Introduction
Navigating the intricate landscape of medical device regulations is a formidable task that demands a comprehensive understanding of the Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR). These regulatory frameworks impose stringent requirements on safety, performance, classification, and market access, shaping the development and entry of medical devices into the market. Since the IVDR's publication in 2017, significant advancements have been made to align with technological progress and improve patient safety, emphasizing the critical analysis and presentation of clinical data, including often overlooked post-market surveillance data.
Industry leaders stress the urgency of adapting to these evolving demands to ensure compliance and operational efficiency. Effective strategies for regulatory document preparation are crucial, especially given the increasing complexity of packaging, labeling requirements, and the need for alignment across various documents, such as clinical evaluation plans and risk management files. Recent developments, such as the UK's MHRA roadmap for new medical device regulations and the European Commission's proposal to expedite the implementation of EUDAMED, highlight the dynamic nature of these regulations and the importance of staying informed.
Understanding and adhering to these regulations is essential for structuring technical files correctly, ensuring meticulous analysis and presentation of necessary data. By adopting efficient compliance strategies and staying abreast of regulatory initiatives, medical device professionals can navigate this complex terrain effectively, maintaining market access and operational efficiency.
Understanding MDR and IVDR Regulations
Ensuring compliance with medical device regulations necessitates a thorough understanding of the Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR) pertinent to your region. These governing frameworks establish stringent requirements for safety, performance, classification, and market access. Published on May 25, 2017, the IVDR, for instance, introduced comprehensive rules to align with the latest technological advancements and improve patient safety. This regulation emphasizes the need for detailed analysis and coherent presentation of clinical data, including post-market surveillance data, which is crucial yet often underutilized.
The changing environment of compliance requirements significantly influences the development and market entry of medical devices. Industry experts highlight the urgency of adapting to these changes to ensure compliance and efficiency. Practical approaches involve improving the efficiency of regulatory and safety document preparation, which minimizes delays and errors. This is particularly important given the increasing complexity of packaging and labeling requirements, as well as the necessity to align various documents like the clinical evaluation plan and risk management files.
