Overview
Mexico stands out as a prime medtech trial hub, leveraging its robust healthcare infrastructure, diverse patient demographics, and the specialized expertise of organizations like bioaccess® that streamline trial management and ensure regulatory compliance. This article underscores Mexico's strategic advantages, including lower operational costs and a notably high enrollment rate for medical trials, which collectively position it as an appealing destination for expedited medical device research.
As the medtech landscape evolves, the role of bioaccess in addressing key challenges becomes increasingly vital, fostering a collaborative environment that enhances the efficiency of clinical research. Ultimately, the synergy between Mexico's resources and bioaccess's capabilities not only facilitates successful trials but also sets the stage for future innovations in medical technology.
Moving forward, stakeholders are encouraged to consider the benefits of collaboration in this thriving ecosystem.
Introduction
In the rapidly evolving field of medical technology, Mexico emerges as a promising hub for clinical trials, offering a unique blend of advantages that cater to the needs of Medtech companies.
With a robust healthcare infrastructure, a diverse patient population, and a cost-effective environment, the country is strategically positioned to facilitate innovative clinical studies.
However, navigating the complexities of regulatory requirements and local healthcare dynamics presents its own set of challenges.
This article delves into the multifaceted landscape of Mexico's healthcare system, exploring essential strategies and best practices for conducting successful Medtech trials while highlighting the invaluable role of local expertise and partnerships in overcoming potential obstacles.
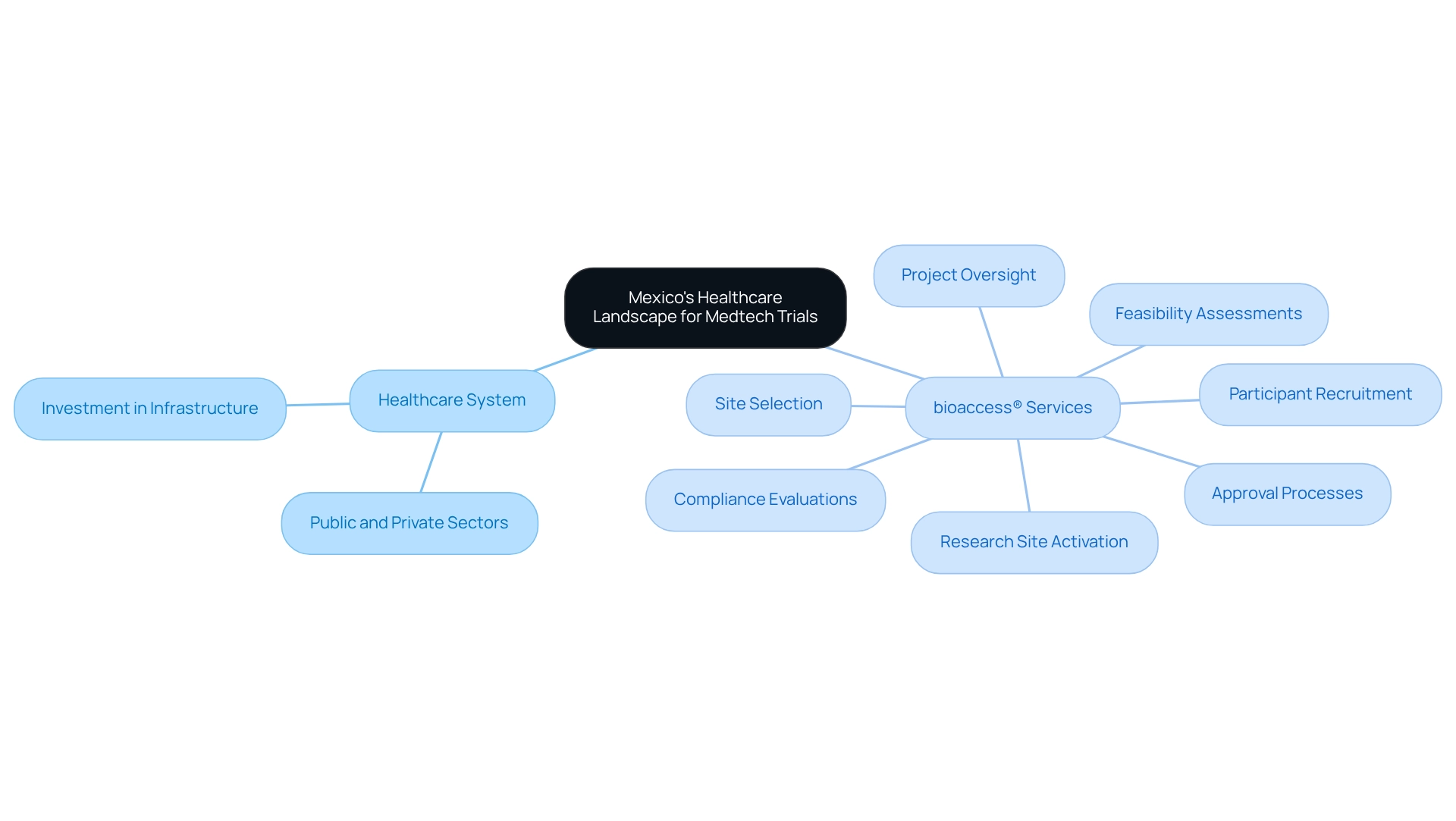
Understanding Mexico's Healthcare Landscape for Medtech Trials
Mexico's healthcare system represents a robust blend of public and private sectors, encompassing over 4,000 hospitals, a significant portion of which are public institutions. This dual framework not only caters to a diverse patient demographic but is also crucial for research, particularly for Medtech firms aiming to leverage the capabilities of bioaccess®. This positions Mexico as a prominent medtech trial hub. The nation has made substantial investments in healthcare infrastructure, enhancing its capacity to conduct medical research efficiently.
