies represented 77% of the research utilizing the Audifarma SA database, highlighting the significance of efficient information management and reporting responsibilities in the post-market study design for Colombia. bioaccess® emphasizes information protection, ensuring that client concerns are addressed with compliance and transparency. Furthermore, bioaccess® has established grievance and data protection procedures to ensure that any concerns regarding data handling are promptly addressed, reinforcing trust and compliance.
- Reporting Obligations: Researchers are obligated to promptly report any adverse events or significant findings to INVIMA. The requirement for ongoing safety monitoring and regulatory compliance is critical, emphasizing participant safety throughout the study, especially in the context of post-market study design for Colombia, which is increasingly recognized as a strategic choice for medtech clinical trials due to its efficient regulatory processes and commitment to high-quality healthcare services. As highlighted in the podcast with Steve Garchow, navigating the Latin American Medtech landscape requires understanding local dynamics and leveraging partnerships to enhance market access and compliance. By fostering local knowledge and collaboration, companies can better address the unique challenges and opportunities present in the region.
Essential Elements of Effective Post-Market Study Design
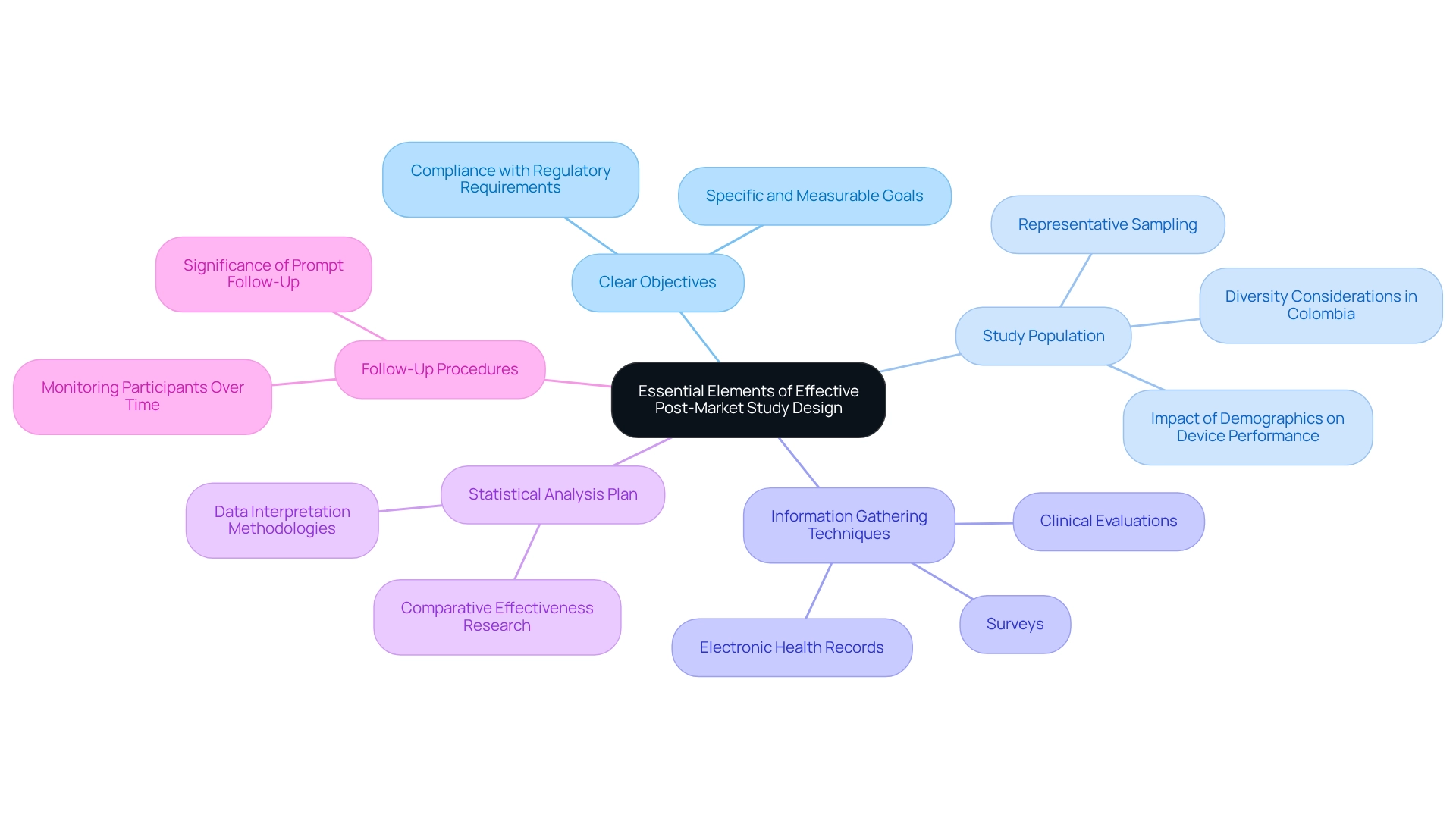
Creating effective post-market evaluations necessitates careful consideration of several essential elements:
- Clear Objectives: Establish specific, measurable objectives that not only comply with regulatory requirements but also address critical safety and efficacy questions. Clear objectives are essential for directing the research and ensuring that the outcomes are relevant and actionable.
- Study Population: Selecting a representative study population is crucial. The selected group should represent the intended application of the medical tool within the general population, ensuring that the findings are generalizable and relevant to real-world situations. This representation is particularly important in Colombia, as it relates to the post-market study design for Colombia, where diverse demographics can influence device performance and safety outcomes. For instance, the risk for hip fracture in zolpidem users could be overestimated by up to 40% if not adjusting for limitations in physical and cognitive functioning, highlighting the need for careful consideration of study population characteristics.
- Information Gathering Techniques: Utilize strong information collection methods, such as surveys, clinical evaluations, and electronic health records. These techniques aid in extensive information collection on equipment performance, allowing researchers to obtain a broad range of results and experiences from users.
