Overview
Optimizing recruitment for pivotal studies in Latin America requires a comprehensive understanding of the target patient population, ethical considerations, and innovative recruitment strategies. The article emphasizes the importance of demographic analysis, community engagement, informed consent, and adaptive trial designs, all of which are crucial for enhancing participant recruitment and retention in clinical research within the region.
Introduction
In the realm of clinical trials, particularly within the diverse landscapes of Latin America, the importance of strategic recruitment cannot be overstated. As the region experiences a demographic transformation, understanding the unique health needs and cultural contexts of various populations becomes crucial for successful participation in clinical studies.
This article delves into the multifaceted approaches necessary for optimizing recruitment efforts, including:
- Ethical considerations
- The formation of dedicated teams
- Innovative trial designs
- The application of data analytics
By exploring these elements, stakeholders can enhance engagement and retention rates, ultimately contributing to the advancement of medical technology and improved health outcomes across communities.
Identifying the Target Patient Population in Latin America
To effectively optimize recruitment for pivotal studies in Latin America, accurately identifying the target patient population is paramount. Begin by conducting a thorough analysis of demographic characteristics, health needs, and cultural nuances that may influence potential participants. Recent estimates from the U.S. Census Bureau indicate significant diversity among Hispanic/Latino subgroups, with:
- Mexican Americans at 58.9%
- Other Hispanic/Latino at 23.5%
- Various Central and South American populations contributing to this rich demographic tapestry.
This diversity underlines the importance of utilizing local health data and collaborating with regional health authorities to gain insights into these communities. Engaging with community leaders is equally crucial, as they can provide perspectives on cultural factors that shape health behaviors and attitudes. Key demographic factors to consider include:
- Age
- Gender
- Socioeconomic status
- Prevalent health conditions
These factors can vary significantly across regions.
This essential comprehension will not only guide your hiring strategies but also play a crucial role in optimizing recruitment for pivotal studies in Latin America, assisting in customizing messaging that connects with the intended audience and ultimately boosting involvement and participation rates in research studies. Significantly, partnerships such as those between bioaccess™ and Caribbean Health Group, backed by Colombia's Minister of Health, illustrate effective frameworks for trials in the region, attaining over 50% decrease in participant acquisition time and 95% retention rates. Furthermore, with the urban labor force in Latin America expected to reach 337 million by 2050, it is essential to focus on optimizing recruitment for pivotal studies in Latin America through a reevaluation of hiring policies and strategies to align with these demographic shifts.
For instance, the average life expectancy for non-Hispanic White males is 75.1 years, providing a comparative context that can inform health needs assessments in target populations. Utilizing such extensive insights, along with expedited medical device trial services that encompass:
- Early-Feasibility
- First-In-Human
- Pilot
- Pivotal
- Post-Market Follow-Up Trials
can result in enhanced enrollment outcomes and participant retention.
Ensuring Ethical Recruitment: The Role of Informed Consent
Informed consent serves as a cornerstone of ethical recruitment in clinical trials. To ensure that potential participants are thoroughly informed, it is essential to craft clear and comprehensive consent forms that detail the project's purpose, procedures, risks, and benefits. Interaction with local communities plays a crucial role in this process, as it allows researchers to contextualize the importance of the research and address any concerns that may arise.
Media coverage, especially from sources like Clinical Leader, underscores the importance of optimizing recruitment for pivotal studies in Latin America and Colombia, highlighting local participation and its implications for job creation, economic growth, and healthcare improvement. Research indicates that comprehension rates for informed consent hover around 59.8% (95% CI: 0.384, 0.811), with significant variability across different components, underscoring the need for enhanced communication strategies. For instance, translating materials into local languages and incorporating culturally relevant examples can significantly improve understanding.
The Verheggen 1996 research involving 198 adult patients across 26 trials further illustrates this variability, as it evaluated participants' understanding of informed consent components. Furthermore, revisiting and updating consent procedures consistently is essential to reflect any changes in the research protocol, thereby maintaining transparency and fostering trust throughout the selection phase. Significantly, the meta-analysis showed that open-ended questions frequently result in reduced understanding of the research's purpose and participants' rights, indicating the need for more structured and supportive communication approaches.
