Overview
The regional advantages of Latin America for research success include its diverse cultures, strong economic growth, and cost-effectiveness compared to North America and Europe, which collectively enhance the region's appeal for clinical studies. The article illustrates this by discussing initiatives like partnerships for medical studies, significant reductions in recruitment times, and lower operational costs, all of which position Latin America as a promising landscape for innovative research and development in healthcare.
Introduction
Latin America is rapidly transforming into a dynamic hub for clinical research, offering a unique blend of cultural diversity, economic growth, and evolving healthcare landscapes. This region has become increasingly attractive for Medtech companies looking to navigate the complexities of clinical trials, as evidenced by initiatives aimed at addressing local challenges such as regulatory barriers and resource fragmentation.
With countries like Colombia, Brazil, and Argentina at the forefront, researchers are discovering that understanding local nuances is not just beneficial, but essential for the success of their studies. As the landscape continues to evolve, the region presents an unprecedented opportunity for innovation and collaboration, positioning itself as a key player in the global clinical research arena.
Unlocking Latin America's Unique Research Landscape
The regional advantages of Latin America for research are showcased by its lively tapestry of cultures, languages, and demographics, establishing it as an ideal environment for diverse studies. On March 29, 2019, during a meeting in Miami, FL, bioaccess™ and Caribbean Health Group announced their partnership to introduce medical studies in Barranquilla, aiming to position it as a premier location for such studies, a decision openly endorsed by Colombia's Minister of Health. This initiative is crucial as it addresses the challenges faced by Medtech companies in the region, such as regulatory hurdles, language barriers, and fragmented resources, which hinder seamless communication and collaboration with American trial clients.
The area is presently experiencing strong economic growth, leading to increased investments in healthcare and development sectors. Nations like Brazil, Mexico, and Argentina are swiftly positioning themselves as significant participants in the global research arena by utilizing the regional advantages of Latin America for research. This emergence is especially important as Brazil's continuous vaccine development initiatives are anticipated to strengthen its market position further, showcasing the regional advantages of Latin America for research studies.
For researchers, recognizing the regional advantages of Latin America for research, including cultural nuances and local healthcare frameworks, is critical for tailoring their methodologies effectively. This understanding not only enhances the relevance of studies but also guarantees their impact, particularly in addressing the diverse health challenges prevalent in the context of the regional advantages of Latin America for research. The diverse disease environment in South America, including increasing occurrences of cancer, heart disease, and diabetes—as emphasized in the case study on disease trends—along with a significant pediatric demographic, showcases the regional advantages of Latin America for research by enhancing access to specialized patient groups for research trials.
Furthermore, partnerships like that of GlobalCare Clinical Trials with bioaccess™ have achieved over a 50% reduction in recruitment times and 95% retention rates, illustrating the regional advantages of Latin America for research in providing effective solutions to Medtech challenges. As Anil Kumar P., a Research Manager in Healthcare, states, 'Whether collaborating with small start-ups or large enterprises, I consistently provide high-quality analysis that supports informed decision-making and drives impactful results in the healthcare industry.' This viewpoint strengthens the idea that informed and culturally aware inquiry strategies, leveraging the regional advantages of Latin America for research, are essential for achieving success in the healthcare sector.
Furthermore, with approximately 65% of Horizon Databook's revenue originating from competitive and market intelligence teams, the financial support for studies in the region underscores the regional advantages of Latin America for research, further reinforcing its potential for expansion.