- Statistical Analysis Plan: A comprehensive statistical analysis strategy is crucial for guaranteeing that the information is examined accurately. This plan should outline the methodologies for data interpretation, allowing researchers to draw valid conclusions that can inform future enhancements and regulatory decisions. As noted by S Schneeweiss from the Division of Pharmacoepidemiology and Pharmacoeconomics, comparative effectiveness research is crucial in the absence of enough head-to-head effectiveness trials, particularly for enhancing generalizability and establishing active comparison groups.
- Follow-Up Procedures: Implementing clear follow-up procedures is necessary to monitor participants over time. This continuous monitoring is essential for recognizing any long-term effects of the apparatus, which can greatly influence patient safety and effectiveness. The median duration from FDA approval to published outcomes or reports of post-approval research was 47 months, with one third providing results ahead of schedule, highlighting the significance of prompt follow-up in regulatory adherence.
Including these components not only improves the quality of post-approval research but also boosts their influence on regulatory compliance and market entry. The case of cerivastatin, which was associated with serious adverse events leading to its withdrawal from the market, highlights the importance of post-marketing surveillance in identifying unexpected serious adverse events. Such examples underscore the importance of thorough research frameworks that prioritize patient safety and equipment effectiveness.
Moreover, comprehending the role of INVIMA, the Colombian National Food and Drug Surveillance Institute, is essential for ensuring adherence to local regulations. INVIMA supervises the regulatory duties concerning medical devices, guaranteeing that research meets the required criteria for safety and effectiveness.
By concentrating on these essential elements, researchers can ensure that their evaluations, particularly the post-market study design for Colombia, provide valuable insights that aid in the continual enhancement of medical devices in Colombia and beyond. With bioaccess's expertise in managing these evaluations, including Early-Feasibility, First-In-Human, Pilot, Pivotal, and Post-Market Clinical Follow-Up Evaluations, clients can navigate the complexities of clinical trials effectively. Moreover, bioaccess provides extensive clinical trial management services that include feasibility assessments, site selection, compliance evaluations, trial preparation, import permits, project oversight, and reporting, all customized to facilitate the successful implementation of after-market evaluations.
