Overview
The article provides a comprehensive guide on improving patient recruitment for clinical trials by outlining strategic steps such as defining eligibility criteria, engaging with the community, utilizing technology, and building trust with participants. These strategies are supported by evidence that emphasizes the importance of tailored outreach, collaboration with healthcare providers, and leveraging digital tools to enhance visibility and participation, ultimately fostering a more effective recruitment process.
Introduction
In the intricate realm of clinical trials, the patient recruitment process stands as a crucial determinant of success, particularly in competitive environments such as Colombia. With its unique advantages, including a robust healthcare system and significant R&D tax incentives, Colombia presents a fertile ground for innovative approaches to attract participants.
This article delves into the multifaceted strategies essential for optimizing patient recruitment, from defining eligibility criteria and engaging communities, to leveraging digital tools and building trust with participants.
By exploring these comprehensive methods, researchers can enhance enrollment efficiency and ensure that clinical trials are not only successful but also ethically conducted, ultimately advancing medical knowledge and improving patient outcomes.
Understanding the Patient Recruitment Process in Clinical Trials
The patient recruitment process in clinical trials is a multifaceted endeavor that encompasses several essential stages, particularly in a competitive landscape like Colombia's, which offers notable advantages for first-in-human studies:
- Defining Eligibility Criteria: Establishing precise inclusion and exclusion criteria is fundamental in targeting the appropriate population for each study. These criteria not only streamline the selection process but also enhance the likelihood of successful patient recruitment, thereby aligning with the specific goals of the trial.
- Creating a Hiring Strategy: A thorough hiring strategy is essential, outlining the target population, hiring methodologies, timelines, and necessary resources. This plan should remain adaptable to address any challenges or changes encountered during patient recruitment, ensuring that objectives are met efficiently.
- Engaging with the Community: Actively engaging with the community plays a crucial role in patient recruitment and raising awareness about clinical trials. Collaborations with local healthcare providers, community organizations, and patient advocacy groups can effectively foster trust and interest among potential participants, which is crucial for patient recruitment and aligns with the growing trend of seeking diverse sources for participation. In Colombia, where approximately 95% of the population is covered by universal healthcare, such engagement can improve patient recruitment success. The recent statistic that Walgreens signed 15 contracts for clinical studies in Q3 of 2023, up from 8 contracts in Q2, highlights the competitive landscape.
- Utilizing Technology: The integration of technology is paramount in reaching a wider audience. Utilizing digital platforms and social media to disseminate information about trials—through online advertisements, informational webinars, and dedicated trial websites—can significantly boost visibility and improve patient recruitment. The case study titled "Comparability of Data Collection Methods" indicates that optimizing electronic measures for diverse populations is crucial, suggesting a shift in focus from comparability debates to practical applications in patient recruitment strategies.
- Building Trust: Establishing a foundation of trust with prospective participants is essential. Clear communication regarding the study’s objectives, procedures, and potential risks can alleviate concerns and improve patient recruitment. In Colombia, where the healthcare system ranks among the top five globally, fostering trust is vital for patient recruitment to harness the competitive advantages presented by the local ecosystem. According to industry expert Brant, there is a pressing need for patient recruitment efforts to expand beyond the U.S. to meet competitive goals, underscoring the importance of trust and clarity in this process.
- Utilizing R&D Tax Incentives: Colombia provides substantial R&D tax incentives that can improve the financial feasibility of research studies. Investments in science, technology, and innovation projects can receive a 100% tax deduction, a 25% tax discount, and a 50% future tax credit, along with approximately $10 million in government grants. These incentives can make carrying out experiments in Colombia not only feasible but also financially appealing.
These strategic phases are essential in enhancing patient recruitment initiatives in Colombia, ultimately resulting in better results in research studies and maximizing the advantages of the supportive regulatory environment, cost efficiencies, and quality healthcare accessible in the region.