The Cost-Effectiveness of Clinical Research in Latin America
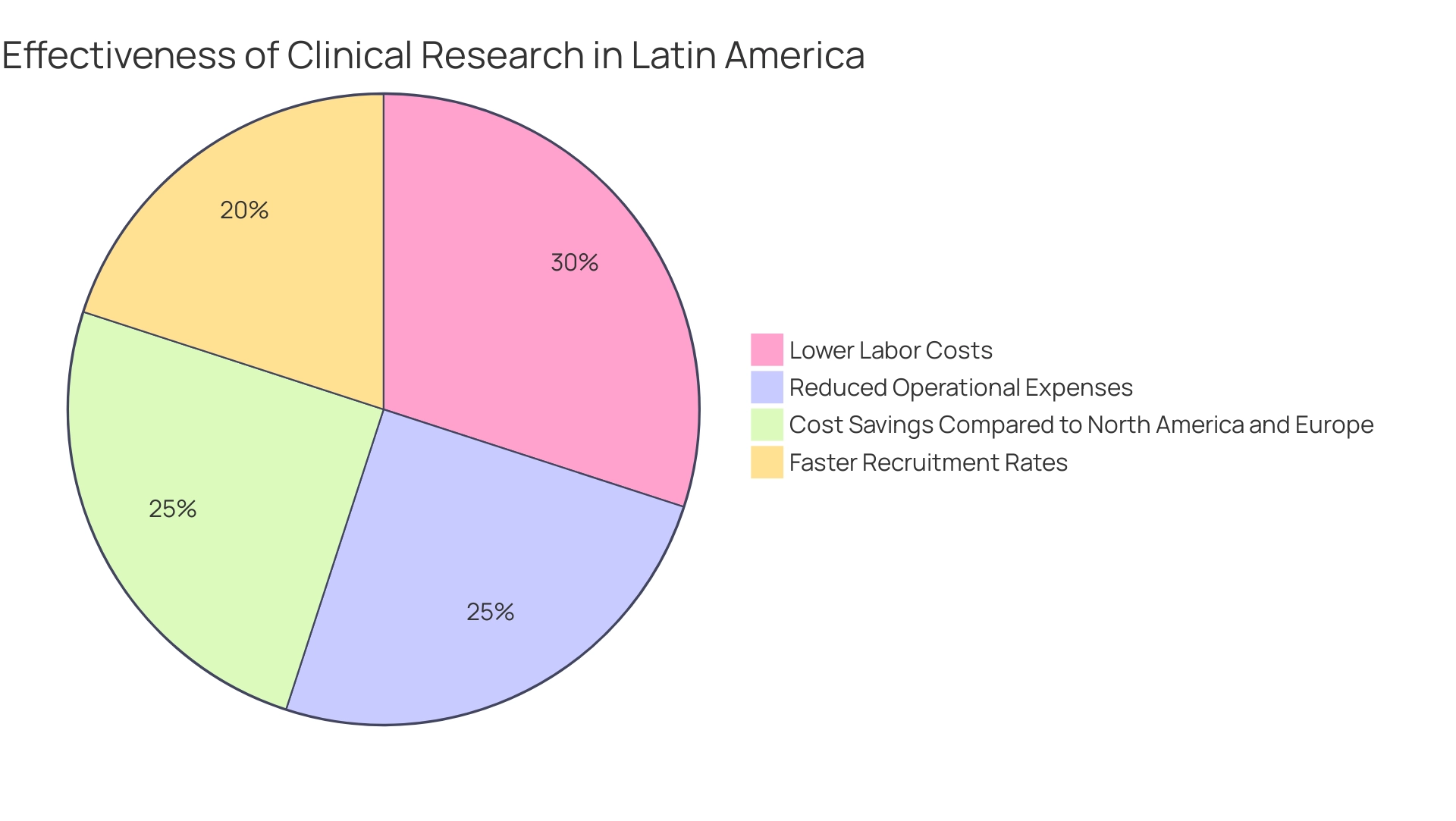
Carrying out medical research in South America takes advantage of the regional advantages of Latin America for research, providing substantial cost benefits in comparison to North America and Europe. A recent analysis indicates that site fees in the U.S. can be approximately 30–50% higher than those in Eastern Europe and Asia, showcasing the regional advantages of Latin America for research as an attractive alternative. The regional advantages of Latin America for research, including lower labor costs and reduced operational expenses, enable companies to allocate resources more effectively.
Furthermore, the diverse patient populations present in countries like Peru, Colombia, and Chile exemplify the regional advantages of Latin America for research by facilitating quicker recruitment and retention rates. With over 20 years of experience in Medtech, bioaccess® specializes in comprehensive clinical study management services, including:
- Early-Feasibility Studies
- First-In-Human Studies
- Post-Market Follow-Up Studies
This ensures a seamless process from setup to reporting. Our tailored strategy tackles linguistic, cultural, and socio-economic obstacles to informed consent, which can influence the success of studies in these areas.