Recent news underscores the dynamic nature of these regulations. For instance, the UK Medicines and Healthcare products Regulatory Agency (MHRA) has announced a roadmap for new medical device regulations, prioritizing patient safety and facilitating access to necessary devices. Similarly, the European Commission's proposal aims to accelerate the mandatory implementation of parts of EUDAMED by late 2025, enhancing transparency and compliance harmonization across the EU.
Understanding and adhering to these regulations is essential for structuring your technical file correctly, ensuring that all necessary data is meticulously analyzed and presented. By staying informed about governance initiatives and adopting efficient compliance strategies, you can navigate the complexities of the medical device industry and maintain market access.
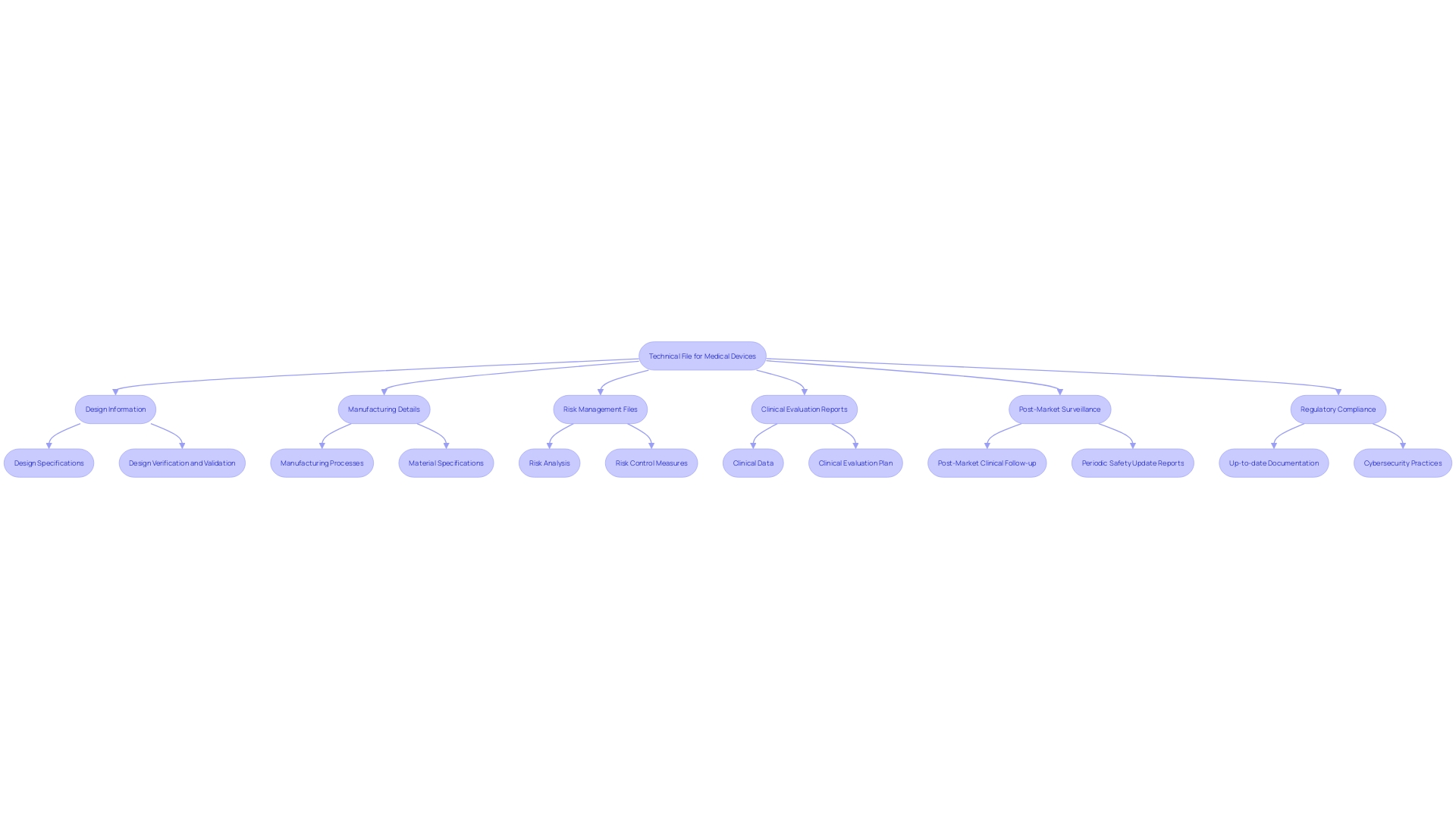
Key Components of a Technical File
A technical file must include thorough documentation, comprising design and manufacturing specifics, management files, and clinical evaluation reports. Each element is critical for demonstrating compliance with regulatory requirements. Design information should include specifications, drawings, and materials used, ensuring alignment with the latest quality standards. Manufacturing details should cover processes, validation protocols, and quality control measures to maintain consistency and safety across production batches.
Risk management files should be detailed, outlining identified risks, mitigation strategies, and ongoing monitoring plans. This is especially pertinent in light of the FDA’s recent guidance on incorporating cybersecurity into medical device development. The FDA emphasizes planning for the 'Total Product Life Cycle,' highlighting the importance of integrating secure practices from the initial design phase through to post-market surveillance.
Clinical evaluation reports must be thorough, incorporating information analysis and coherent presentation to connect conclusions with the actual information. Post-market surveillance data, derived from similar and own products, should be rigorously analyzed to ensure continuous compliance and safety.
Keeping all documents current and reflective of the latest design and manufacturing processes is essential. This encompasses revising files to reflect alterations in materials, processes, or compliance necessities, along with guaranteeing consistency across different documents, such as the clinical evaluation plan, clinical evaluation report, and management files.
