Introduction
Panama is positioning itself as a pivotal player in the clinical research landscape, particularly within the medical devices sector. With a strategic geographic location and a commitment to enhancing healthcare standards, the country has become an attractive destination for clinical trials. The medical device market in Panama is on an upward trajectory, projected to grow at a compound annual growth rate of 10% over the next five years.
This growth is bolstered by favorable regulatory frameworks that simplify approval processes, thereby inviting both local and international stakeholders to engage in research initiatives. As clinical trials flourish, they not only advance medical technology but also enhance Panama's reputation as a center of clinical excellence in Latin America. This article delves into the dynamics of clinical research in Panama, exploring the registration processes, stakeholder engagement strategies, and the ethical standards essential for successful trials in this burgeoning market.
Overview of Clinical Research Landscape for Medical Devices in Panama
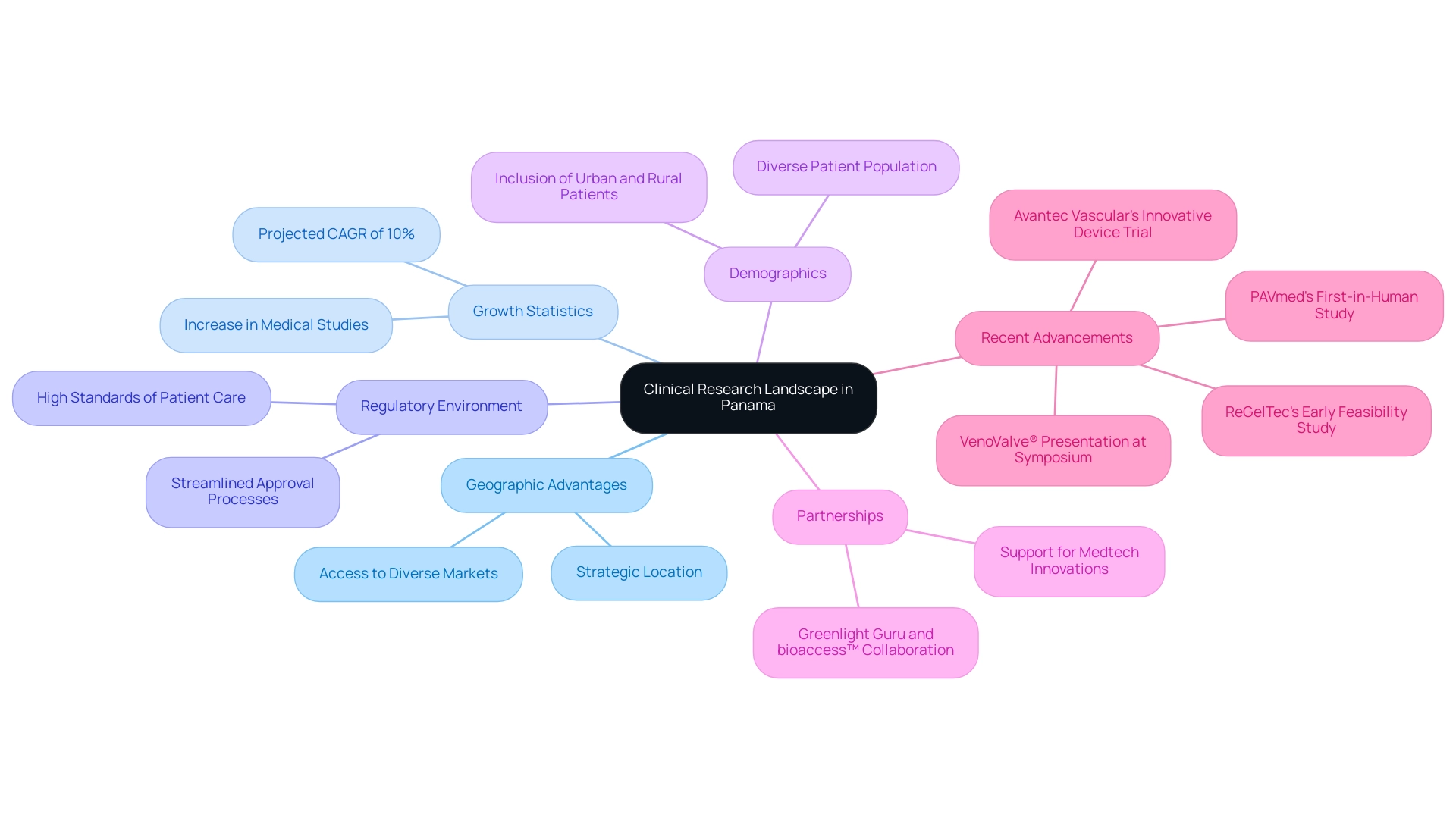
Panama is quickly becoming a key center for research in Latin America, particularly in the field of medical devices. The nation's strategic geographic location and steadfast dedication to improving healthcare standards have greatly drawn many research studies. Recent statistics indicate that the medical device market in Panama is projected to grow at a compound annual growth rate (CAGR) of 10% over the next five years, reflecting its increasing importance in the region. Favorable regulations implemented by the Panamanian government streamline the approval processes for medical devices, thus making Panama an appealing destination for both sponsors and researchers.