This understanding is essential for companies seeking to navigate the regional advantages of Latin America for research. The strategic application of tools and expert guidance offered by bioaccess® can improve operational efficiency, particularly when assessing research costs and optimizing budgets. Furthermore, insights from the case study titled 'Next Tier Opportunities in Clinical Trials' reveal that while the region in South America presents viable opportunities, operational readiness and patient availability must be carefully assessed.
As emphasized by Patricio Ledesma, Head of Clinical Operations at bioaccess®, 'My dedication is in supporting biotech leaders with the planning and implementation of research studies across diverse areas, including South America, where operational efficiency can be optimized.' This strategic focus not only streamlines testing processes but also positions companies to bring innovative products to market more swiftly, contributing to local economies through job creation and healthcare improvements.
Navigating the Regulatory Landscape for Clinical Trials
Navigating the regulatory environment for research studies in Latin America highlights the regional advantages of Latin America for research, requiring a nuanced understanding of the distinct regulations that differ from nation to nation. Each country sets its own criteria, necessitating diligent adherence to local regulations overseeing research studies to ensure ethical and successful outcomes. For instance, Colombia, recognized as a Level 4 health authority by PAHO/WHO, has established a robust framework through INVIMA, which oversees medical device regulation and classification.
Since 2000, all examined research sites in Peru have met regulations, demonstrating a significant compliance achievement in the region. Engaging with local regulatory bodies early in the planning stages can significantly facilitate smoother approval processes and mitigate potential delays. Recent discussions surrounding the pharmaceutical sector in Mexico and Brazil emphasize the necessity for ongoing awareness of regulatory changes, which can affect execution of studies.
Moreover, establishing robust connections with local stakeholders—including ethics committees and regulatory bodies—is crucial in improving the success of research studies. The challenges posed by language barriers and cultural differences underscore the critical need for precise translation of regulatory and patient-related materials, as emphasized in case studies, ensuring informed consent and ethical treatment are upheld. As Julio G. Martinez-Clark, CEO of bioaccess, notes, 'Colombia has recognized these benefits and has an ambitious science, technology, and innovation plan for 2022–2031 to become a knowledge economy.'
To assist researchers, bioaccess provides extensive services that include:
- Feasibility studies
- Project setup
- Project management
- Compliance assessments
Ensuring that all elements of the research process are handled efficiently. Considering these factors, creating a thorough strategy that emphasizes local involvement and adherence is essential for researchers seeking to navigate the intricate terrain of trials in America, especially by utilizing the regional advantages of Latin America for research. By leveraging the expertise of CROss like bioaccess, researchers can effectively overcome the challenges and seize the opportunities present in this dynamic region.
Fostering Regional Collaboration for Enhanced Research Opportunities
Regional cooperation plays a vital role in utilizing the regional advantages of Latin America for research in health studies. By establishing partnerships among nations, researchers can effectively share resources, expertise, and best practices. Initiatives such as collaborative studies and cross-border experiments not only enhance the abilities of individual nations but also address widespread health issues collectively.