, and the arrows indicate the sequential flow from one stage to the next. Each box represents a stage in the patient recruitment process, and the arrows indicate the sequential flow from one stage to the next.](https://images.tely.ai/telyai/skasxate-each-box-represents-a-stage-in-the-patient-recruitment-process-and-the-arrows-indicate-the-sequential-flow-from-one-stage-to-the-next.webp)
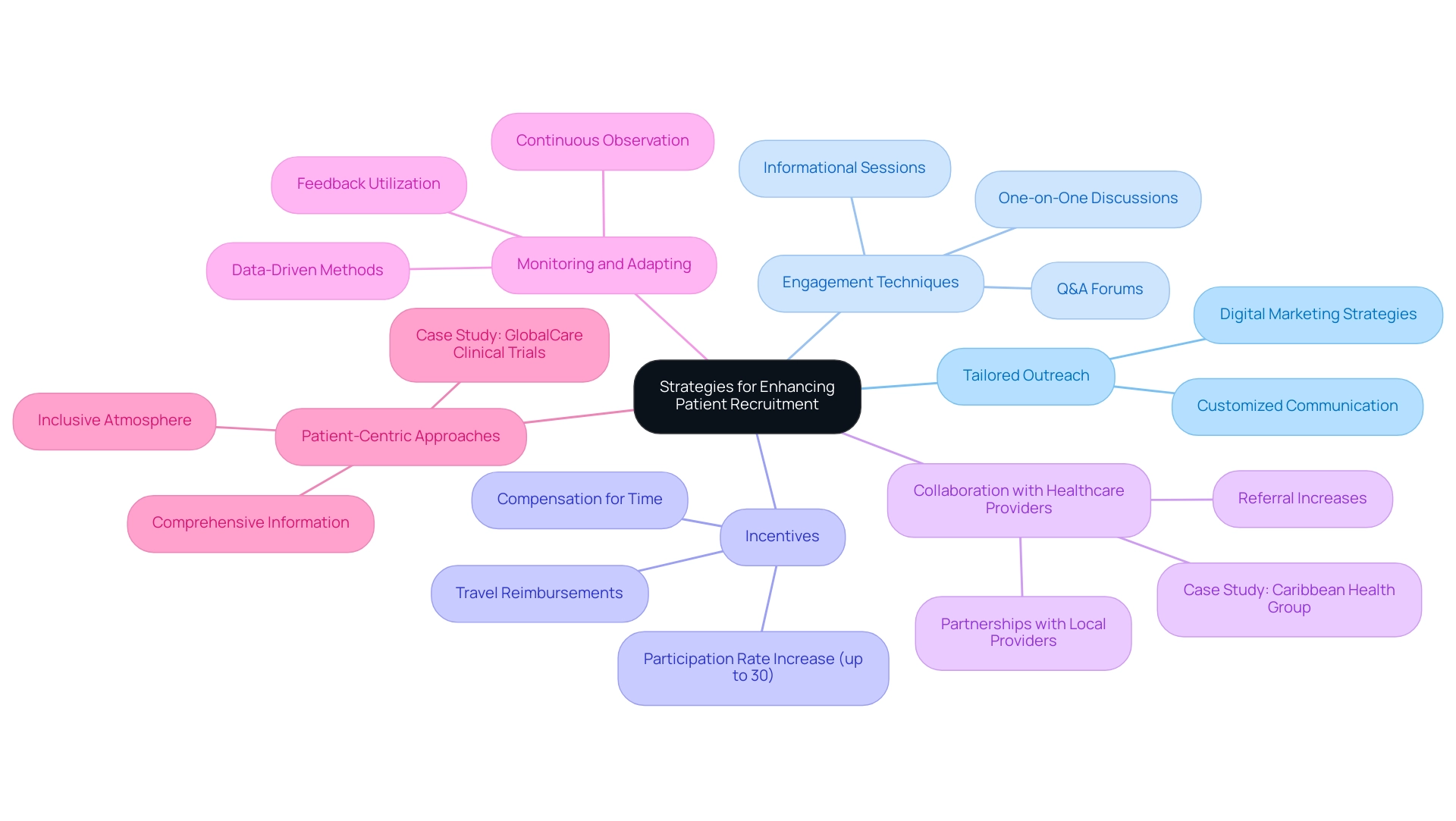
Effective Strategies for Enhancing Patient Recruitment
A multifaceted approach is essential to enhance patient recruitment in clinical trials. Consider the following strategies:
-
Tailored Outreach: Customize outreach efforts to align with specific demographics based on the study's eligibility criteria.