Understanding the demographics, which include a substantial population with varied health challenges, is vital for Medtech firms seeking to recruit participants for studies. Bioaccess® offers comprehensive trial management services, encompassing:
- Approval processes
- Research site activation
- Participant recruitment
- Feasibility assessments
- Site selection
- Compliance evaluations
- Project oversight
This ensures a streamlined approach to trials. Furthermore, bioaccess®’s expertise in navigating regulatory processes and delivering tailored solutions positions it as an invaluable partner for Medtech startups, particularly those looking to utilize Mexico as a medtech trial hub.
Mexico's geographical proximity to the United States facilitates smoother collaboration and logistics for international firms, further reinforcing its status as a medtech trial hub for expedited medical device research services.
Navigating Regulatory Requirements for Medtech Trials in Mexico
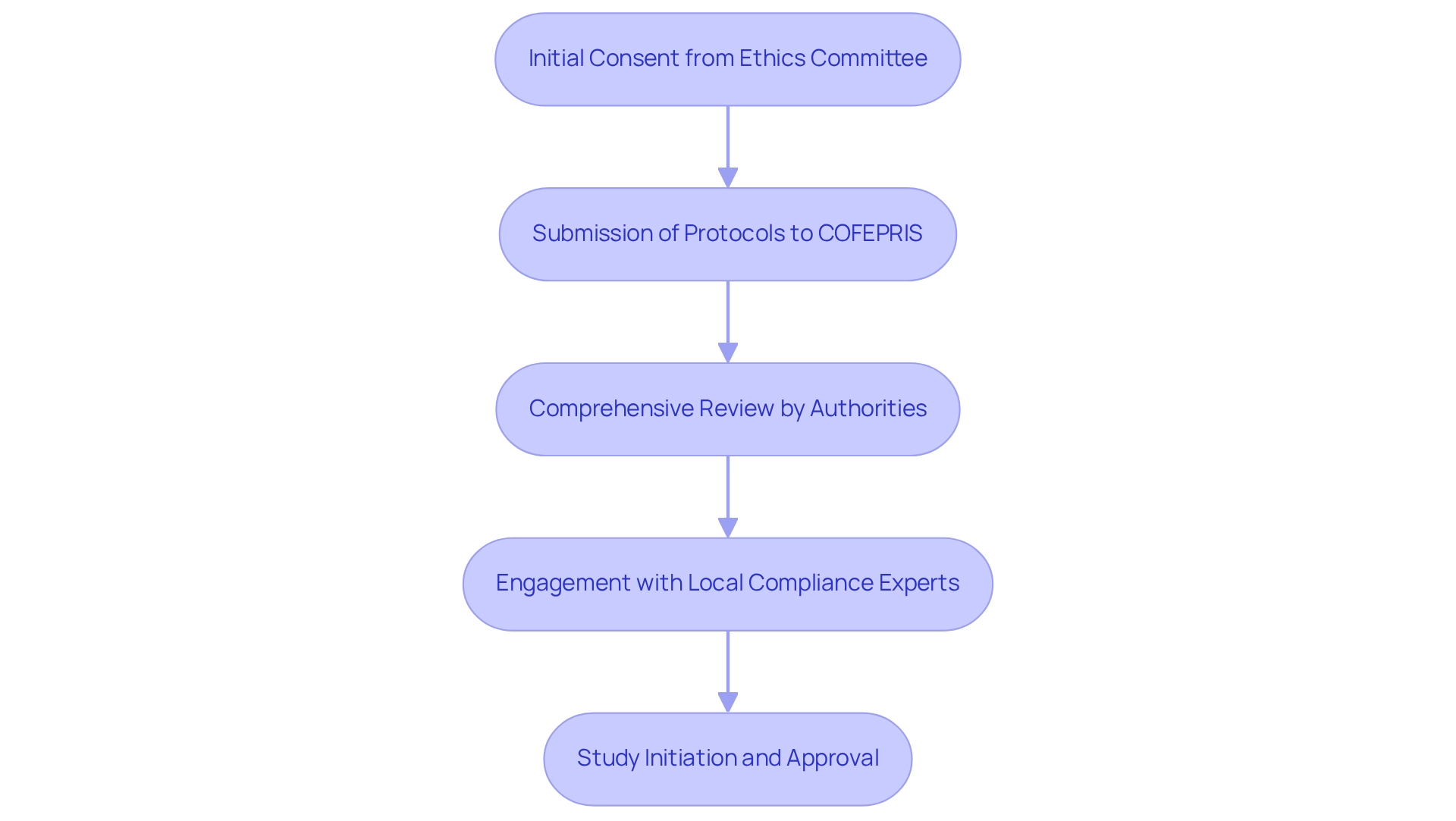
Conducting clinical trials in Mexico necessitates strict adherence to the regulations established by COFEPRIS (Federal Commission for Protection against Sanitary Risk). Companies are required to submit detailed protocols for approval, which encompass ethical considerations and safety measures. This process typically begins with securing initial consent from an ethics committee, followed by a comprehensive review by the appropriate authorities.
