Introduction
In the complex landscape of medical device development, clinical evaluation stands as a cornerstone for ensuring safety and efficacy. This systematic process involves the rigorous collection and analysis of clinical data, which is essential for substantiating the performance of medical devices. In regions like Latin America, where regulatory challenges and resource fragmentation pose significant hurdles, understanding the intricacies of clinical evaluation becomes paramount.
The article delves into the essential steps of conducting clinical evaluations, including:
- The importance of compliance with regulatory standards
- The critical role of literature reviews in reinforcing the evaluation process
By navigating these elements effectively, stakeholders can foster innovation in Medtech, ultimately enhancing healthcare outcomes and driving economic growth.
Understanding Clinical Evaluation: A Foundation for the Procedure
The assessment evaluation is a structured and systematic clinical evaluation procedure dedicated to assessing the performance and safety of medical devices or interventions. This clinical evaluation procedure involves the meticulous collection and analysis of medical data to substantiate that a product is both effective and safe for its intended purpose. Considering the unique challenges encountered by Medtech firms in Latin America, such as compliance obstacles and fragmented resources, it becomes essential to navigate this complex environment effectively.
Expertise in the regulatory requirements, especially ISO 14155 and the Medical Device Regulation (MDR), is crucial for ensuring compliance and facilitating trials in the region. As emphasized by Tom Melvin from the Healthcare Products Regulatory Authority, 'The best way to ascertain what levels of evidence should be required before approval of new devices is through scientific investigation.' Moreover, the clinical evaluation procedure involves dynamic medical assessment reports that are frequently updated throughout a product's lifecycle to ensure continuous review of safety and effectiveness.
This ongoing clinical evaluation procedure is particularly important in the context of conditional evidence development schemes for high-risk medical devices, which aim to enhance transparency in regulatory processes. Additionally, future research aims to incorporate diverse data sources, such as CTIS and EUDAMED, into evaluations, improving post-market surveillance and facilitating better retrieval of relevant documents. By integrating specific service capabilities such as:
- Feasibility studies
- Site selection
- Trial setup
- Project management
and by directly addressing the need for a solution-driven approach to bridge gaps in research, we can foster collaboration between stakeholders.
This will ultimately speed up Medtech innovations and trials in Latin America, benefiting local economies through job creation and enhanced healthcare.
Step-by-Step Breakdown of the Clinical Evaluation Procedure
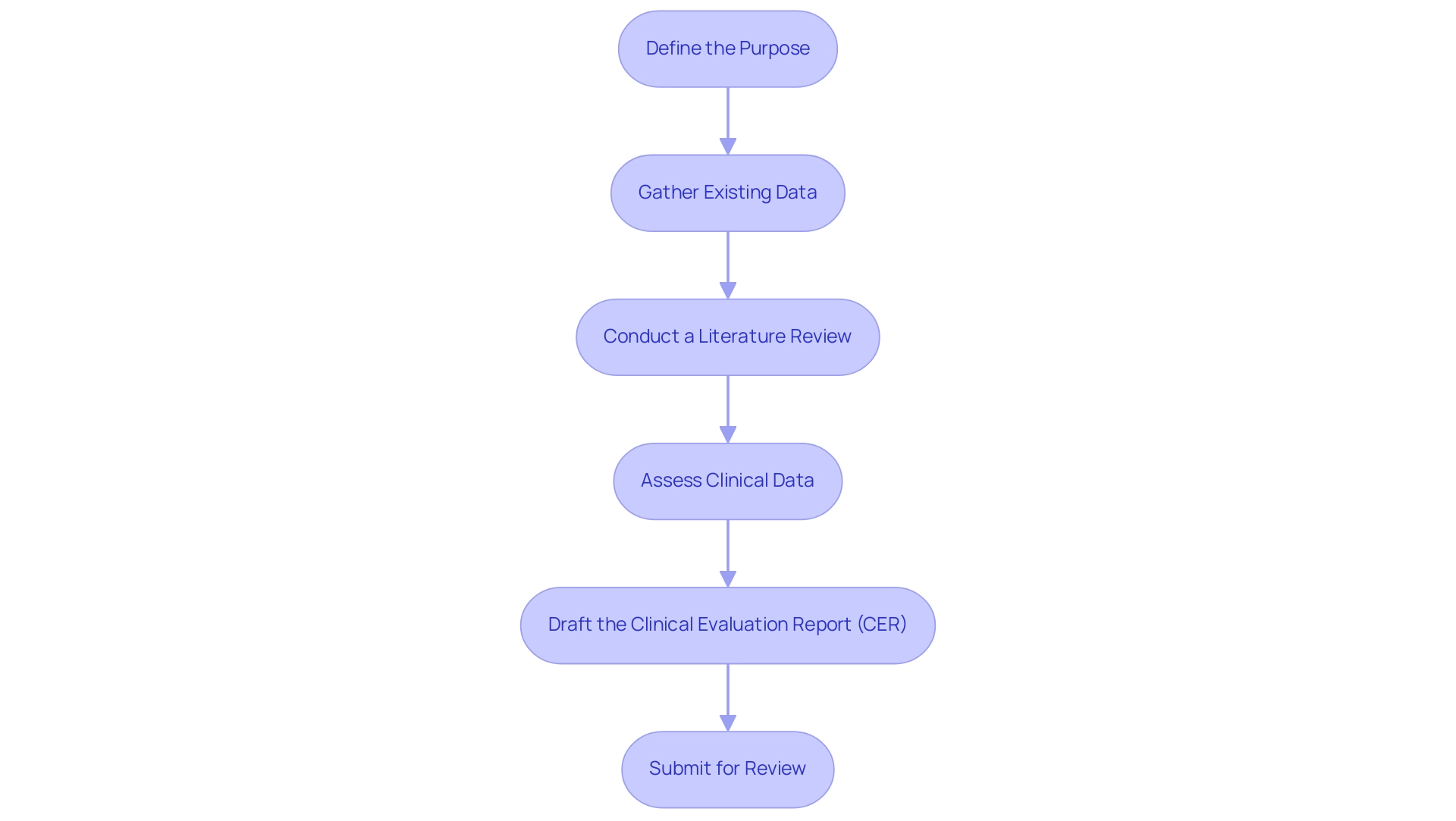