This entails utilizing customized communication methods that align with potential individuals' interests and needs, thus enhancing engagement. Digital marketing strategies enable researchers to conduct targeted campaigns that directly address these unique factors.
-
Engagement Techniques: Implement engagement techniques such as informational sessions, Q&A forums, and one-on-one discussions.
These strategies enable researchers to directly tackle questions and concerns from prospective contributors, fostering a sense of community and support. As Teuteberg emphasizes,
Our main focus at Splash is on patient engagement and ensuring the patient does not fall into the cracks leading them to drop out of the study.
This approach emphasizes the significance of interpersonal skills and insights gained from the hiring process, which are essential for establishing trust and rapport with participants.
-
Incentives: Offering incentives for participation, such as travel reimbursements or compensation for time, can significantly motivate potential participants to enroll.
For instance, studies show that offering incentives can increase participation rates by up to 30%. This statistic highlights the significance of well-structured incentives for patient recruitment as a vital strategy in hiring efforts.
-
Collaboration with Healthcare Providers: Forge partnerships with local healthcare providers who can refer patients to the study.
Building strong relationships with these providers increases trust and, consequently, referral rates, which enhances the patient recruitment pipeline. Significantly, bioaccess™ has partnered with Caribbean Health Group to improve research services in Barranquilla, backed by Colombia's Minister of Health, during a meeting conducted on March 29, 2019, at PROCOLOMBIA's office in Miami, FL.
This collaboration exemplifies the power of such partnerships in positioning Barranquilla as a leading destination for clinical trials in Latin America.
-
Monitoring and Adapting: Continuously observe hiring efforts and remain flexible based on feedback and hiring data.
Examining effective tactics and recognizing areas for enhancement can improve the hiring strategy.
The study titled Limitations and Future Directions acknowledged the need for experimental designs and advanced analytics to systematically assess hiring strategies. For example, using data-driven methods can help identify which hiring channels yield the best results, allowing for more efficient allocation of resources.
-
Patient-Centric Approaches: Prioritize the needs and preferences of potential contributors by providing comprehensive information about the study, including its benefits and contributions to advancing medical knowledge.
This patient-focused method not only enhances patient recruitment efforts but also cultivates a more inclusive atmosphere for participants, ultimately resulting in better retention in studies.