As observed by Singh, "Numerous investigations have indicated that there appears to be negative prejudices against African Americans and Latinos in obtaining access to care, and that may also include biases against involving them in experiments," highlighting the significance of ethical practices that emphasize inclusivity and comprehension. Thus, a diverse strategy that integrates clear communication, community involvement, and ongoing feedback systems is vital for optimizing recruitment for pivotal studies in Latin America, which will ultimately aid the wider influence of Medtech initiatives in local economies.
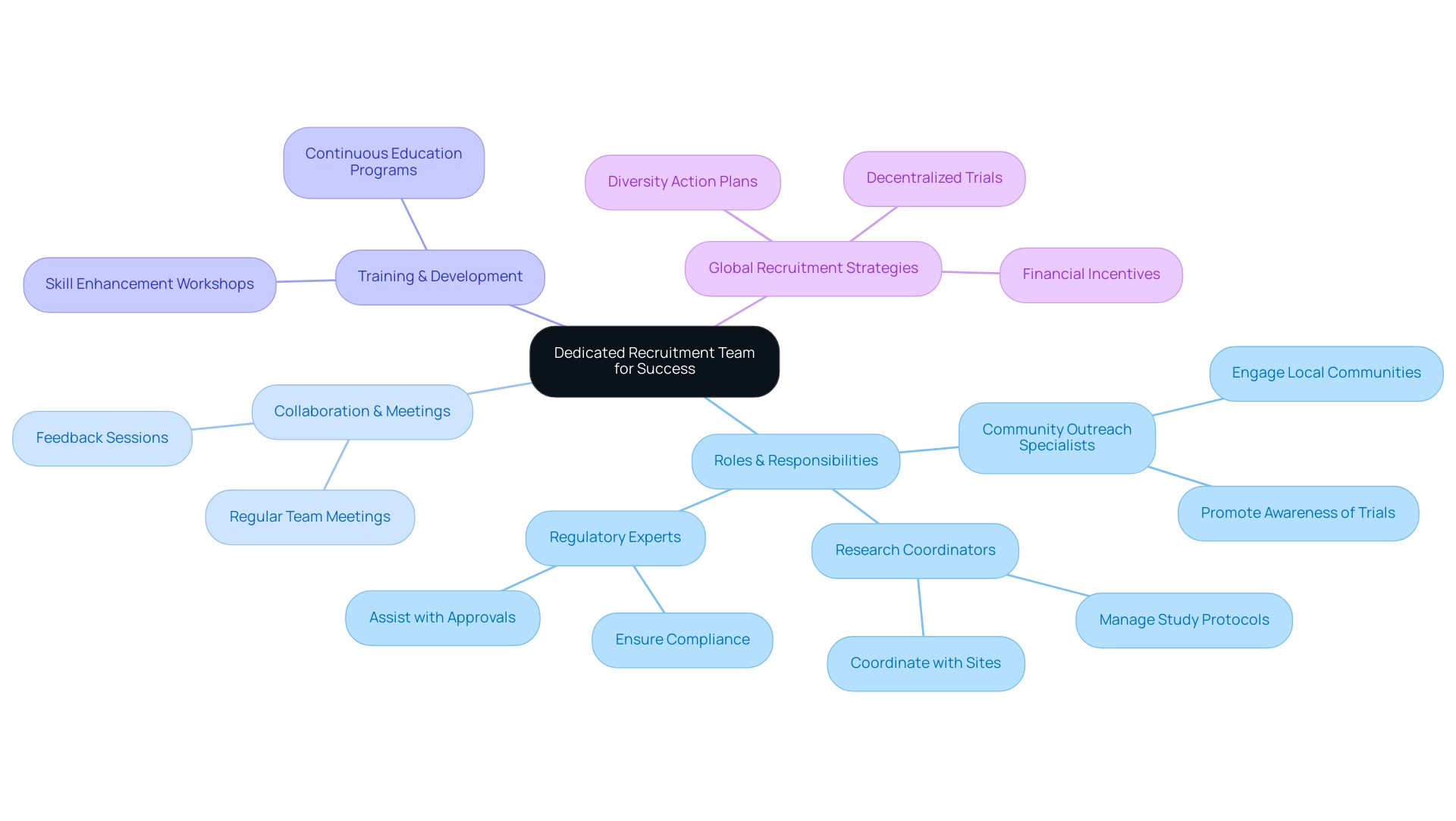
Building a Dedicated Recruitment Team for Success
To enhance hiring initiatives in research studies, it is essential to form a dedicated group focused on optimizing recruitment for pivotal studies in Latin America, which includes:
- Research coordinators
- Community outreach specialists
- Regulatory experts
Each member’s roles and responsibilities should be clearly defined to ensure accountability and operational efficiency. Establishing a cooperative atmosphere is essential; conducting regular meetings enables the team to align on hiring objectives, tackle challenges, and celebrate achievements.