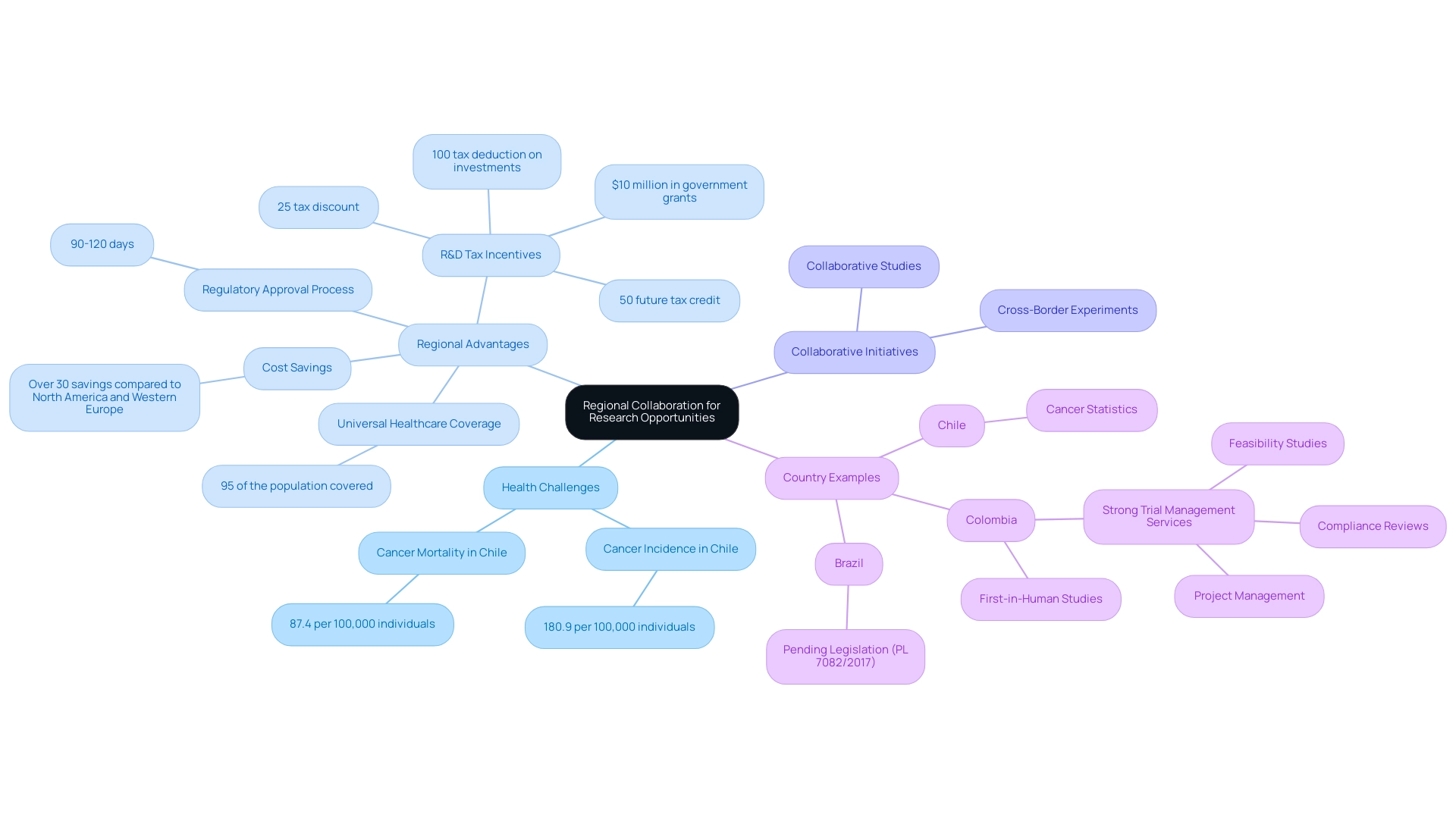
For instance, in Chile, the cancer incidence rate stands at 180.9 per 100,000 individuals, with a mortality rate of 87.4 per 100,000 individuals, highlighting a significant health challenge that can be addressed through collaborative efforts. In this landscape, Colombia emerges as a premier destination for first-in-human studies, highlighting the regional advantages of Latin America for research, which include significant benefits like:
- Over 30% savings in research expenses compared to North America and Western Europe
- A rapid regulatory approval process of 90-120 days
- A healthcare system recognized as one of the best worldwide
Collaborating with local institutions and universities in Colombia not only provides invaluable insights and access to a broader talent pool but also enhances patient recruitment, with 95% of the population covered by universal healthcare.