The collaboration between GlobalCare Clinical Trials and bioaccess™ has accomplished over a 50% decrease in enrollment time and a remarkable 95% retention rate, showcasing the effectiveness of patient-centric strategies.
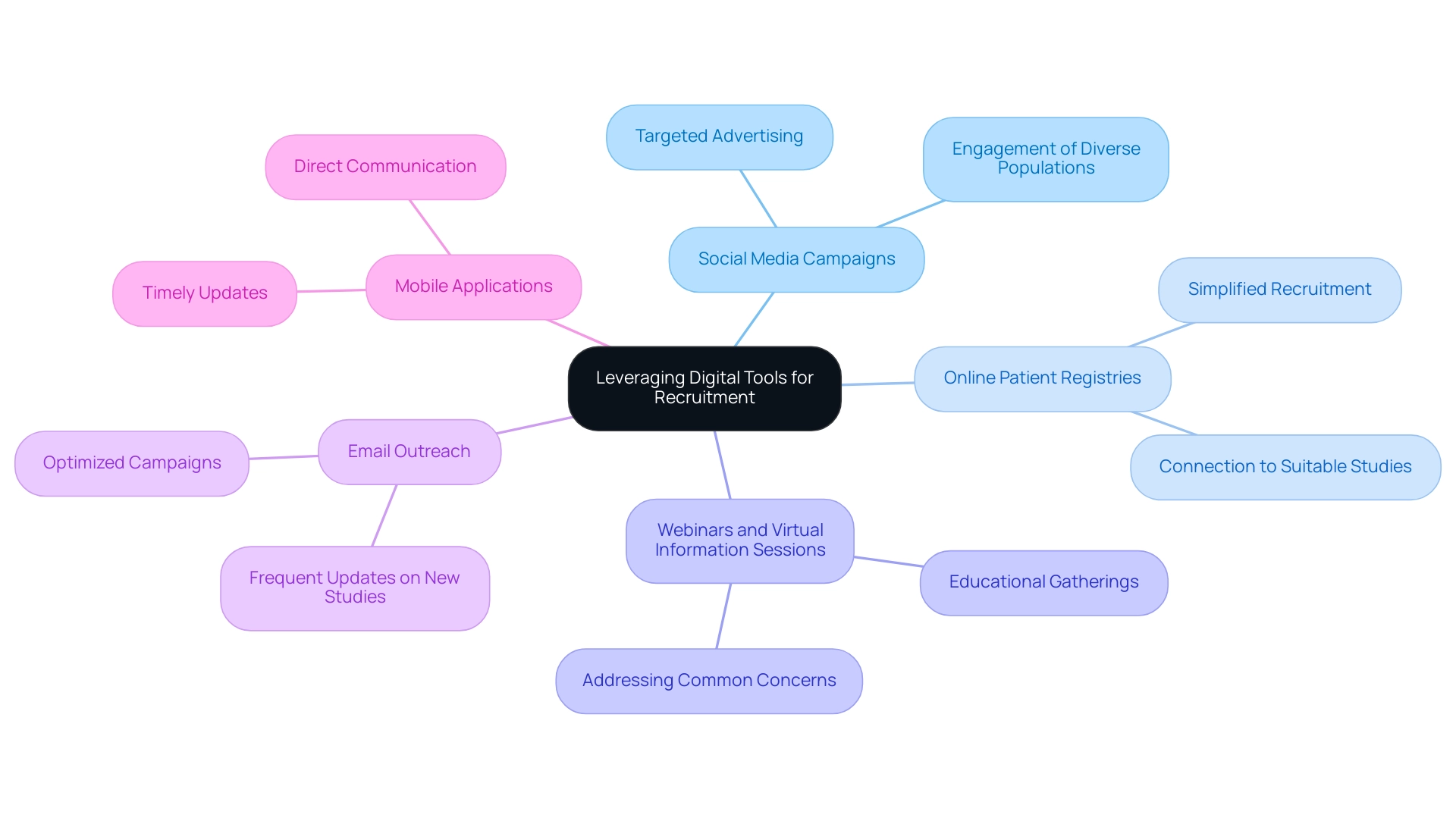
Leveraging Digital Tools for Recruitment
To effectively leverage digital tools for patient recruitment in clinical trials, consider implementing the following strategies:
- Social Media Campaigns: Utilize platforms such as Facebook, Twitter, and Instagram to develop targeted advertising campaigns. These campaigns can be customized to engage prospective individuals based on particular demographics and interests, significantly improving enrollment efforts. Successful social media campaigns have demonstrated their potential to engage diverse populations, facilitating broader reach and participation.
- Online Patient Registries: Work together with established online patient registries that link patients with pertinent research studies. These platforms not only simplify patient recruitment but also connect qualified individuals with suitable studies, thereby enhancing enrollment efficiency.
- Webinars and Virtual Information Sessions: Arrange online educational gatherings to inform prospective attendees about the research study. These sessions can address common concerns, provide detailed information about study protocols, and foster interaction, ensuring that individuals feel informed and valued.
- Email Outreach: Implement optimized email campaigns to engage previous participants or individuals who have shown interest in research studies. This approach has been shown to increase enrollment rates compared to traditional direct mailing methods, with statistics indicating a significant improvement in engagement. Frequent updates on new studies can promote continued involvement and sustain interest.
- Mobile Applications: Explore the creation or modification of mobile applications that offer extensive information about ongoing research studies. These applications can facilitate direct communication, allowing patients to express interest, ask questions, and receive timely updates. As Tim K. Mackey observes, utilizing digital technologies in patient recruitment can significantly enhance the inclusivity of these trials, particularly for ethnic and racial minority groups.
Moreover, a case study titled "General practice and digital methods to enlist stroke survivors for a mobility study" highlights the comparative effectiveness of digital enrollment techniques for patient recruitment against traditional approaches, demonstrating that while digital methods may lead to fewer participants over a shorter time, they can still be valuable in specific contexts. Furthermore, findings suggest that while online hiring is faster and cheaper, offline methods may yield better conversion rates for patient recruitment, providing a balanced view of the effectiveness of different hiring strategies.