The healthcare system in Panama is well-regulated, ensuring high standards of patient care and safety. Furthermore, the diverse patient population, encompassing multiple ethnic groups and socioeconomic backgrounds, offers a rich and varied demographic for conducting thorough research studies. For example, the inclusion of patients from both urban and rural settings ensures that medical devices are tested for efficacy across different environments, which is crucial for their success in broader markets.
As stated by Dr. Maria Gonzalez, a prominent investigator in the area and an important contributor to various studies, "The growth of experimental studies in Panama not only supports our local healthcare system but also establishes us as a competitive participant in the worldwide scientific landscape." This is further illustrated by recent partnerships, such as the one between Greenlight Guru and bioaccess™, aimed at accelerating Medtech innovations and research in the region. Noteworthy advancements include:
- PAVmed's first-in-human study in Colombia
- The presentation of one-year data on the VenoValve® by Dr. Jorge Hernando Ulloa at the Charing Cross International Symposium, highlighting significant developments in vascular medicine
- ReGelTec's Early Feasibility Study on HYDRAFIL™ for addressing chronic low back pain in Colombia
- Avantec Vascular's first-in-human trial of an innovative vascular device
These showcase the dynamic potential of Latin America for research. Recent data shows a significant rise in the quantity of medical studies carried out in Panama, fueled by both local initiatives and global interest. These trials not only contribute to the advancement of medical technologies but also enhance the global reputation of Panama as a hub for healthcare excellence. With the increasing demand for innovative medical devices, stakeholders can expect a rise in opportunities to perform research in this vibrant region.
Navigating the Medical Devices Registration Process in Panama
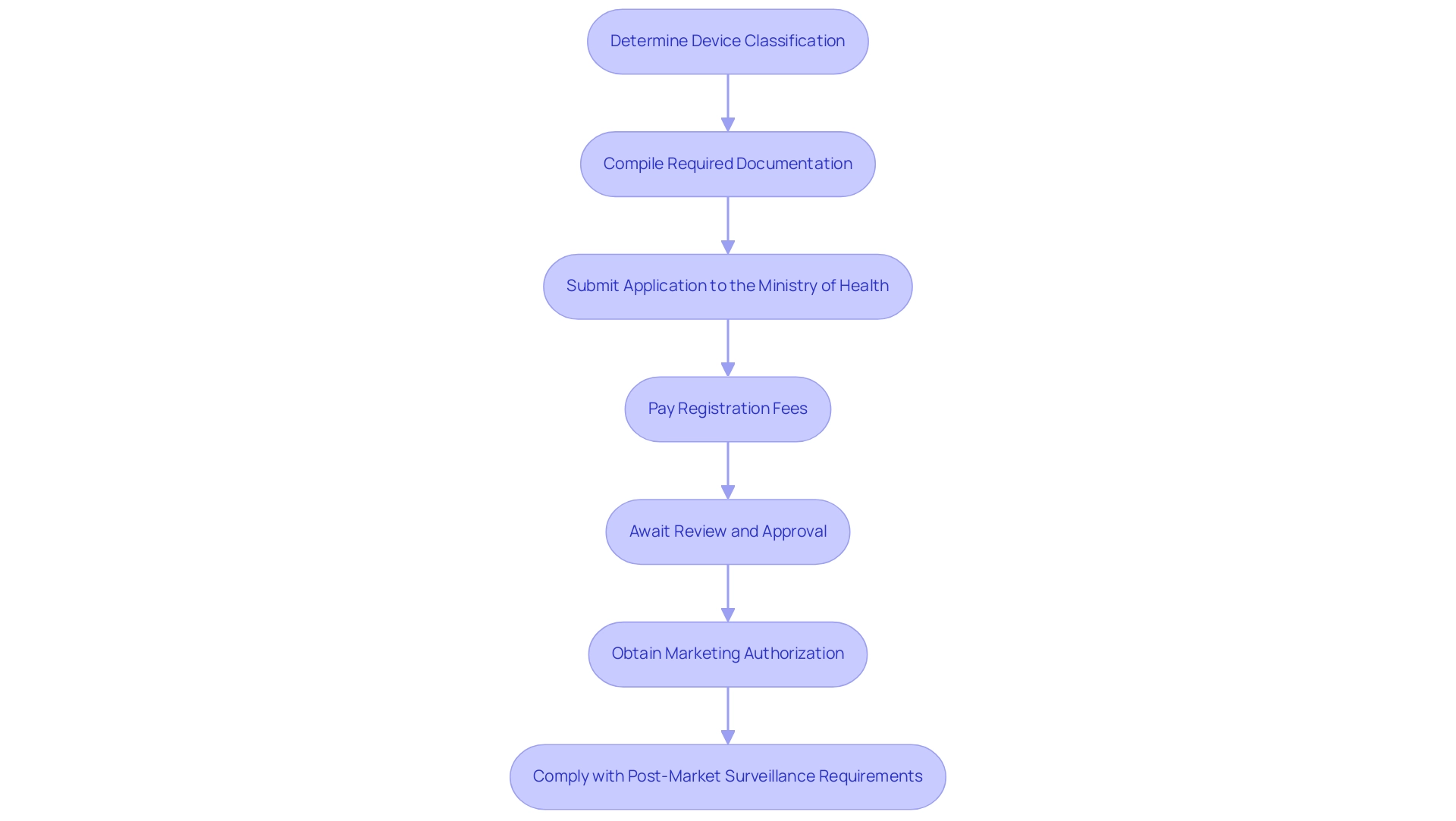
To successfully register medical devices in Panama, follow these steps:
-