- Define the Purpose: Establishing clear objectives for the medical assessment is essential. Identify the specific questions you aim to address regarding the safety and performance of the medical device. Grasping the expected results can assist in simplifying the assessment process.
- Gather Existing Data: Compile all pertinent clinical data, including insights from prior studies, post-market surveillance documentation, and relevant academic literature. This foundational step ensures that your assessment is grounded in existing knowledge and findings, which is critical for effective trial management in compliance with regulations during the clinical evaluation procedure.
- Conduct a Literature Review: Engage in a comprehensive literature review to uncover existing evidence that supports the device's safety and efficacy. This strengthens your case and provides context for your evaluation, aiding in identifying gaps that may require further investigation.
- Assess Clinical Data: Analyze the collected data meticulously to ascertain its relevance and quality. Assess if the data complies with set standards and is appropriate for guiding safety and performance evaluations, as part of the clinical evaluation procedure that is especially significant considering the oversight environment managed by organizations such as INVIMA in Colombia.
- Draft the Clinical Evaluation Report (CER): Document your findings and methodology in a structured Clinical Evaluation Report (CER) that complies with compliance standards. A well-structured clinical evaluation procedure (CER) efficiently conveys your assessment process and serves as an essential document for compliance review, particularly during submissions to entities like INVIMA, which supervises medical device adherence as a Level 4 health authority by PAHO/WHO.
- Submit for Review: Once the CER is finalized, prepare it for submission to the relevant regulatory bodies. This signifies a significant step in the medical assessment process and must be approached with precision and care to meet all necessary guidelines.
In the context of ongoing research, studies like the Drugs and Evidence Based Medicine in the Elderly (DEBATE) Study illustrate the importance of thorough medical assessments. The DEBATE Study aims to assess the effect of multifactorial prevention on composite major cardiovascular events in elderly patients with atherosclerotic diseases, underscoring the critical role of well-structured assessments in advancing medical knowledge. Additionally, taking into account the occurrence of metabolic syndrome among US adults, as noted in the third National Health and Nutrition Examination Survey, emphasizes the significance of focused medical assessments.
Utilizing Bayesian reasoning helps in comprehending how test outcomes change the likelihood of illness in individual patients, further highlighting the importance of strong medical assessment techniques. Significantly, bioaccess® focuses on overseeing different kinds of studies, such as Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Follow-Up Studies (PMCF), supported by more than 20 years of experience in Medtech, guaranteeing a swift and compliant assessment process.