To effectively navigate these complexities, it is essential for companies to engage local compliance experts, such as those from bioaccess®, who specialize in ensuring adherence to all legal requirements, including documentation and reporting standards. Their expertise in overseeing the extensive procedure—from feasibility assessments and site selection to testing set-up, initiation, and approval—can significantly enhance the testing process. A solid understanding of the General Health Law and its secondary regulations is vital for a seamless experience, particularly in addressing the challenges faced by medical device startups, including regulatory obstacles and recruitment issues.
Moreover, bioaccess® provides specialized services in reporting on study status and serious and non-serious adverse events, alongside their expertise in conducting Early-Feasibility Studies and First-In-Human Studies.
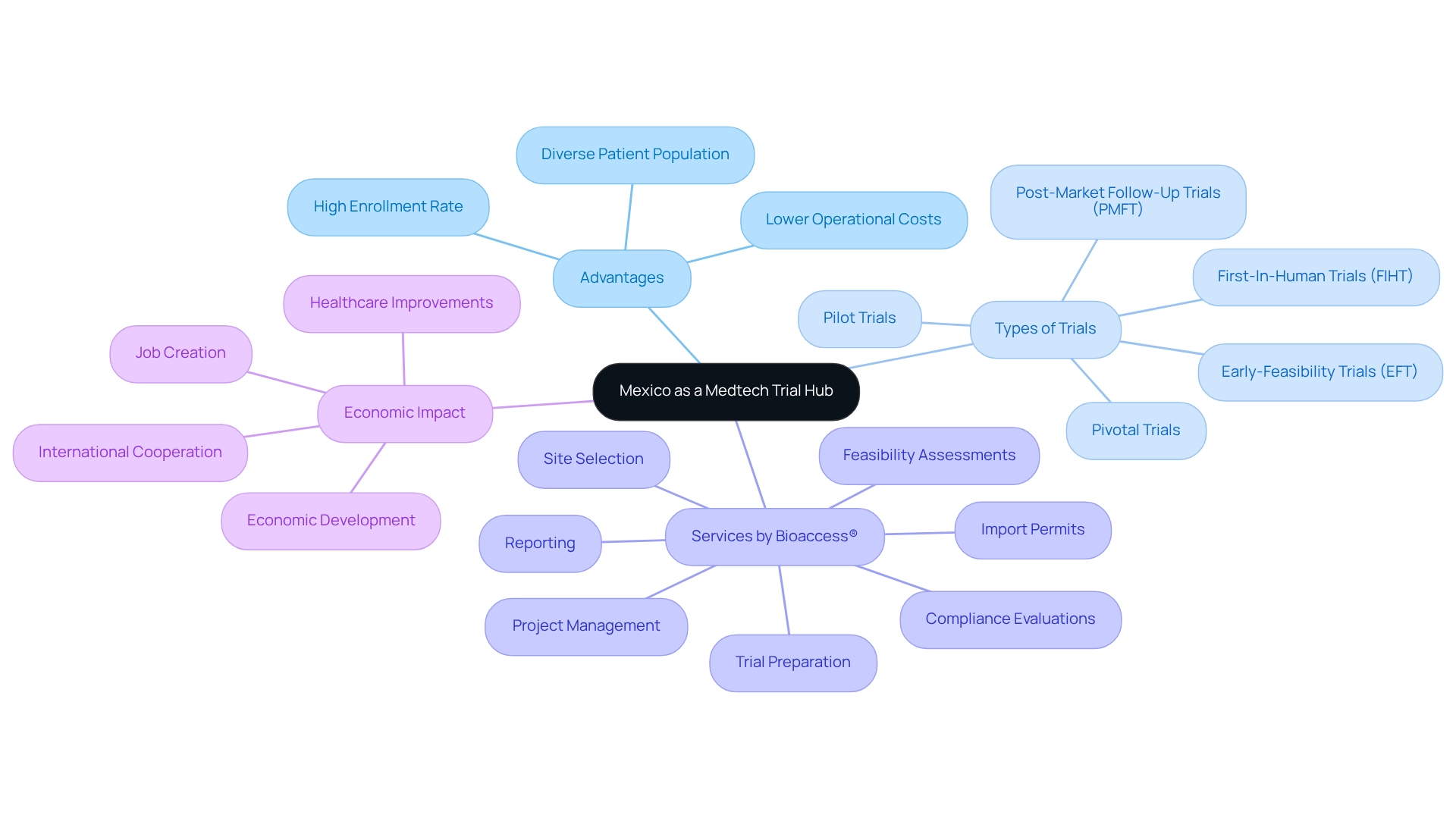
Leveraging Mexico's Advantages for Medtech Clinical Trials
Mexico serves as a prominent medtech trial hub, offering numerous advantages for medtech studies, including lower operational costs compared to the U.S. and Europe. The country provides access to a diverse patient population, which is crucial for studies requiring varied demographics. Additionally, Mexico boasts a high enrollment rate for medical trials within its healthcare system, significantly expediting the recruitment process.