Furthermore, recent conversations regarding blockchain technology suggest possibilities for improving study management and automating procedures such as e-consent, despite certain scalability obstacles. As these digital tools advance, they offer distinct chances to enhance patient recruitment and retention in medical studies, ensuring a more varied participant pool.
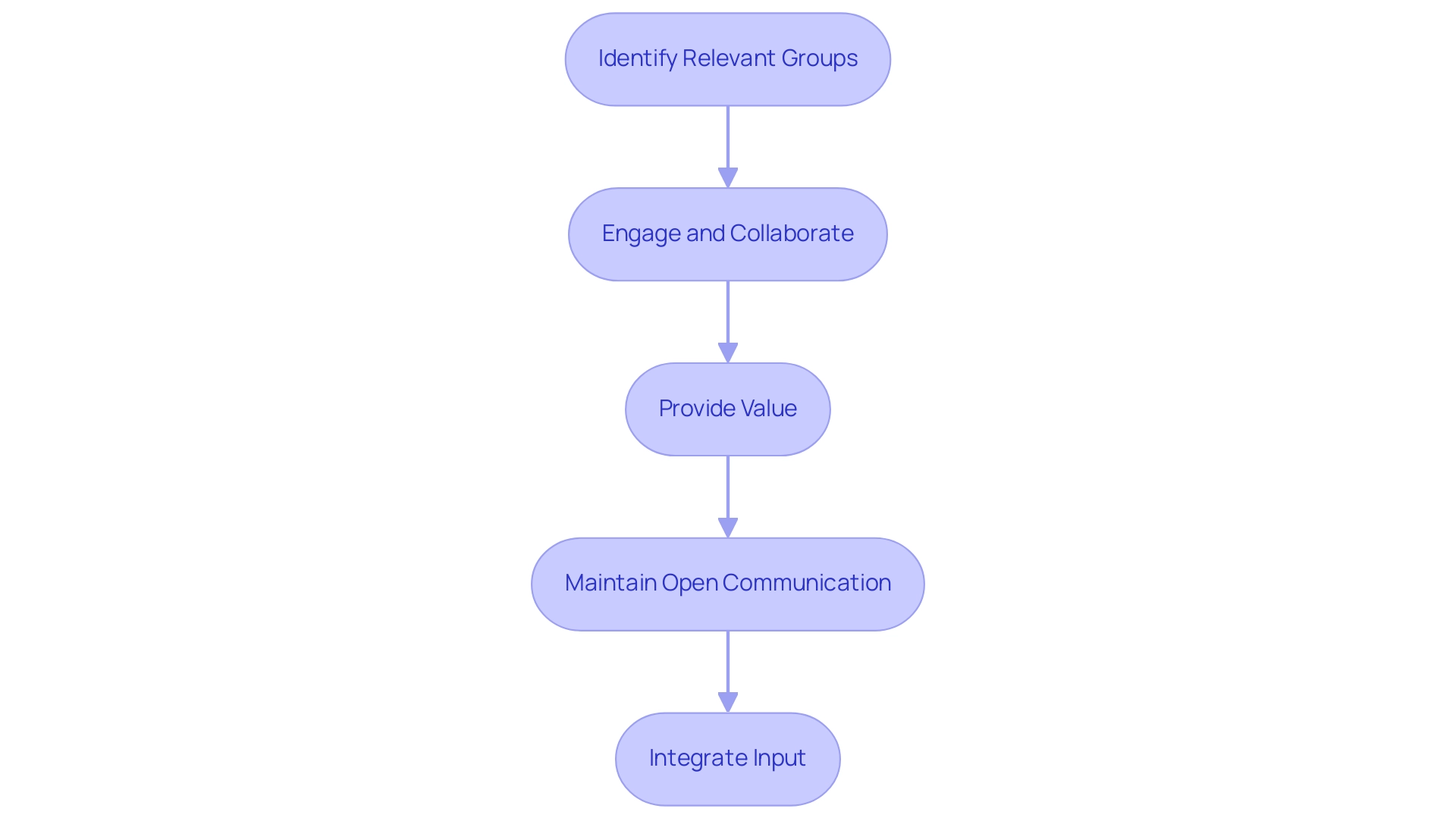
Building Relationships with Patient Advocacy Groups
Establishing strong connections with patient advocacy organizations is crucial for successful patient recruitment and retention in research. A recent cross-sectional survey across the Delhi National Capital Region after the COVID-19 pandemic indicated an increase in awareness about medical research to 87%, emphasizing the significance of collaborating with advocacy groups in the present environment. Here are key steps to consider:
- Identify Relevant Groups: Start by investigating advocacy organizations that correspond with the specific therapeutic field of your study. Understanding their mission and objectives will help in establishing meaningful connections.