Ensuring Compliance in Clinical Evaluations: Key Considerations
Adherence is vital in medical assessments, and several key factors must be considered to ensure conformity to ethical and legal standards:
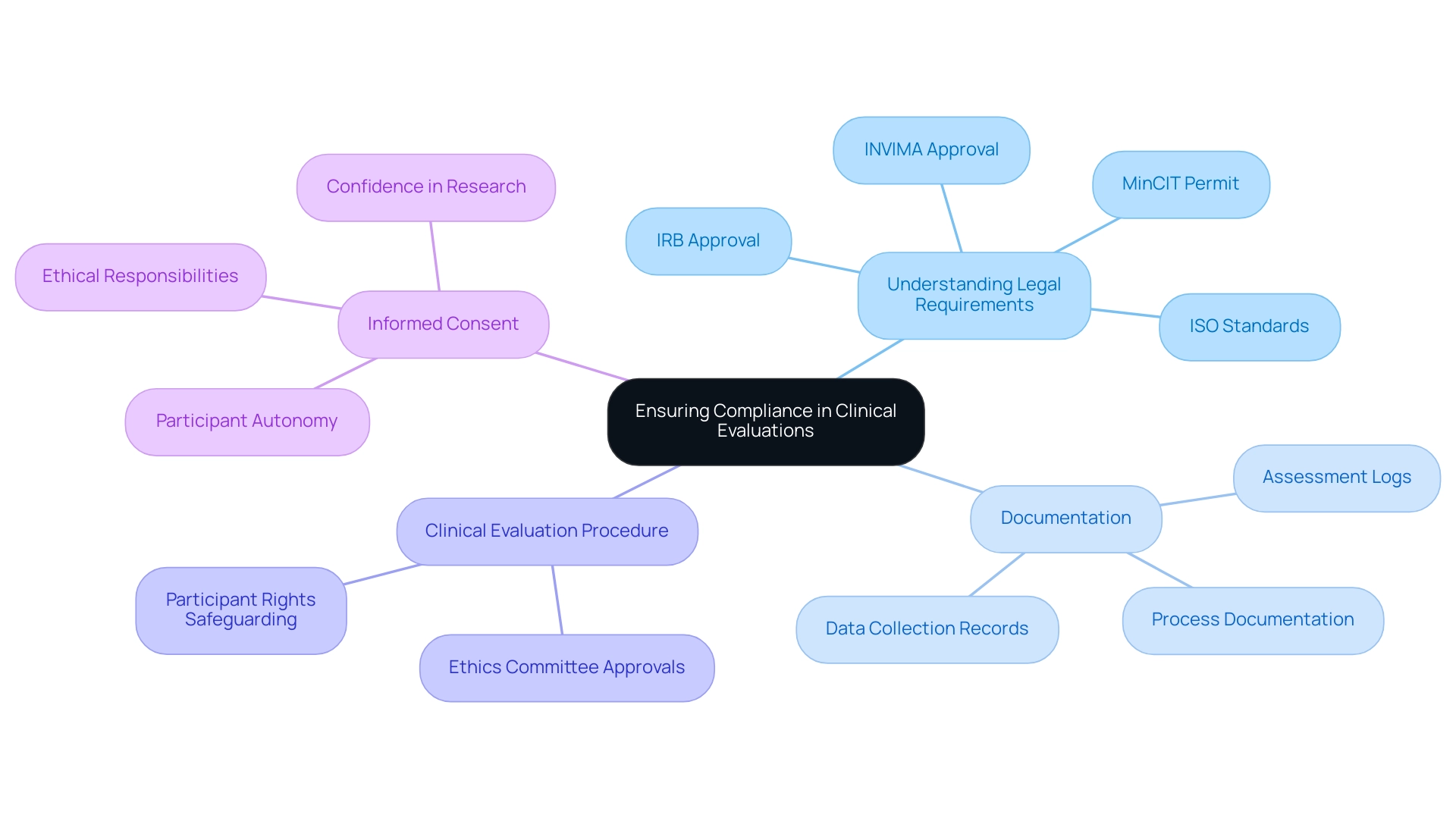
- Understanding Legal Requirements: It is crucial to familiarize yourself with applicable guidelines, including ISO standards and local laws that govern medical assessments. In Colombia, this includes obtaining ethics committee (IRB) approval, INVIMA approval, and the MinCIT import permit. Adhering to these regulations not only guarantees that ethical standards are upheld but also safeguards the integrity of the study.