The presence of experienced research organizations (CROs), such as bioaccess®, enhances the feasibility of conducting studies effectively. Bioaccess® specializes in comprehensive trial management services, which include:
- Early-Feasibility Trials (EFT)
- First-In-Human Trials (FIHT)
- Pilot Trials
- Pivotal Trials
- Post-Market Follow-Up Trials (PMFT)
Their services also encompass feasibility assessments, site selection, compliance evaluations, trial preparation, import permits, project management, and reporting. With over two decades of experience in the Medtech field, companies can leverage bioaccess's considerable expertise.
Moreover, Mexico's status as a medtech trial hub streamlines approval processes by aligning with international regulatory standards. The impact of medtech research transcends individual firms, contributing to local economies through job creation, economic development, healthcare improvements, and fostering international cooperation.
Types of Clinical Studies Suitable for Mexico's Medtech Environment
Mexico is exceptionally positioned for a range of medical investigations, including early feasibility assessments (EFS), pivotal examinations, and post-market medical follow-up evaluations (PMCF). With over 20 years of experience in the Medtech sector, bioaccess® leverages its robust healthcare framework to support first-in-human trials, establishing Mexico as a medtech trial hub and a prime location for innovative medtech firms seeking to conduct expedited medical device research. Furthermore, Mexico's diverse patient demographic facilitates research requiring specific demographic characteristics, thereby enhancing the credibility of results.
To improve recruitment and retention rates, companies must strategically assess local healthcare dynamics and leverage the expertise of bioaccess®, a leading contract research organization, in navigating the complexities of research management services, including:
- site selection
- compliance reviews
- setup
- project management
Additionally, the impact of medical studies on local economies—such as job creation and healthcare improvements—underscores the importance of these initiatives in fostering international collaboration and economic development.

Overcoming Challenges in Clinical Research Management in Mexico
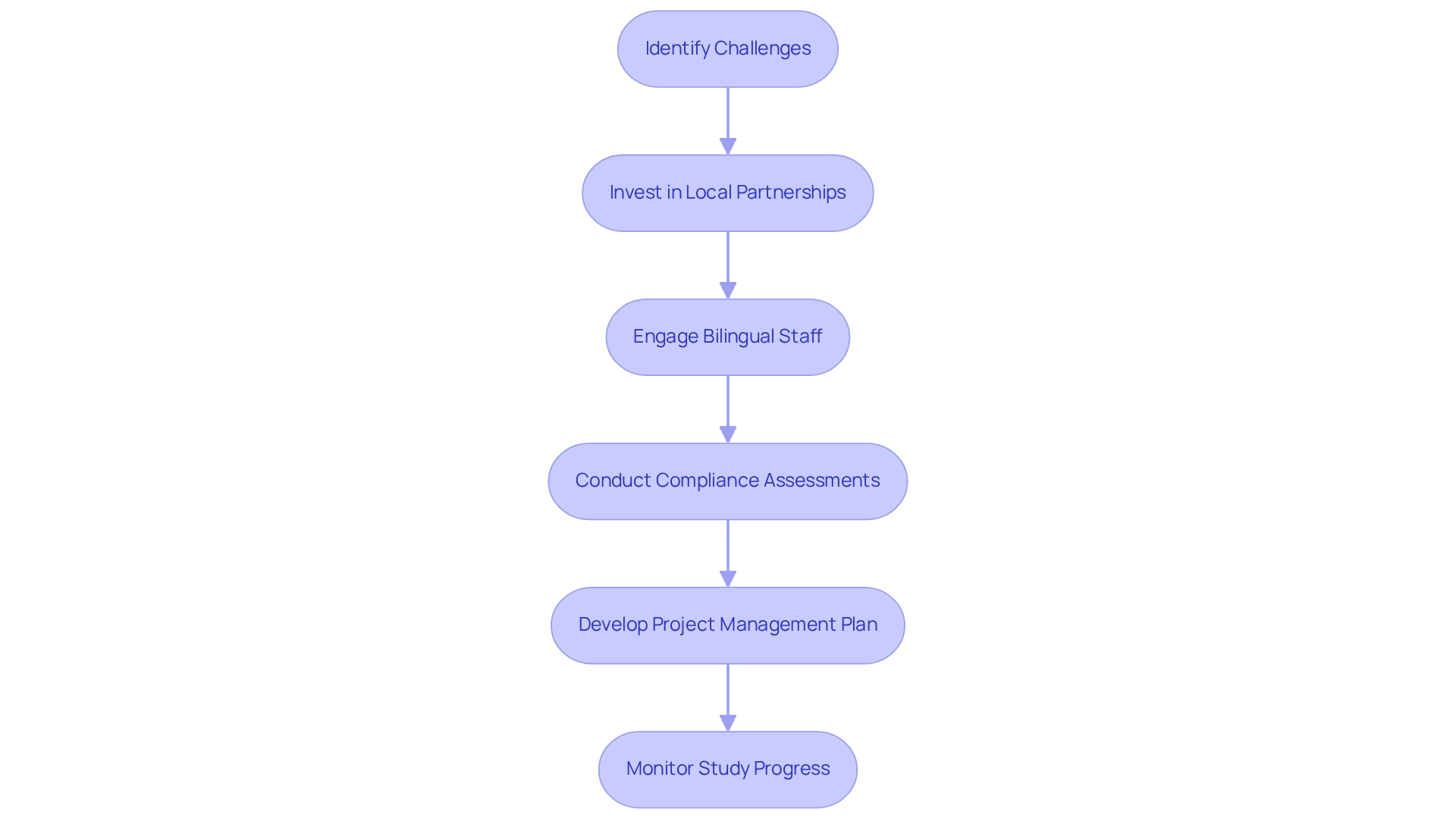