- Engage and Collaborate: Initiate contact with these groups to explore potential collaborative opportunities, such as co-hosting informational events or sharing educational resources. This engagement fosters a sense of partnership and shared goals.
- Provide Value: It’s crucial to offer valuable support to advocacy organizations. Share educational materials about the clinical study that they can distribute to their members, ensuring that the information is both accurate and accessible.
- Maintain Open Communication: Establish and nurture ongoing communication with advocacy groups to keep them informed about trial progress, updates, and outcomes. This transparency is vital for building trust and reliability in the partnership.
- Integrate Input: Actively seek and integrate feedback from advocacy organizations regarding hiring strategies and concerns of those involved. This collaborative approach not only enhances engagement efforts but also showcases a commitment to ethical hiring practices and the welfare of participants.
Alongside these strategies, employing thorough clinical study management services can greatly improve enrollment and retention initiatives. Services like feasibility studies and site selection ensure that experiments are carried out in optimal environments. The trial setup process, which involves securing necessary approvals from ethics committees and health ministries, is essential for ensuring compliance and facilitating timely participant enrollment.
Furthermore, compliance reviews streamline processes to meet regulatory requirements. Reporting metrics, including study status, inventory, and adverse event reporting, provide valuable insights into participant engagement and retention, allowing for data-driven adjustments to strategies.
As emphasized by a principal investigator, "The partnership of our Pages and the Consortium has been very effective toward achieving our common mission of advancing understanding of these diseases, developing more effective treatments, and assuring that all patients have access to current correct information." By adhering to these steps and learning from examples such as the RDCRN's experience, which demonstrates effective patient involvement, researchers can establish lasting collaborations that improve enrollment initiatives and ensure that patient perspectives are essential to the research process. Additionally, the impact of MedTech research studies on local economies, including job creation and healthcare improvements, further underscores the importance of these partnerships in fostering international collaboration.
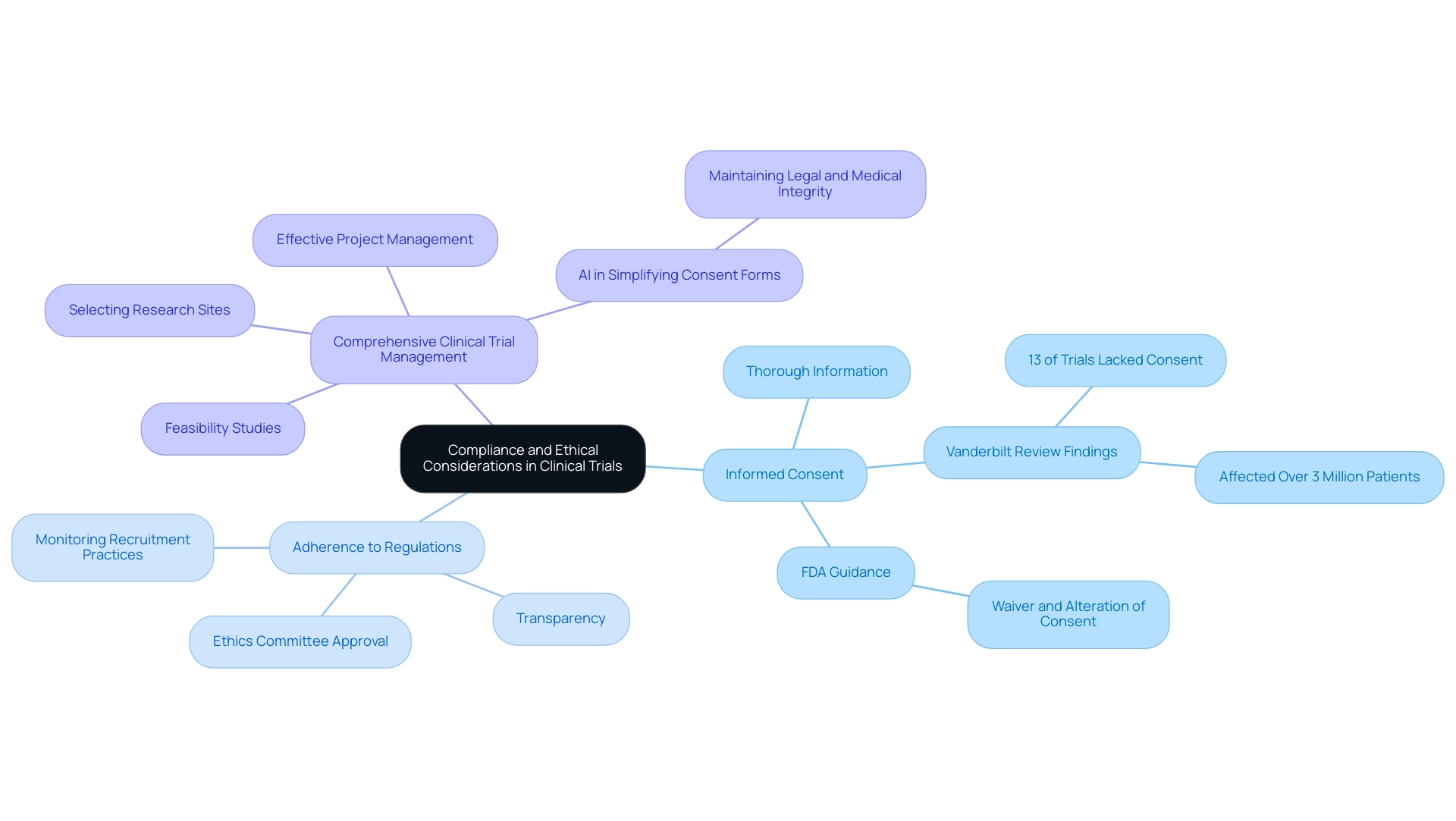
Ensuring Compliance and Ethical Considerations
To ensure compliance and uphold ethical considerations in patient recruitment for clinical studies, it is essential to implement the following strategies:
-
Informed Consent: It is essential that potential participants receive thorough and comprehensible information regarding the study, encompassing all associated risks and benefits. This transparency enables individuals to make informed decisions about their participation. Recent evaluations, such as the Vanderbilt Systematic Review, revealed that 13% of studies did not require informed consent, impacting over 3 million patients, thereby underscoring the necessity for diligent consent processes. Furthermore, with the upcoming FDA guidance on waiver and alteration of consent, researchers must stay informed about regulatory changes that could affect these practices.
-
Adherence to Regulations: Familiarity with both local and international regulations governing clinical trials is imperative. Adhering to these standards not only safeguards the rights of individuals but also boosts the credibility of the research.
- Ethics Committee Approval: Prior to initiating recruitment, obtaining approval from an ethics committee or institutional review board (IRB) is essential. This step ensures that the study adheres to ethical guidelines and safeguards the well-being of those involved.
- Transparency: Maintaining open communication with potential participants about the study's objectives, methodologies, and any potential conflicts of interest is vital. Such transparency fosters trust and contributes to ethical hiring practices.
- Monitoring Recruitment Practices: Regular reviews of recruitment strategies are necessary to ensure ongoing compliance with ethical standards. This process allows for timely adjustments to address any emerging concerns and enhances the overall integrity of the trial.
-
Comprehensive Clinical Trial Management: Engaging in thorough feasibility studies and selecting appropriate research sites and principal investigators (PIs) is crucial. This not only streamlines the setup and approval process with ethics committees and health ministries but also ensures adherence to local regulations regarding import permits and the nationalization of investigational devices. Effective project management and monitoring, alongside meticulous reporting of study status and adverse events, further bolster the trial’s success.
In addition to these ethical considerations, it is important to recognize the significant economic impacts of research trials. They can drive job creation and contribute to economic growth within local communities by fostering international collaboration and improving healthcare systems. As the landscape of medical research evolves, particularly with the anticipated FDA guidance on consent processes, it is essential to consider innovative solutions that simplify consent while preserving legal and medical integrity.