- Documentation: Meticulous documentation of all processes, data collection, and assessments is vital. This practice offers clarity and trackability, which are essential for both compliance with regulations and the trustworthiness of the research findings.
- Clinical Evaluation Procedure: Before starting the clinical evaluation procedure, it is essential to obtain the required approvals from ethics committees or institutional review boards. This step is instrumental in safeguarding participant rights and ensuring that the research is conducted ethically.
- Informed Consent: All participants must provide informed consent prior to their involvement in any research, thereby respecting their rights and autonomy. This procedure not only meets ethical responsibilities but also boosts participant confidence in the research process.
The National Institutes of Health (NIH) emphasizes the importance of compliance, pointing out that an astonishing 90% of pharmaceutical development attempts fail, highlighting the essential need for strict adherence to guidelines. Our services, including review and feedback on study documents, compliance reviews, and reporting on serious and non-serious adverse events, are designed to mitigate such risks and enhance the likelihood of successful outcomes. Recent trends in the research industry, such as the acquisition of a contract research organization (CRO) specializing in trial management and the merger between two research firms, signify a movement towards consolidating expertise in compliance and oversight matters.
These strategic maneuvers reflect the industry's commitment to enhancing drug development capabilities and ensuring strict adherence to compliance frameworks. Our comprehensive trial management services include the clinical evaluation procedure, feasibility studies, site selection, compliance reviews, trial setup, import permits, project management, and reporting, ensuring that your medical device trials progress smoothly and efficiently.
Crafting an Effective Clinical Evaluation Report (CER)
Developing an effective clinical evaluation procedure requires a systematic approach that complies with regulatory requirements and best practices, particularly in the context of comprehensive trial management services. Follow these steps to ensure a comprehensive and compliant CER:
-
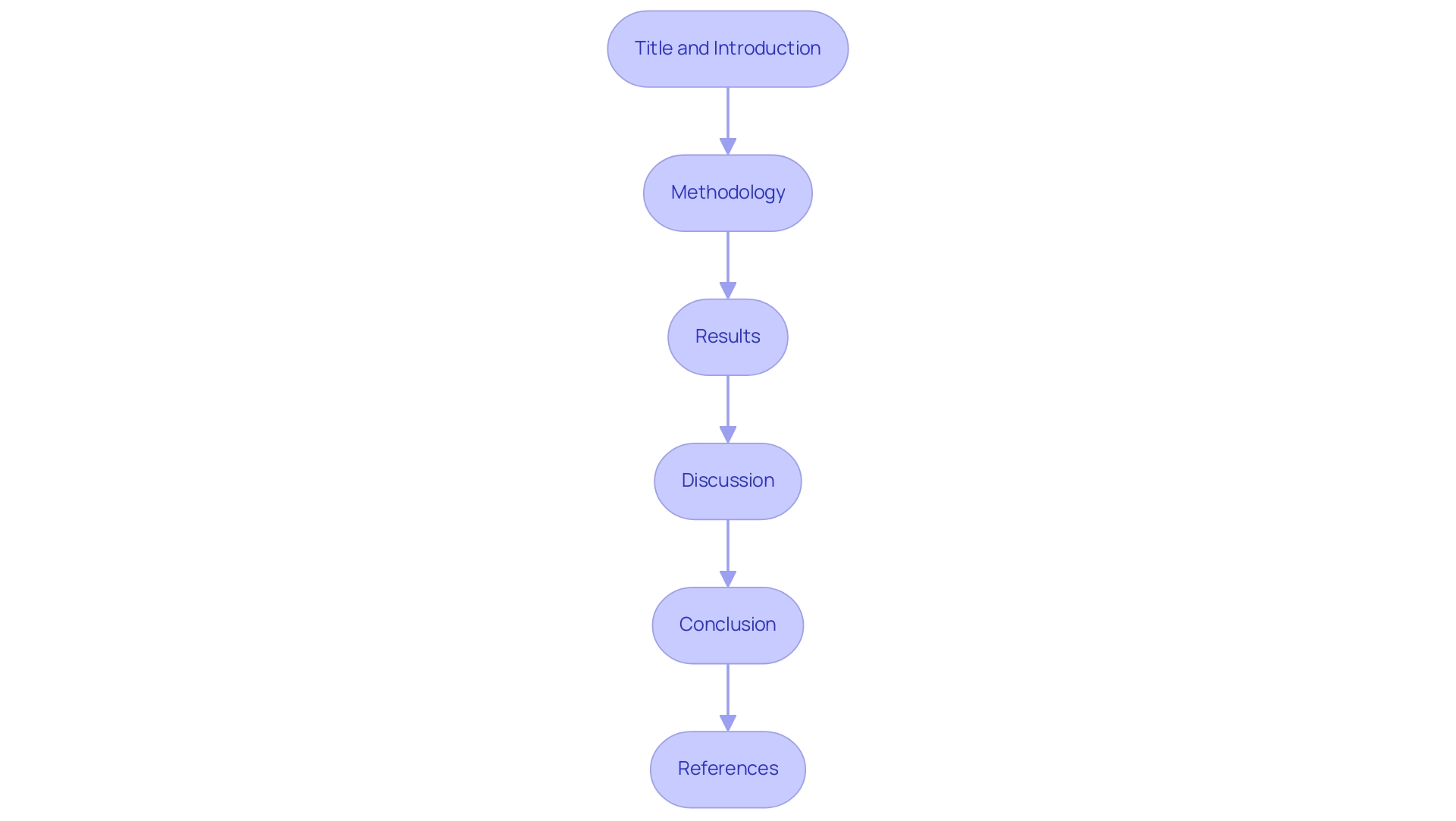
Title and Introduction: Begin with a clear title that reflects the content of the report.
The introduction should express the purpose of the CER, offering essential background information about the medical device, including its intended use and the medical context in which it operates.
-
Methodology: Detail the methodologies employed for data collection and analysis. This section must ensure transparency, allowing readers to understand how data integrity is maintained.
Notably, the term 'state of the art,' as defined in MEDDEV 2.7.1 Rev 4, refers to applicable standards, information on the medical condition, benchmark devices, and alternatives available to the target population. As Niki Price states,
'state of the art' refers to the best practices currently available in the marketplace,