Encouraging open communication and constructive feedback fosters a culture of continuous improvement in strategies. Training sessions further prepare the team with the essential skills and knowledge to engage effectively with the target population, adapting to the changing environment of research studies. Significantly, with the FDA now mandating diversity action plans for all phase 3 studies, optimizing recruitment for pivotal studies in Latin America has become more essential in hiring strategies.
Furthermore, the global effort to broaden participant recruitment beyond the United States is motivated by the increase of decentralized and virtual studies. Comprehensive clinical research management services, including:
- Feasibility assessments
- Site selection
- Compliance reviews
- Setup
- Import permits
- Nationalization of investigational devices
play a critical role in this expansion. As emphasized in the case study 'Challenges and Solutions in Recruitment and Retention,' ongoing education campaigns, streamlined study designs, and financial incentives are vital in overcoming obstacles such as limited awareness and participant fatigue.
As observed by Anthony Haywood, Vice President of Clinical Trials Optimization at MEDiSTRAVA, the 2024 trends in attracting and retaining participants emphasize a more patient-centric, technologically advanced, and inclusive approach, which highlights the significance of well-structured teams in driving global health enhancement through Medtech innovation. Moreover, promoting global cooperation is crucial for optimizing recruitment for pivotal studies in Latin America and ensuring varied participant involvement in research studies.
Exploring Alternative Trial Designs to Enhance Recruitment
To enhance participant acquisition in research studies, it is crucial to focus on optimizing recruitment for pivotal studies in Latin America, along with examining innovative study designs, including adaptive formats, decentralized approaches, and hybrid models.
Adaptive studies, as defined by the FDA CDRH, are characterized by the ability to make modifications based on interim results, thereby enhancing flexibility and responsiveness to participant needs. Recent developments, such as the ICECAP study—a randomized, response-adaptive investigation into the optimal duration of induced hypothermia for neuroprotection in comatose survivors of cardiac arrest—exemplify the effectiveness of response-adaptive designs in optimizing clinical outcomes and improving recruitment strategies.
Decentralized studies utilize technology for optimizing recruitment for pivotal studies in Latin America, facilitating remote participation and making them particularly beneficial for engaging participants in rural or underserved areas. These experiments not only enhance accessibility but also enrich participant diversity, leading to more robust data. Additionally, the case analysis titled 'Educational Strategies for Adaptive Designs' emphasizes the significance of improving comprehension and awareness of adaptive techniques within the research community, which can promote efficiency in experiments and aid in participant enlistment.
Hybrid models, which integrate both traditional and innovative approaches, play a crucial role in optimizing recruitment for pivotal studies in Latin America by maximizing reach and participant engagement. Adopting these trial designs can significantly increase convenience and appeal for potential participants, thereby enhancing overall enrollment outcomes.