Despite its advantages, conducting medical studies in Mexico as a medtech trial hub presents significant obstacles, including regulatory delays and cultural differences that can affect patient recruitment. Companies frequently encounter challenges in navigating the local healthcare landscape, which varies considerably from region to region. Furthermore, language barriers can complicate communication with participants and local stakeholders.
To effectively address these challenges, companies should invest in local partnerships and engage bilingual staff to facilitate smoother interactions. At bioaccess, we provide comprehensive clinical study management services that directly tackle these issues. Our expertise includes viability assessments, site selection, and the careful selection of principal investigators (PIs) to ensure that studies are conducted efficiently.
We also conduct thorough compliance assessments of research documents to meet local regulatory standards, assisting in the setup and approval processes from ethics committees and health ministries. Developing a robust project management plan that addresses these potential issues is essential for maintaining timelines and ensuring compliance. Our dedicated project management and monitoring services keep studies on track, while our reporting mechanisms ensure transparency regarding study status, inventory, and adverse events.
By leveraging our expertise, companies can navigate the complexities of medical studies in Mexico as a medtech trial hub more effectively, ultimately contributing to job creation, economic development, and healthcare improvement in the region.
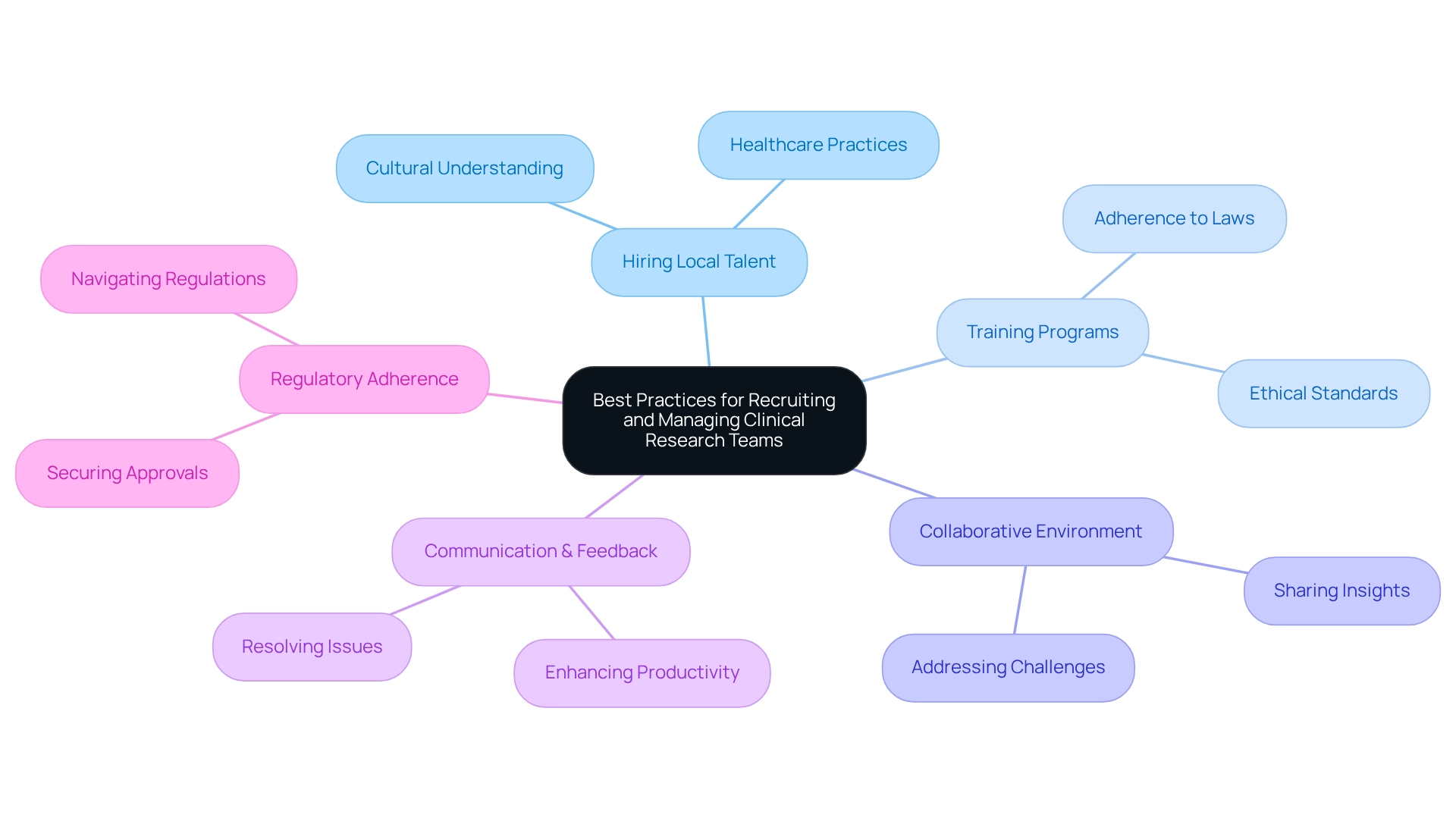
Best Practices for Recruiting and Managing Clinical Research Teams
Recruiting and managing a skilled clinical research team is paramount for the success of studies in Mexico. Companies must prioritize hiring local talent who possess a deep understanding of cultural nuances and healthcare practices. This local expertise is vital for navigating the complexities of the Latin American Medtech landscape. Furthermore, implementing training programs that emphasize adherence to laws and ethical standards is essential. Such programs ensure that team members are well-prepared to meet the specific requirements of each country, including securing the necessary approvals from ethics committees and health ministries.