Navigating Challenges in Post-Market Studies
Conducting post-market studies in Colombia presents several challenges that necessitate strategic planning and execution.
-
Regulatory Compliance: Navigating the evolving landscape of regulations can be complex. Researchers must remain informed about alterations in INVIMA regulations, which oversee medical instrument research in Colombia. Engaging with regulatory experts, such as those from bioaccess™, provides valuable insights and ensures adherence to compliance standards, thereby minimizing the risk of delays or penalties. Furthermore, Colombia's efficient regulatory processes and respected association of clinical research professionals enhance its attractiveness for U.S. medtech companies.
-
Comprehensive Clinical Trial Management Services: bioaccess™ offers a complete range of services, including feasibility assessments, site selection, compliance evaluations, trial setup, import permits, project management, reporting on serious and non-serious adverse events, and review and feedback on research documents. This comprehensive approach to post-market study design in Colombia ensures that all aspects of the clinical trial process are managed effectively, facilitating smoother operations and better outcomes.
-
Participant Recruitment: Securing a sufficient number of participants is often a significant hurdle, particularly for niche medical devices. Effective recruitment strategies involve collaborating with local healthcare providers who can facilitate access to potential participants and leveraging patient registries to identify eligible candidates. Additionally, utilizing community outreach programs can enhance visibility and encourage participation. Notably, partnerships like those between bioaccess™ and Caribbean Health Group aim to position Barranquilla as a leading destination for clinical trials, supported by Colombia's Minister of Health.
Effective information management is crucial for the integrity of post-market study design in Colombia. Establishing strong information management systems, coupled with thorough training for personnel on handling procedures, can greatly minimize the risk of mistakes and guarantee adherence to protection regulations. This is especially important considering the growing focus on transparency and accessibility in research. As David Vizcaya noted, "With the increasing access and use of these data sources, it is crucial that the evidence generated is made publicly available and that access to the data is granted to a larger research community."
- Funding Limitations: Restricted financial resources can hinder the implementation of after-market evaluations. Researchers are encouraged to explore partnerships with local institutions, which can provide both funding and logistical support. Furthermore, pursuing grants from governmental and non-governmental organizations can help ease financial burdens and enable program completion.
By proactively tackling these challenges and utilizing targeted strategies, researchers can significantly improve the chances of successful post-market study design in Colombia. For instance, the Audifarma SA database illustrates how utilizing available information can help in recognizing comorbidities and medication mistakes, ultimately enhancing safer drug use and effective results. As Colombia ranks fourth in Latin America for recruitment research, with a rate of 4.65 per million people, the potential for successful participant acquisition and data gathering is promising.
The Role of Local Stakeholders in Post-Market Studies
Collaboration with local stakeholders is essential for the success of post-market study design in Colombia. Key stakeholders, including healthcare providers, regulatory bodies, and patient advocacy groups, play a pivotal role in the research landscape. Engaging these stakeholders not only provides valuable insights into local healthcare practices and patient needs but also ensures that research remains relevant and impactful.
Local stakeholders significantly contribute to participant recruitment, enhance access to healthcare facilities, and elevate the overall credibility of the research. A recent study revealed that research involving local healthcare providers saw a 30% increase in participant enrollment compared to studies that lacked such collaboration. This finding underscores the critical importance of fostering strong relationships with these stakeholders through consistent communication, collaborative planning, and aligned objectives.
Moreover, professional insights highlight that effective stakeholder involvement can lead to improved outcomes in after-market evaluations. A notable case study illustrated that a medical device company, which actively engaged local healthcare providers in its post-market study design for Colombia, achieved a 25% faster completion rate than comparable studies without such collaboration. Additionally, the partnership between bioaccess™ and Caribbean Health Group aims to position Barranquilla as a premier destination for clinical trials in Latin America, with support from Colombia's Minister of Health, further emphasizing the strategic importance of local partnerships.