The potential of AI to streamline consent forms can play a significant role in this regard, making them more accessible to participants. According to Sean Khozin, MD, MPH, from the Laboratory for Financial Engineering at MIT, innovative solutions are vital for ensuring scientists can access the wealth of data generated by research trials. By proactively addressing these ethical considerations and compliance requirements, researchers can enhance their patient recruitment efforts and contribute to the advancement of clinical research.
Additionally, it is worth noting that the Flesch-Kincaid Grade Level for the Confidentiality clause decreased to 5.70 after simplification, highlighting the importance of making consent forms easier to read and understand.
Conclusion
In the competitive landscape of clinical trials, particularly in Colombia, the patient recruitment process is pivotal to success. This article has explored essential strategies for optimizing recruitment, emphasizing the importance of:
- Defining eligibility criteria
- Developing comprehensive recruitment plans
- Actively engaging with local communities
By leveraging digital tools and building trust with potential participants, researchers can significantly enhance enrollment efficiency and ensure ethical practices throughout the trial process.
Moreover, the integration of technology, such as social media campaigns and online patient registries, offers innovative approaches to reach a broader audience. Collaborations with healthcare providers and patient advocacy groups further strengthen recruitment efforts, facilitating a more robust pipeline of participants. The emphasis on patient-centric approaches not only fosters greater engagement but also improves retention rates, ultimately contributing to the advancement of medical knowledge.
In conclusion, as the clinical trial landscape continues to evolve, particularly with the unique advantages presented by Colombia's healthcare system and R&D tax incentives, adopting a multifaceted recruitment strategy is essential. By prioritizing ethical considerations and compliance, researchers can navigate the complexities of patient recruitment effectively, ensuring that clinical trials are not only successful but also contribute positively to the communities they serve.
Frequently Asked Questions
What are the essential stages in the patient recruitment process for clinical trials in Colombia?
The essential stages include defining eligibility criteria, creating a hiring strategy, engaging with the community, utilizing technology, building trust, and utilizing R&D tax incentives.
Why is defining eligibility criteria important in clinical trials?
Defining eligibility criteria helps target the appropriate population for each study, streamlining the selection process and enhancing the likelihood of successful patient recruitment.
What should a hiring strategy encompass in the patient recruitment process?
A hiring strategy should outline the target population, hiring methodologies, timelines, and necessary resources, and it should be adaptable to address challenges during recruitment.
How does community engagement contribute to patient recruitment?
Engaging with the community fosters trust and interest among potential participants, especially through collaborations with local healthcare providers and patient advocacy groups, which is crucial for successful recruitment.
What role does technology play in patient recruitment?
Technology, particularly digital platforms and social media, helps reach a wider audience by disseminating information about trials through online advertisements, webinars, and dedicated trial websites.
How can trust be established with prospective participants?
Trust can be built through clear communication regarding the study’s objectives, procedures, and potential risks, which alleviates concerns and improves recruitment.
What financial incentives does Colombia offer for research and development?
Colombia offers substantial R&D tax incentives, including a 100% tax deduction, a 25% tax discount, a 50% future tax credit, and approximately $10 million in government grants, making research financially appealing.
What are some tailored outreach methods for patient recruitment?
Tailored outreach methods include customizing communication strategies based on specific demographics and utilizing digital marketing for targeted campaigns.
What engagement techniques can be used to improve patient recruitment?
Engagement techniques such as informational sessions, Q&A forums, and one-on-one discussions help address questions and concerns from potential participants, fostering community support.
How can incentives impact patient recruitment rates?
Offering incentives, such as travel reimbursements or compensation for time, can significantly motivate potential participants, potentially increasing participation rates by up to 30%.
What is the importance of collaboration with healthcare providers in recruitment?
Collaborating with local healthcare providers can enhance trust and referral rates, thereby improving the patient recruitment pipeline.
How should recruitment efforts be monitored and adapted?
Recruitment efforts should be continuously observed and adjusted based on feedback and hiring data to identify effective tactics and areas for enhancement.
What is a patient-centric approach in recruitment?
A patient-centric approach prioritizes the needs and preferences of potential participants by providing comprehensive information about the study, which enhances recruitment efforts and retention rates.