The patient recruitment process is streamlined due to this extensive coverage, allowing for quicker access to diverse patient populations. Furthermore, the regional advantages of Latin America for research are highlighted by Colombia's significant R&D tax and financial incentives, which include:
- A 100% tax deduction on investments in science, technology, and innovation projects
- A 25% tax discount
- A 50% future tax credit
- Approximately $10 million in government grants
This makes it an attractive option for medical device companies. Pending legislation in Brazil (PL 7082/2017) could greatly enhance the regulatory environment for medical studies in the area, potentially influencing the dynamics of medical experiments throughout South America.
This cooperative method establishes South America as a developing center for research studies, showcasing the regional advantages of Latin America for research, particularly as the area aims to enhance its scientific output in sectors like oncology. While Brazil currently leads in scientific output, Colombia’s strong trial management services—including feasibility studies, compliance reviews, and project management—highlight significant opportunities for growth in cancer studies and beyond.
Harnessing Local Talent for Research Excellence
Attracting and efficiently overseeing local talent is essential for attaining excellence in clinical studies, leveraging the regional advantages of Latin America for research. Project directors should prioritize building strong partnerships with local universities and academic institutions to leverage the rich talent pool available. By implementing comprehensive training and development programs, organizations can significantly enhance the skills of local investigators, fostering a culture of continuous improvement that benefits the entire academic landscape.
Furthermore, creating an inclusive and collaborative work environment not only encourages innovation but also boosts engagement among team members. These components are essential for the success of medical studies, as demonstrated by FOMAT Medical Research, which emphasizes that, in South America, patient retention is outstanding. Physicians and patients in these areas have strong bonds that result in high rates of patient compliance and study retention.
Significantly, the involvement in medical studies in Latin America rose from 6.04% in 2015 to 8.54% in 2021, which highlights the regional advantages of Latin America for research in global medical investigations. Julio Martinez-Clark, CEO of bioaccess, has been a prominent advocate for operationalizing research trials in this region, highlighting the regional advantages of Latin America for research by leveraging his extensive experience in supporting over 100 Medtech companies and fostering collaborations such as the one with Caribbean Health Group to position Barranquilla as a leading destination for research trials. This partnership has received backing from Colombia's Minister of Health, indicating a wider dedication to improving the medical study environment.
However, it is essential to recognize that Latin American patients often contribute to studies that primarily benefit populations in wealthier regions, raising important ethical considerations. This dynamic is further examined in the case study titled 'Social Injustice in Clinical Trials,' which addresses the ethical and epistemic costs of trials and the necessity to tackle the misalignments of interests impacting traditionally underrepresented groups. By investing in local talent and nurturing these relationships, organizations can ensure more effective and ethically sound research outcomes.
Innovating in Medtech: Latin America's Role as a Testing Ground
Latin America has emerged as a critical testing ground for Medtech innovations, highlighting the regional advantages of Latin America for research through its diverse patient populations and a variety of healthcare challenges. This diversity provides companies with the opportunity to pilot new technologies and gather a wealth of data that showcases the regional advantages of Latin America for research, which is instrumental for product development. Notably, the regional advantages of Latin America for research are exemplified by Colombia and Paraguay being leading countries for Early Feasibility Studies (EFS) and First-in-Human (FIH) studies, which enhances the region's competitive landscape.
Collaborating with local healthcare providers and institutions enriches the feedback loop and helps refine solutions that leverage the regional advantages of Latin America for research. Paraguay exemplifies this potential; despite concerns around corruption and regulatory oversight, it has established a solid framework for medical research, making it a preferred location for cardiovascular medical technology experiments. Our comprehensive clinical trial management services at bioaccess® include:
- Feasibility studies
- Site selection
- Compliance reviews
- Trial setup
- Import permits
- Project management
This ensures a streamlined process for your studies.
As highlighted by Guillaume Corpart, CEO and founder of Global Health Intelligence, 'We cover roughly 90% of all the hospitals in South America within our database, with more than 140 data points for each.' This extensive coverage highlights the regional advantages of Latin America for research and development initiatives in Medtech. Moreover, with Paraguay's urbanization rate at 61.6%, in contrast to the regional average of 84.6%, understanding these demographic nuances is essential for successful experiments.