Determine Device Classification: Identify the classification of your medical device, which will dictate the registration requirements. Devices are classified into three categories (Class I, II, and III) based on risk levels.
-
Compile Required Documentation: Prepare essential documents, including the device's technical file, clinical evaluation reports, and quality management system certifications. Ensure all documents adhere to regional regulations. bioaccess® can assist in reviewing these documents to ensure they meet all requirements.
-
Submit Application to the Ministry of Health: File your registration application with the Panamanian Ministry of Health (MINSA), including all necessary documentation. It is advisable to work with a local representative, such as bioaccess®, which specializes in navigating the regulatory landscape, to facilitate communication and ensure compliance.
-
Pay Registration Fees: Upon submission, pay the applicable registration fees. Fees vary depending on the device classification and complexity of the review process. bioaccess® can provide insights into the fee structure and help manage this process.
-
Await Review and Approval: The review process can take several weeks to months, depending on the device's classification and completeness of the submitted documentation. Be prepared to respond to any queries from regulatory authorities promptly. Utilizing bioaccess®'s knowledge in research management can improve your response strategy, ensuring prompt communication with regulators.
-
Obtain Marketing Authorization: Once approved, you will receive marketing authorization, allowing you to conduct trials and promote your device within Panama. bioaccess® can guide you through the next steps for launching your product effectively.
-
Comply with Post-Market Surveillance Requirements: After the device is on the market, ensure adherence to ongoing monitoring and reporting obligations as mandated by regional regulations. The expertise of regulatory professionals like Katherine Ruiz at bioaccess® can provide valuable guidance in maintaining compliance post-approval. bioaccess® offers tailored solutions for post-market surveillance, helping you to implement effective monitoring strategies and fulfill reporting obligations.