Additionally, fostering a collaborative environment allows team members to share insights and challenges, significantly enhancing overall productivity. Consistent communication and feedback mechanisms are crucial for quickly resolving issues, ensuring that the process advances seamlessly. By leveraging local expertise and adhering to regulatory guidelines, bioaccess® facilitates a thorough process for advancing medical device evaluations. This approach effectively addresses typical challenges, including recruitment difficulties and regulatory obstacles, thereby reinforcing the importance of collaboration in clinical research initiatives.
Implementing Innovative Methodologies in Medtech Clinical Trials
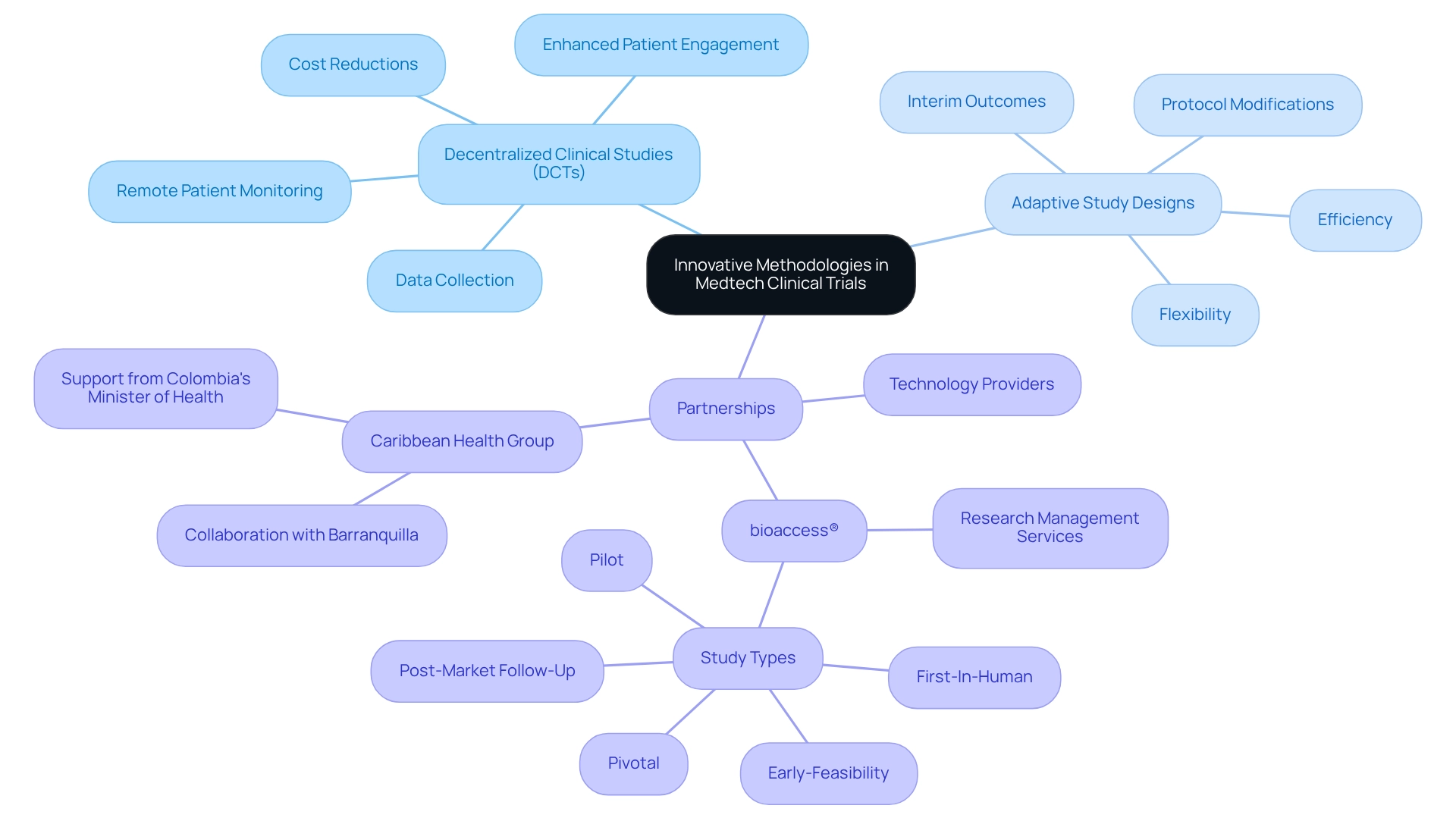
Innovative methodologies, notably decentralized clinical studies (DCTs) and adaptive study designs, are increasingly recognized as vital components of Mexico's emergence as a medtech trial hub. DCTs utilize technology to facilitate remote patient monitoring and data collection, leading to significant cost reductions and enhanced patient engagement. Meanwhile, adaptive designs allow for modifications to study protocols based on interim outcomes, thus improving both flexibility and efficiency.
Companies are encouraged to explore partnerships with technology providers to effectively implement these methodologies, ensuring alignment with regulatory requirements and patient needs. Organizations like bioaccess® are at the forefront, pioneering comprehensive research management services across Latin America. Their focus encompasses Early-Feasibility, First-In-Human, Pilot, Pivotal, and Post-Market Follow-Up studies. The collaboration with Caribbean Health Group aims to position Barranquilla as a premier site for medical studies, supported by Colombia's Minister of Health. This collaboration underscores the importance of international cooperation and innovation in advancing global health outcomes.
Building Strategic Partnerships for Successful Clinical Trials in Mexico
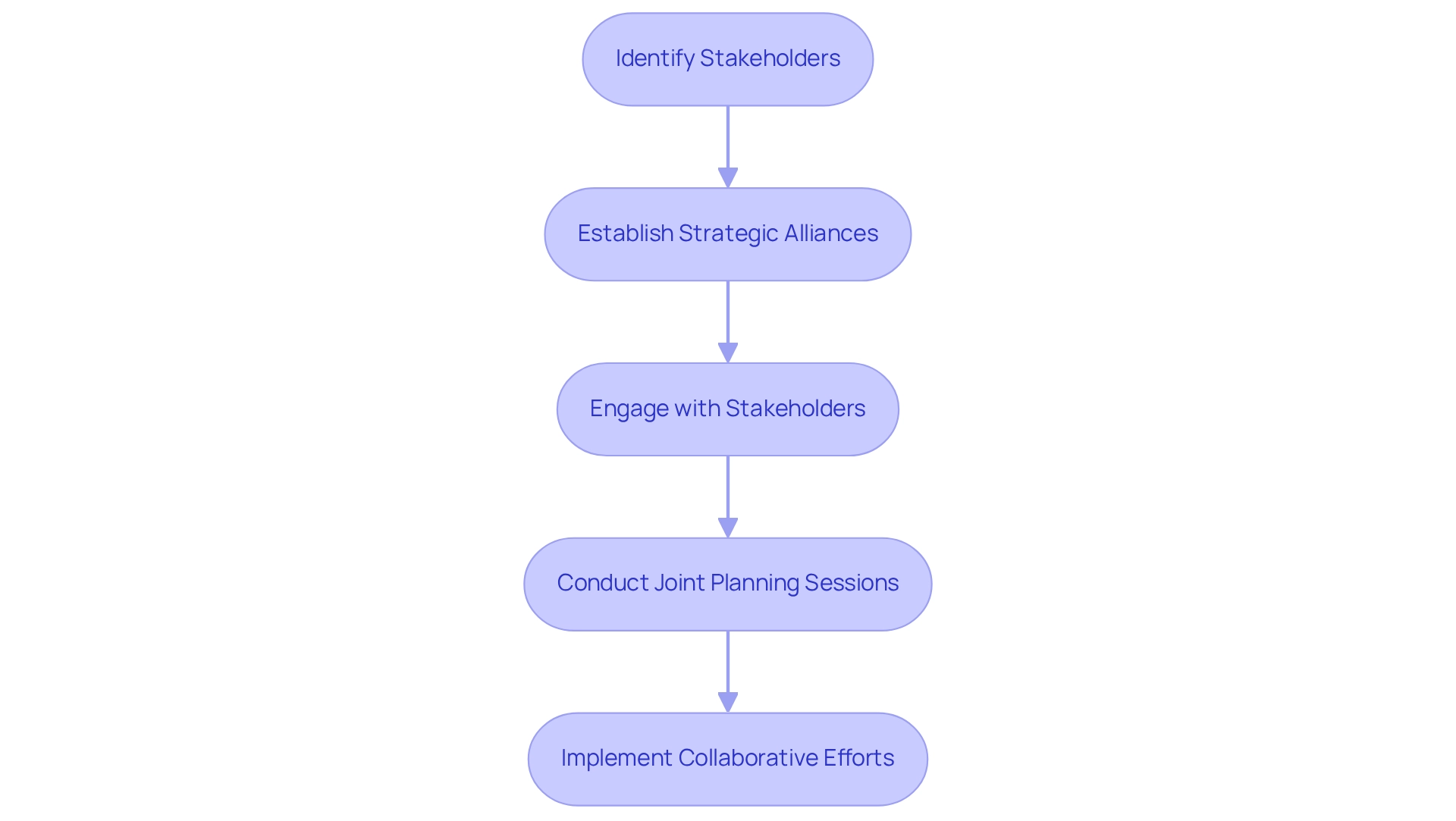
Establishing strategic alliances with local healthcare providers, compliance specialists, and patient advocacy organizations is essential for the success of research studies in Mexico. At bioaccess, our comprehensive trial management services encompass feasibility studies, site selection, compliance reviews, trial setup, import permits, project management, and reporting. These collaborations not only enhance patient recruitment efforts but also streamline regulatory processes, underscoring our commitment to effective clinical research.