Current pilot programs are paving the way for innovation and enhanced research success rates, showcasing the regional advantages of Latin America for research and contributing significantly to job creation, economic growth, and healthcare advancements. Additionally, understanding the regulatory landscape is vital; INVIMA, as the national regulatory authority, plays a crucial role in overseeing medical device trials in Colombia, which exemplifies the regional advantages of Latin America for research by ensuring compliance and safety standards are met. This regulatory support further facilitates the successful execution of clinical studies, reinforcing the positive impact of Medtech clinical studies on local economies.
Conclusion
Latin America is positioning itself as a pivotal player in the global clinical research landscape, characterized by its cultural diversity, economic potential, and a growing commitment to healthcare innovation. The region’s unique attributes, including lower operational costs and diverse patient demographics, are attracting Medtech companies and researchers alike. Initiatives aimed at overcoming regulatory challenges and fostering regional collaboration further enhance Latin America’s appeal, allowing for a more streamlined approach to clinical trials.
The importance of understanding local nuances cannot be overstated. By leveraging regional expertise and engaging with local institutions, researchers can optimize their methodologies, thus ensuring the relevance and impact of their studies. This approach not only addresses pressing health challenges but also cultivates a more ethical framework for research, benefiting both local populations and global health outcomes.
As countries like Colombia and Brazil continue to enhance their regulatory frameworks and invest in research capabilities, the potential for innovation and collaboration grows. With a strong focus on harnessing local talent and fostering partnerships, Latin America is well on its way to becoming a leading hub for clinical research, offering significant opportunities for Medtech advancements that can drive economic growth and improve healthcare outcomes across the region. The time is ripe for stakeholders to embrace this transformation and capitalize on the myriad possibilities that Latin America has to offer in the realm of clinical research.
Frequently Asked Questions
What are the regional advantages of Latin America for research?
Latin America offers a vibrant cultural tapestry, diverse languages, and demographics, making it an ideal environment for various studies. The region is experiencing economic growth, leading to increased investments in healthcare and development, which enhances its research capabilities.
What recent partnership was announced to promote medical studies in Barranquilla?
On March 29, 2019, bioaccess™ and Caribbean Health Group announced a partnership to introduce medical studies in Barranquilla, with the aim of establishing it as a premier location for such research. This initiative is supported by Colombia's Minister of Health.
What challenges do Medtech companies face in Latin America?
Medtech companies face challenges such as regulatory hurdles, language barriers, and fragmented resources, which can hinder communication and collaboration with American trial clients.
How are countries like Brazil, Mexico, and Argentina positioning themselves in the global research arena?
These countries are leveraging their regional advantages to become significant participants in global research by investing in healthcare and utilizing their diverse patient populations and cultural nuances.
Why is understanding local healthcare frameworks important for researchers?
Recognizing local healthcare frameworks and cultural nuances is crucial for researchers to tailor their methodologies effectively, enhancing the relevance and impact of their studies, especially in addressing diverse health challenges.
What diseases are prevalent in South America that highlight the need for research?
There is an increasing occurrence of diseases such as cancer, heart disease, and diabetes, along with a significant pediatric demographic, which presents opportunities for research trials.
How have partnerships like GlobalCare Clinical Trials with bioaccess™ improved research efficiency?
Such partnerships have achieved over a 50% reduction in recruitment times and maintained 95% retention rates, demonstrating effective solutions to Medtech challenges in the region.
What cost advantages does Latin America offer for medical research compared to North America and Europe?
Conducting medical research in Latin America offers substantial cost benefits, with site fees being approximately 30-50% lower than those in the U.S. and Europe, along with lower labor costs and reduced operational expenses.
What services does bioaccess® provide for clinical study management?
Bioaccess® specializes in comprehensive clinical study management services, including early-feasibility studies, first-in-human studies, and post-market follow-up studies.
What is the importance of operational readiness in Latin American research?
While the region presents viable research opportunities, careful assessment of operational readiness and patient availability is essential for successful study execution.
How does bioaccess® support biotech leaders in research planning and implementation?
Bioaccess® focuses on optimizing operational efficiency and streamlining testing processes, which helps biotech leaders bring innovative products to market more swiftly and contributes to local economies.