underscoring the importance of this comparison. This analysis serves as a critical clinical evaluation procedure, especially in the context of compliance with regulations set forth by INVIMA, Colombia's National Food and Drug Surveillance Institute, classified as a Level 4 health authority by PAHO/WHO.
Furthermore, incorporating feedback on study documents is vital to ensure compliance with country requirements and enhance the overall quality of the trial set-up process.
-
Results: Present the findings obtained from the literature review and any medical data collected. Utilize clear tables and figures to enhance comprehension of the results, facilitating a straightforward interpretation of the information.
-
Discussion: In this critical section, analyze the results, discussing their implications for the device's safety and efficacy. Address how the findings align with the clinical performance and safety requirements under the Medical Device Regulation (MDR). Referencing the case study titled 'Analyzing Clinical Data for Compliance' can illustrate the importance of analyzing data to validate the effectiveness and safety of medical devices.
-
Conclusion: Summarize the key findings of the report, emphasizing their relevance to regulatory requirements and the device's performance. This section should emphasize the significance of the evidence gathered and analyzed in the previous steps.
-
References: Conclude with a comprehensive list of all sources cited throughout the report.
This not only provides credibility but also supports your claims, reinforcing the thoroughness of your evaluation. Following these structured steps not only aids in compliance with Article 61 of the MDR but also enhances the overall quality and reliability of the clinical evaluation procedure, ultimately supporting the device's market approval. Moreover, involving specialists such as Katherine Ruiz, who focuses on compliance matters for medical devices and in vitro diagnostics in Colombia, can offer further insights into the complexities of the oversight landscape.
Moreover, efficient project management and reporting during the trial process are crucial to guarantee that all elements of the research conform to compliance expectations.
The Role of Literature Reviews in Clinical Evaluation
Literature reviews are a vital component of clinical assessments, fulfilling several critical functions:
- Data Gathering: They play a pivotal role in identifying existing studies and data that can substantiate the clinical assessment process. By systematically reviewing literature, evaluators can compile comprehensive evidence that informs their clinical evaluation procedure, especially regarding the challenges faced by Medtech companies in Latin America, such as regulatory hurdles and language barriers.
- Assessing Quality: A thorough literature review enables evaluators to scrutinize the quality and relevance of existing data, ensuring that only credible and reliable sources contribute to the evaluation findings. This is vital for preserving the integrity of medical research, especially as companies like Greenlight Guru and bioaccess™ partner to improve trials and innovation in the region.