Engaging with Local Stakeholders
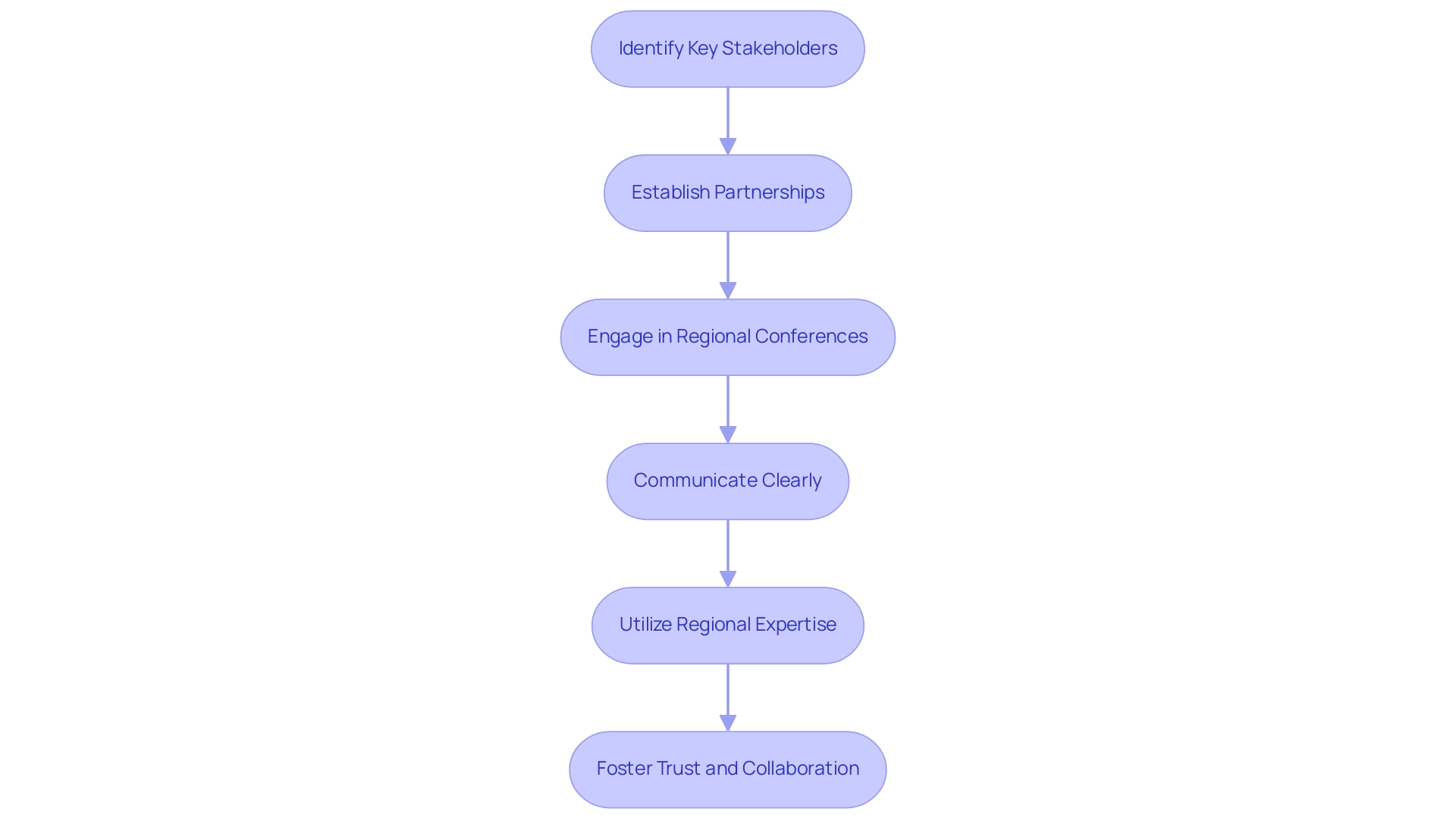
Effectively engaging local stakeholders in Panama's clinical research landscape requires a multifaceted approach:
-
Identify Key Stakeholders: Begin by identifying relevant stakeholders such as healthcare institutions, regulatory authorities, and patient advocacy groups. Understanding their roles and interests is crucial for enhancing collaboration, ensuring that the diverse needs of all involved parties are considered and fostering a more inclusive research environment.
-
Establish Partnerships: Form strategic alliances with local hospitals and clinics experienced in conducting clinical studies. Successful partnerships with establishments such as Clinica Hospital San Fernando have demonstrated advantageous in offering access to varied patient demographics and facilitating logistics for studies. Such partnerships are instrumental in overcoming logistical challenges and ensuring seamless operations. Based on a recent survey by the Panamanian Ministry of Health, 75% of trial sites reported enhanced patient recruitment through community partnerships.
-
Engage in Regional Conferences: Attend medical and scientific conferences in Panama to network with regional professionals and stakeholders. These events provide excellent opportunities to build connections and gain insights into the regional clinical research landscape. Engaging in these forums can also provide valuable exposure to recent advancements and regulatory updates. As mentioned by Dr. Ana Ruiz, a prominent researcher in Panama, "Networking at community conferences has been pivotal in establishing trust with stakeholders."