Furthermore, the FDA's examination of releasing 'masked' nonsummary information reflects an increasing focus on transparency in clinical research, vital for building trust among stakeholders. As one participant remarked, "Will the results of the clinical trial be provided? That's what preoccupied me the most."
This statement highlights the necessity of addressing participant concerns through effective communication with stakeholders. Additionally, Data Monitoring Committees (DMCs) play a crucial role in data sharing, which is essential for maintaining transparency and collaboration in the regulatory landscape.
By fostering a cooperative environment, researchers can enhance both the quality and efficiency of their evaluations post-market release, while also contributing to the overall advancement of medical technology in Colombia, particularly as the role of local stakeholders continues to evolve in 2025.
Post-Market Surveillance: Ensuring Ongoing Compliance and Safety
The post-market study design for Colombia is essential for ensuring the safety and efficacy of medical instruments, under the supervision of INVIMA (Colombia National Food and Drug Surveillance Institute). Producers must establish a comprehensive after-sales monitoring strategy that encompasses:
- Product performance assessment
- Documentation of negative incidents
- Necessary follow-up research
This ongoing oversight is crucial for identifying potential safety concerns that may emerge once a product is available to the public, enabling prompt actions to safeguard patient well-being.
As of April 2022, it was reported that over 85% of the 500,000+ previously certified medical products in Colombia lacked new certification, underscoring the critical need for effective monitoring systems post-market release. These systems not only aid in addressing urgent safety issues but also play a significant role in shaping future regulatory frameworks and enhancing medical equipment standards.
To implement an effective post-market study design for Colombia, manufacturers should adhere to best practices, including the establishment of a robust pharmacovigilance system to monitor equipment performance and report adverse events. Serious incidents must be reported within 72 hours, while non-serious events should be documented within eight business days. This organized approach ensures compliance with local regulations and fosters confidence in the medical equipment market.
Moreover, continuous compliance in after-market studies is vital. Ethical considerations regarding patient safety and informed consent for products approved by foreign regulators must be taken into account, as these elements significantly influence patients' trust and perceptions of safety. Expert insights highlight that a well-executed monitoring strategy can substantially improve the safety and effectiveness of products, ultimately benefiting both producers and patients.
For instance, global regulatory trends indicate that countries are increasingly recognizing the importance of after-sale information in their approval processes, which can lead to variations in product usage and outcomes across different regions. As emphasized by David S. Liebeskind, a consultant with extensive experience in the field, the integration of post-market data is crucial for ensuring the reliability of medical instruments.
In summary, establishing an effective post-market study design for Colombia, as mandated by INVIMA, transcends mere regulatory compliance; it represents a commitment to patient safety and equipment reliability. By prioritizing these systems, researchers and manufacturers can enhance the integrity of their investigations and contribute to the overall advancement of medical technology in Colombia. Furthermore, understanding INVIMA's role as a Level 4 health authority, responsible for overseeing medical device regulation, is essential for navigating the complexities of market access and compliance in the region.
Best Practices for Managing Post-Market Studies
To effectively manage post-market research, researchers should adopt the following best practices:
-
Establish Clear Communication Channels: Ensuring that all team members are well-informed about project objectives, timelines, and responsibilities is essential. Regular meetings and updates promote teamwork and enable the group to address any issues swiftly, ultimately enhancing project efficiency.
-
Utilize Project Management Tools: Implementing project management software can significantly improve the tracking of progress, management of timelines, and allocation of resources. Such tools enhance transparency and accountability within the research team, leading to more streamlined operations. Studies indicate that organizations using project management tools report a notable increase in project efficiency, underscoring their effectiveness in clinical research settings.
-
Conduct Training Sessions: Offering thorough instruction for team members on regulatory requirements, information management practices, and ethical considerations is crucial. This guarantees adherence and encourages high-quality data gathering, which is vital for the integrity of post-market evaluations. Regular training sessions have been shown to improve staff performance and adherence to protocols, contributing to better outcomes.