- Identifying Gaps: Conducting a meticulous literature review can uncover significant gaps in current data, highlighting areas that require further exploration or research. This proactive method is essential for advancing the research agenda, as shown by PAVmed's first-in-human study in Colombia, which demonstrates how targeted reviews can result in significant outcomes. Well-structured literature reviews provide the necessary evidence to substantiate claims regarding the safety and efficacy of medical devices, which is indispensable for regulatory submissions during the clinical evaluation procedure. As mentioned in a pertinent research article, a literature review can be seen as a literature inquiry, highlighting its systematic nature and significance in medical assessments. Furthermore, insights gained from the case study titled 'Parents’ Experiences of Living with a Child with a Long-Term Condition' demonstrate how literature reviews can clarify challenges encountered in medical contexts. This rapid structured review provided critical insights into the difficulties parents encounter, thereby contributing to a broader understanding of patient experiences and informing future research directions. By addressing the challenges of language barriers and resource fragmentation, literature reviews not only enhance the quality of the clinical evaluation procedure but also support collaborative efforts to comply with evolving standards in medical device assessments.
Conclusion
The journey through clinical evaluation in medical device development reveals its integral role in ensuring safety and efficacy, particularly within the complex regulatory landscape of Latin America. By meticulously following the structured steps of:
- Defining purposes
- Gathering data
- Conducting literature reviews
- Drafting comprehensive clinical evaluation reports (CERs)
stakeholders can effectively navigate the challenges inherent in this process.
Compliance with regulatory standards is paramount, as it not only ensures ethical practices but also enhances the credibility of clinical evaluations. Key aspects such as:
- Understanding local regulations
- Securing ethics approvals
- Maintaining rigorous documentation
are essential for fostering trust and transparency in research. The critical nature of these elements is further underscored by the high failure rates in clinical drug development, emphasizing the need for adherence to established guidelines.
Moreover, literature reviews serve as a foundational pillar in clinical evaluations, offering a systematic approach to data gathering, quality assessment, and identification of research gaps. By leveraging existing studies, stakeholders can substantiate claims regarding device safety and efficacy, thereby reinforcing the overall evaluation process.
In conclusion, mastering the intricacies of clinical evaluation not only facilitates regulatory compliance but also drives innovation in the Medtech field. By prioritizing these evaluations, stakeholders can contribute to improved healthcare outcomes and economic growth in the region, ultimately benefiting both the industry and the communities it serves. The path forward is clear: a commitment to rigorous clinical evaluation practices is essential for advancing medical technology and ensuring patient safety.
Frequently Asked Questions
What is the purpose of the assessment evaluation in the context of medical devices?
The assessment evaluation is a structured and systematic clinical evaluation procedure aimed at assessing the performance and safety of medical devices or interventions through meticulous data collection and analysis.
Why is expertise in regulatory requirements important for Medtech firms in Latin America?
Expertise in regulatory requirements, such as ISO 14155 and the Medical Device Regulation (MDR), is crucial for ensuring compliance and facilitating clinical trials in Latin America, where firms face unique challenges like compliance obstacles and fragmented resources.
How does the clinical evaluation procedure support the ongoing assessment of medical devices?
The clinical evaluation procedure involves dynamic medical assessment reports that are frequently updated throughout a product's lifecycle to ensure continuous review of safety and effectiveness, particularly important for high-risk medical devices.
What are some specific service capabilities that can enhance the clinical evaluation process?
Specific service capabilities that can enhance the clinical evaluation process include feasibility studies, site selection, trial setup, and project management.
What is the significance of the Clinical Evaluation Report (CER)?
The Clinical Evaluation Report (CER) documents findings and methodology in a structured format that complies with regulatory standards, serving as an essential document for compliance review during submissions to regulatory bodies like INVIMA.
What steps are involved in conducting a clinical evaluation?
The steps involved include defining the purpose, gathering existing data, conducting a literature review, assessing clinical data, drafting the Clinical Evaluation Report (CER), and submitting it for review to relevant regulatory bodies.
How does ongoing research, such as the DEBATE Study, emphasize the importance of medical assessments?
Ongoing research like the DEBATE Study highlights the critical role of thorough medical assessments in advancing medical knowledge, specifically in evaluating the effects of multifactorial prevention on cardiovascular events in elderly patients.
What types of studies does bioaccess® oversee in the Medtech field?
Bioaccess® oversees various types of studies, including Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Follow-Up Studies (PMCF), ensuring a swift and compliant assessment process.