Evaluating and Adapting Recruitment Strategies for Optimal Outcomes
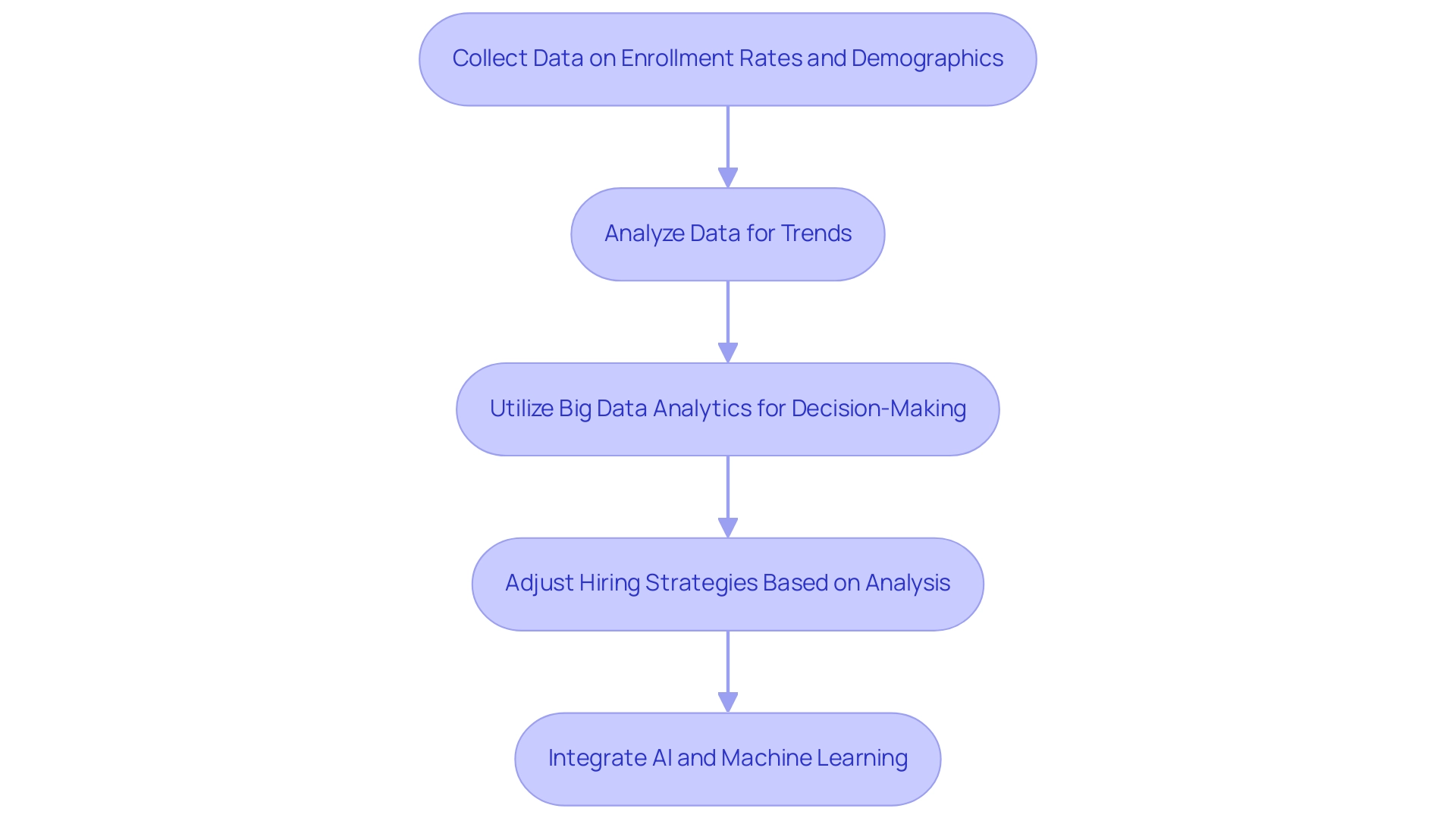
To drive effective hiring in clinical research, it is essential to focus on optimizing recruitment for pivotal studies in Latin America and establish a robust framework for assessing the effectiveness of your strategies. Begin by collecting comprehensive data on enrollment rates, participant demographics, and feedback from both participants and team members. This data forms the backbone of your analysis, allowing for the identification of trends and areas requiring improvement.
Utilizing big data analytics enables hiring leaders to leverage large datasets for predictive analytics and informed decision-making, significantly enhancing your ability to respond to market shifts and emerging trends. Recent studies have demonstrated that data analytics in hiring can enhance forecasting accuracy, resulting in proactive decision-making, as illustrated by the case study on precise forecasting in hiring. This capability enables hiring leaders to adjust strategies in real-time, ensuring they can respond effectively to changing circumstances.
Be prepared to adjust your hiring strategies based on this analysis—whether that involves refining your messaging, broadening outreach efforts, or optimizing recruitment for pivotal studies in Latin America. Moreover, AI and machine learning play a crucial role in automating repetitive tasks, improving hiring quality, and reducing cost-per-hire. Embracing a culture of continuous improvement not only enhances the overall effectiveness of your hiring initiatives but also contributes to optimizing recruitment for pivotal studies in Latin America, ensuring optimal outcomes in clinical trials.