Companies must actively engage with stakeholders to understand their needs and expectations, fostering a sense of shared purpose. Regular communication and joint planning sessions are vital for aligning objectives and ensuring that all parties are working towards common goals. By leveraging local knowledge and resources, companies can navigate the complexities of the Mexican clinical research landscape more effectively. This approach ultimately contributes to job creation, economic growth, and healthcare improvement in the region.
Conclusion
Mexico's healthcare landscape presents a wealth of opportunities for Medtech companies seeking to conduct clinical trials. The country's dual public-private system, coupled with a diverse patient population and an expanding healthcare infrastructure, establishes it as an attractive destination for innovative studies. Engaging local expertise, such as that provided by bioaccess®, is essential for navigating the regulatory complexities and ensuring compliance with COFEPRIS standards, thereby facilitating smoother trial processes.
The advantages of conducting clinical trials in Mexico extend beyond operational cost savings; they encompass a high enrollment rate and access to a varied demographic, critical for obtaining valid trial outcomes. By leveraging local partnerships and innovative methodologies, such as decentralized clinical trials, companies can enhance patient engagement and streamline their research efforts. Furthermore, the collaborative spirit fostered through strategic partnerships with local stakeholders not only aids in overcoming recruitment challenges but also contributes to the overall improvement of the healthcare system and local economies.
In conclusion, while challenges exist in conducting clinical trials in Mexico, the potential benefits far outweigh these hurdles. By prioritizing local knowledge, regulatory compliance, and innovative practices, Medtech companies can successfully navigate the complexities of the clinical research landscape. This approach not only accelerates the development of medical technologies but also fosters international collaboration, ultimately leading to improved healthcare outcomes and economic growth in the region. Embracing these strategies will ensure that Mexico continues to emerge as a vital hub for clinical trials in the Medtech sphere.