-
Communicate Clearly: Maintain open and transparent lines of communication with regional partners. Clearly express your study objectives and actively seek their feedback to align your aims with community needs. This collaborative approach fosters mutual understanding and enhances the overall efficacy of the research process.
-
Utilize Regional Expertise: Engage regional experts who possess a deep understanding of the regulatory environment and cultural context. Their insights are invaluable in navigating potential challenges and optimizing study designs. For instance, regional experts can provide guidance on regulatory compliance, which is essential for the successful execution of clinical trials. A case study involving a partnership with a nearby university highlighted that expert involvement reduced the average approval timeline by 30%.
-
Foster Trust and Collaboration: Building trust with community stakeholders is paramount. Demonstrating transparency and mutual respect can lead to more robust collaborative relationships. A recent study on community engagement highlighted that fostering trust is a critical component of successful public health initiatives. By showing a commitment to ethical practices and community well-being, you can strengthen stakeholder engagement and enhance trial outcomes. The Director of an advocacy group stated, "Trust is the foundation of our partnerships; without it, our efforts would be futile."
By applying these strategies, you can effectively involve local stakeholders in Panama, ultimately aiding the success and integrity of medical initiatives. Furthermore, integrating comprehensive trial management services—such as feasibility studies, site selection, compliance reviews, trial setup, import permits, project management, and reporting—will significantly influence job creation, economic growth, and healthcare enhancement, while fostering international collaboration and advancing global health improvement through innovation in Medtech.
Ensuring Compliance with Ethical Standards
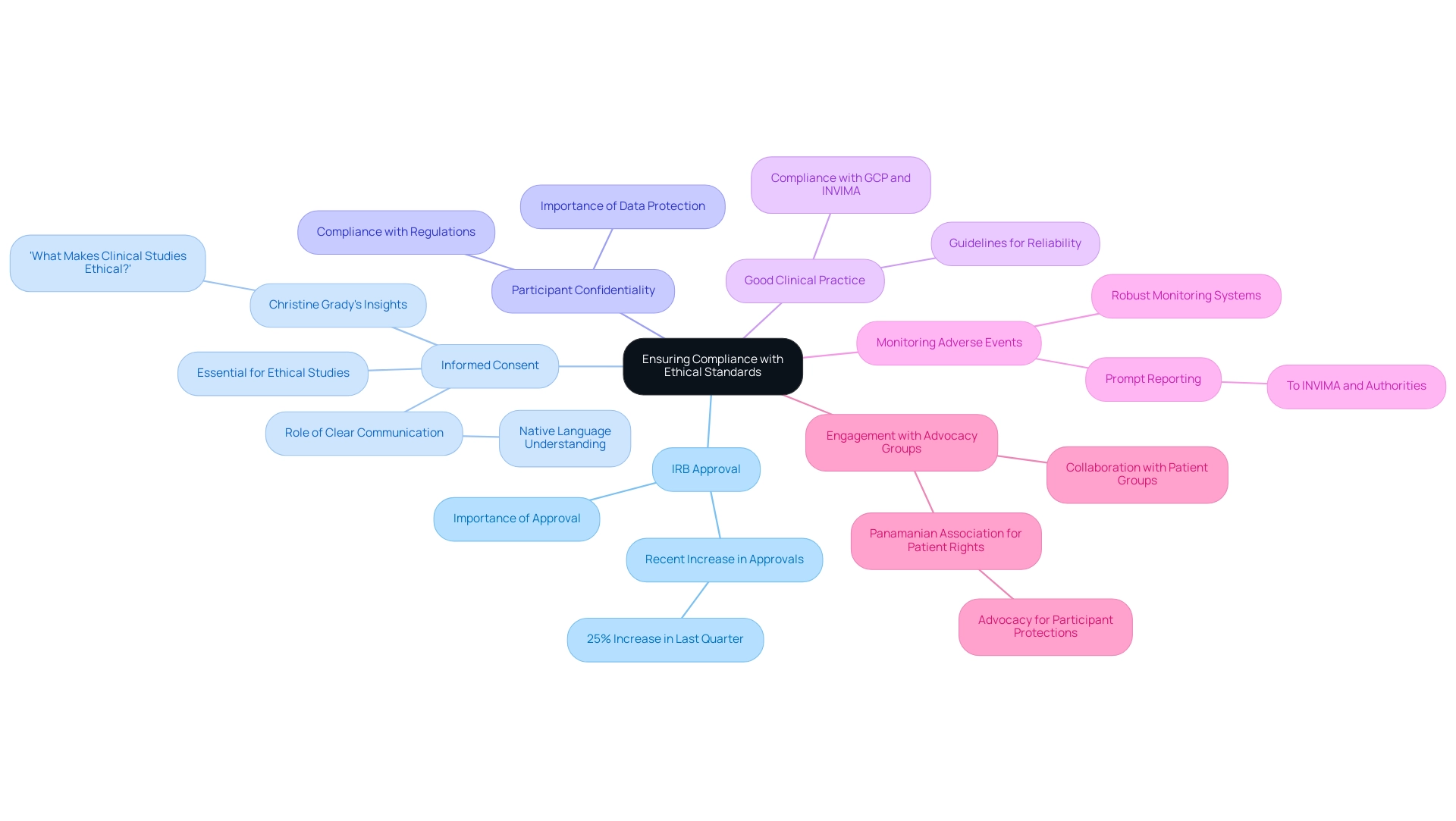
To ensure compliance with ethical standards in clinical research in Panama, follow these essential guidelines:
-
Obtain Institutional Review Board (IRB) Approval: Securing approval from a local IRB is a crucial first step. This approval confirms that the study adheres to ethical standards and safeguards the rights of participants. Recent statistics indicate that in the last quarter, IRB approvals for research trials in Panama increased by 25%, reflecting the thorough review processes in place.