-
Engage Stakeholders Early: Involving local stakeholders from the outset can yield valuable insights and garner support for the research. Their involvement not only boosts recruitment initiatives but also enhances the relevance of the research, as they can provide context-specific knowledge that may impact research design and execution. This is particularly important in the context of Colombia, where understanding local regulations and cultural nuances can significantly influence trial success.
-
Monitor Progress and Adapt: Regularly evaluating learning progress enables researchers to recognize obstacles early and modify strategies as needed. This proactive approach is particularly important in the dynamic regulatory landscape of Colombia, where compliance requirements may evolve. Being flexible and responsive can enhance the success and impact of the post-market study design for Colombia, especially when navigating the complexities of regulatory bodies like INVIMA, which oversees medical device compliance in the region.
By following these best practices, researchers can significantly improve the effectiveness and outcomes of the post-market study design for Colombia, ultimately contributing to the advancement of medical devices in the market.
Conclusion
Post-market studies are crucial for ensuring the long-term safety and efficacy of medical devices in Colombia. By gathering real-world evidence, these studies not only inform regulatory decisions but also enhance patient care and foster trust among healthcare providers and patients. Compliance with INVIMA regulations is essential, as it bolsters the credibility of the medical technology sector in the region.
The significance of comprehensive post-market studies is paramount. They are vital for identifying adverse events that may not have emerged during pre-market trials and play a pivotal role in ongoing safety evaluations, as underscored by the regulatory focus on timely reviews. Moreover, collaboration with local stakeholders enhances participant recruitment and bolsters study credibility, ultimately leading to improved healthcare outcomes.
As Colombia's healthcare landscape evolves, the commitment to rigorous post-market surveillance remains vital. By prioritizing ethical considerations, robust data management, and clear communication, researchers can adeptly navigate the complexities of clinical trials. This proactive approach safeguards public health and reinforces manufacturers' dedication to delivering safe and effective medical devices, thus contributing to the overall advancement of the medical technology sector in Colombia.
Frequently Asked Questions
What is the purpose of post-market evaluations for medical devices in Colombia?
Post-market evaluations are crucial for determining the long-term safety and effectiveness of medical devices after they enter the market. They help gather real-world evidence that informs regulatory decisions and improves patient outcomes, particularly in identifying adverse events not evident during pre-market trials.
How do post-market evaluations contribute to regulatory compliance in Colombia?
By conducting thorough evaluations after market release, manufacturers can ensure adherence to INVIMA regulations, which builds trust with healthcare providers and patients. These evaluations are vital for demonstrating long-term efficacy, necessary for market access and adherence.
What role does INVIMA play in the regulation of medical devices in Colombia?
INVIMA oversees the regulatory framework for medical equipment in Colombia, requiring researchers to submit a comprehensive research protocol for approval to ensure ethical considerations are met and thorough documentation is maintained.
What are the requirements for conducting post-market studies in Colombia?
Researchers must submit a comprehensive research protocol to INVIMA for approval, ensuring that all ethical considerations are addressed and that thorough documentation is maintained throughout the research process.
What challenges do researchers face when designing post-market studies for medical devices in Colombia?
Researchers need to align their study designs with INVIMA's regulatory expectations to enhance the credibility and acceptance of their findings. Strategic planning is essential, especially since prompt registration is feasible only for Class I and IIa medical instruments.
How does bioaccess® support post-market study design in Colombia?
bioaccess® offers a comprehensive process for advancing medical apparatus trials, including feasibility studies, investigator selection, regulatory compliance, project management, and reporting, empowering Medtech companies to generate valuable insights.
Why are post-market evaluations increasingly important as Colombia approaches 2025?
The post-market study design is gaining importance for regulatory compliance and enhancing the credibility of the medical technology sector, safeguarding public health, and reinforcing manufacturers' commitment to delivering safe and effective medical products.