Frequently Asked Questions
What is the structure of Mexico's healthcare system?
Mexico's healthcare system is a robust blend of public and private sectors, encompassing over 4,000 hospitals, with a significant portion being public institutions. This dual framework caters to a diverse patient demographic and is crucial for medical research.
Why is Mexico considered a prominent medtech trial hub?
Mexico is considered a prominent medtech trial hub due to its substantial investments in healthcare infrastructure, a diverse population with varied health challenges, and geographical proximity to the United States, which facilitates smoother collaboration and logistics for international firms.
What services does bioaccess® provide for clinical trials?
Bioaccess® offers comprehensive trial management services, including approval processes, research site activation, participant recruitment, feasibility assessments, site selection, compliance evaluations, and project oversight.
What regulations must companies follow when conducting clinical trials in Mexico?
Companies must adhere to the regulations established by COFEPRIS (Federal Commission for Protection against Sanitary Risk), which require the submission of detailed protocols for approval, ethical considerations, and safety measures.
How can companies navigate the regulatory complexities in Mexico?
Companies can effectively navigate regulatory complexities by engaging local compliance experts, such as those from bioaccess®, who specialize in ensuring adherence to legal requirements, documentation, and reporting standards.
What specialized services does bioaccess® offer regarding study reporting?
Bioaccess® provides specialized services in reporting on study status and serious and non-serious adverse events, as well as expertise in conducting Early-Feasibility Studies and First-In-Human Studies.
What is the importance of understanding the General Health Law in Mexico?
A solid understanding of the General Health Law and its secondary regulations is vital for a seamless experience in conducting clinical trials, particularly in addressing challenges faced by medical device startups, including regulatory obstacles and recruitment issues.