-
Informed Consent: Informed consent is essential to ethical medical studies. All participants must be fully aware of the study's purpose, procedures, risks, and benefits before agreeing to take part. Recent case studies illustrate the critical role of informed consent, showing that clear communication in a participant's native language significantly improves understanding and compliance. Christine Grady, PhD, notes that informed consent is integral to the ethical framework of clinical studies, emphasizing that, 'What Makes Clinical Studies Ethical?' highlights the necessity of informed consent.
-
Protect Participant Confidentiality: Maintaining the confidentiality of participant information is paramount. Compliance with local regulations and international guidelines ensures that personal data is safeguarded against unauthorized access or disclosure.
-
Adhere to Good Clinical Practice (GCP): Good Clinical Practice guidelines must be followed throughout the study process to guarantee the reliability and integrity of data. This adherence helps maintain high ethical standards and enhances the credibility of the research findings. Our service capabilities also encompass set-up, start-up, and approval processes, ensuring compliance with GCP and INVIMA regulations.
-
Monitor and Report Adverse Events: Establish a robust system for monitoring adverse events. Prompt reporting to the relevant authorities, including INVIMA, is essential for participant safety and ethical compliance. Recent news emphasizes how domestic and international human rights organizations function without governmental limitations, underscoring the significance of openness and responsibility in research studies.
-
Engage with Participant Advocacy Groups: Collaboration with patient advocacy groups is vital for addressing participant concerns. These groups offer important insights and assist in making certain that participants' voices are acknowledged throughout the study process. For instance, the Panamanian Association for Patient Rights has successfully advocated for improved participant protections in research trials, demonstrating the impact of such collaborations. This engagement is a practical application of human rights principles, similar to how public-sector workers organized protests to advocate for their rights, despite facing significant barriers.
By following these guidelines, researchers can maintain the highest ethical standards in research, fostering trust and integrity in their work. Furthermore, as we engage in clinical studies, the impact on local economies fosters job creation, promotes economic growth, and enhances healthcare outcomes, ultimately contributing to international collaboration and innovation in the Medtech field.
Conclusion
Panama's emergence as a significant hub for clinical research in the medical devices sector is underscored by its strategic location and commitment to healthcare excellence. The country's medical device market is poised for remarkable growth, with a projected compound annual growth rate of 10% over the next five years. This growth is facilitated by favorable regulatory frameworks that streamline approval processes, making Panama an attractive destination for both local and international clinical trials.
Engaging effectively with local stakeholders is crucial for success in this landscape. By identifying key players, establishing partnerships, and maintaining clear communication, researchers can foster collaboration that enhances trial logistics and participant recruitment. Furthermore, ensuring compliance with ethical standards reinforces the integrity of clinical research, safeguarding participant rights and promoting trust within the community.
Ultimately, the dynamism of Panama's clinical research environment not only advances medical technology but also enhances its reputation as a center of clinical excellence in Latin America. As the demand for innovative medical devices continues to rise, stakeholders are presented with vast opportunities to contribute to this vibrant market, driving forward both local and global health advancements. The future of clinical research in Panama is bright, with the potential to make significant contributions to the field of Medtech and beyond.