As Yuma Heymans notes,
Only 12% of hiring professionals currently use AI in recruitment, indicating a significant opportunity for those who leverage technology and data-driven approaches to gain a competitive edge in recruitment success.
Conclusion
The optimization of recruitment strategies in clinical trials within Latin America hinges on a comprehensive understanding of the region's diverse patient populations. By meticulously analyzing demographic characteristics, health needs, and cultural contexts, stakeholders can craft tailored approaches that resonate with potential participants. The importance of collaboration with local authorities and community leaders cannot be overstated, as these partnerships facilitate deeper engagement and trust, ultimately enhancing recruitment and retention rates.
Ethical considerations, particularly regarding informed consent, are foundational to successful recruitment efforts. Clear and culturally sensitive communication strategies are essential to ensure participants fully understand the study's purpose and implications. As the data suggests, improving comprehension rates through accessible materials is critical for fostering trust and inclusivity in clinical trials.
The establishment of dedicated recruitment teams plays a vital role in streamlining efforts and ensuring accountability. By fostering a collaborative environment and incorporating innovative trial designs, such as adaptive and decentralized approaches, recruitment strategies can be significantly enhanced. This adaptability not only addresses participant needs but also aligns with the growing emphasis on diversity in clinical trials, as mandated by regulatory bodies.
Finally, leveraging data analytics and technology is paramount for evaluating and refining recruitment strategies. The ability to analyze enrollment trends and participant feedback allows for proactive adjustments, ensuring that recruitment initiatives remain effective and responsive to market dynamics. Embracing these multifaceted approaches will not only improve recruitment outcomes but also contribute to the advancement of medical technology and the overall health of communities across Latin America.
Frequently Asked Questions
Why is accurately identifying the target patient population important for recruitment in Latin America?
Accurately identifying the target patient population is crucial because it helps tailor recruitment strategies to meet the specific demographic characteristics, health needs, and cultural nuances that influence potential participants.
What demographic factors should be considered when optimizing recruitment in Latin America?
Key demographic factors to consider include age, gender, socioeconomic status, and prevalent health conditions, as these can vary significantly across different regions.
How does the diversity among Hispanic/Latino subgroups impact recruitment strategies?
The significant diversity among Hispanic/Latino subgroups, such as the varying proportions of Mexican Americans and other Hispanic/Latino populations, necessitates the use of local health data and collaboration with regional health authorities to effectively engage these communities.
What role do community leaders play in the recruitment process?
Engaging with community leaders is essential as they can provide valuable insights into cultural factors that shape health behaviors and attitudes, aiding in the recruitment process.
What are some successful examples of partnerships in recruitment for studies in Latin America?
Partnerships like those between bioaccess™ and Caribbean Health Group have demonstrated effective frameworks, achieving over a 50% decrease in participant acquisition time and 95% retention rates.
How can understanding life expectancy statistics inform recruitment strategies?
Understanding statistics such as the average life expectancy for non-Hispanic White males can provide a comparative context that informs health needs assessments in target populations, guiding recruitment efforts.
What is the significance of informed consent in clinical trial recruitment?
Informed consent is a cornerstone of ethical recruitment, ensuring potential participants are fully informed about the project's purpose, procedures, risks, and benefits.
How can comprehension rates for informed consent be improved?
Comprehension can be enhanced by translating materials into local languages, using culturally relevant examples, and ensuring clear communication strategies are in place.
What does research indicate about understanding informed consent among participants?
Research shows that comprehension rates for informed consent are around 59.8%, with variability across different components, highlighting the need for improved communication strategies.
Why is it important to revisit and update consent procedures?
Consistently updating consent procedures is vital to reflect any changes in the research protocol, maintaining transparency and fostering trust during the selection phase.
What biases may affect recruitment of diverse populations in clinical trials?
Negative prejudices against African Americans and Latinos regarding access to care may also extend to their involvement in clinical trials, underscoring the need for ethical practices that promote inclusivity and comprehension.
What strategies are essential for optimizing recruitment in Latin America?
A diverse strategy that integrates clear communication, community involvement, and ongoing feedback systems is vital for optimizing recruitment and enhancing the impact of Medtech initiatives in local economies.