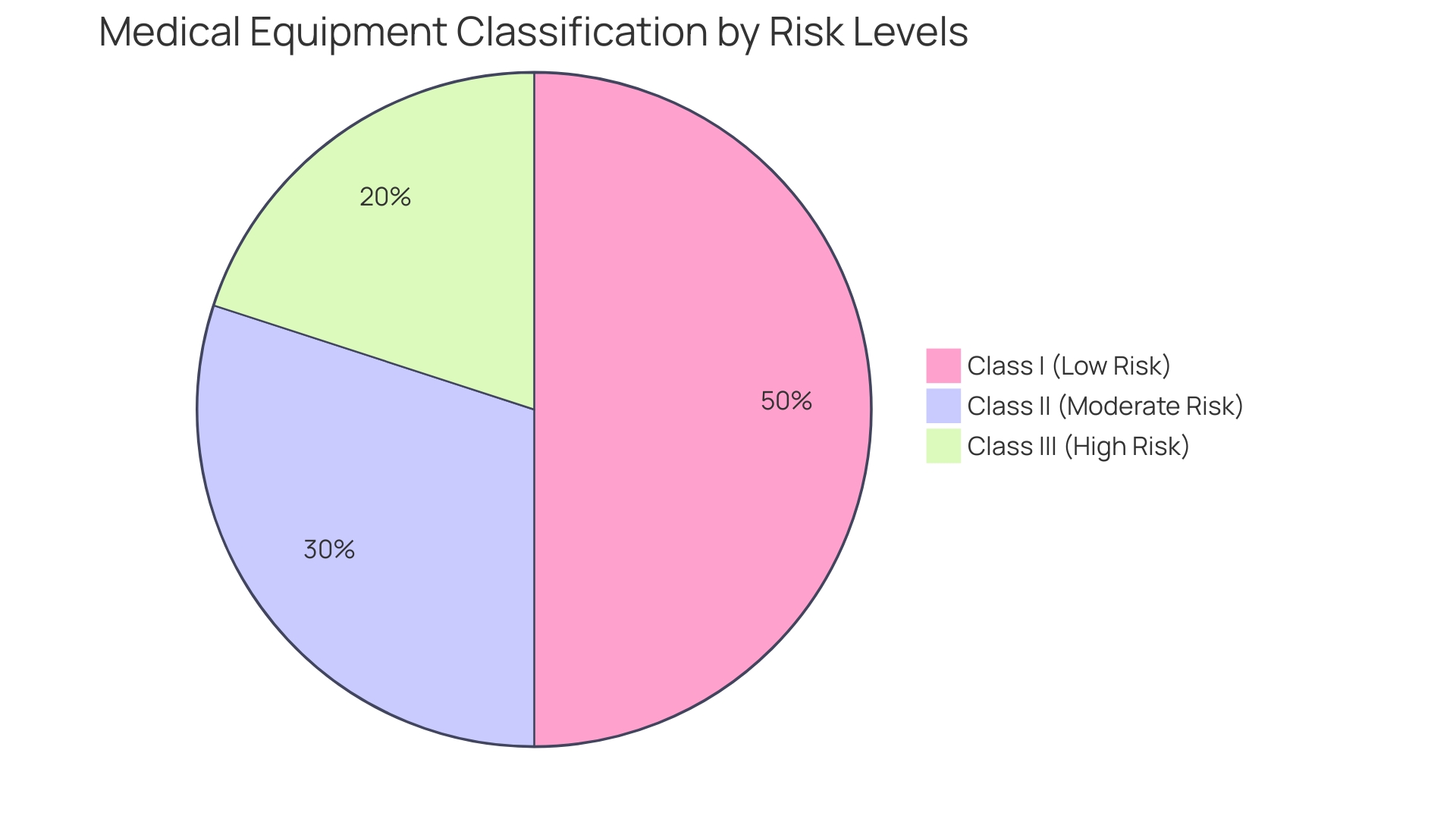
Classification of Medical Devices
Determining the classification of your medical equipment is crucial as it dictates the extent of regulatory oversight and the necessary documentation. 'Medical instruments are categorized into three classes based on risk: Class I (low risk), Class II (moderate risk), and Class III (high risk).'. For example, a Software as a Medical Product (Sand) meeting the FDA's criteria for a Class III classification would be subject to the most stringent controls. To determine the suitable classification, consult the FDA’s classification database, which arranges over 1,700 types of equipment by medical specialty under 21 CFR Parts 862-892. This classification impacts the technical file, determining the detail level and types of assessments required. For instance, the categorization of neurological instruments emphasizes the importance of patient perspectives and real-world data, as reflected in the FDA's 2021 guidance on Implanted Brain-Computer Interface (BCI) Systems. Staying informed about evolving regulatory demands and utilizing practical approaches to ensure compliance are essential for navigating this complex landscape efficiently.
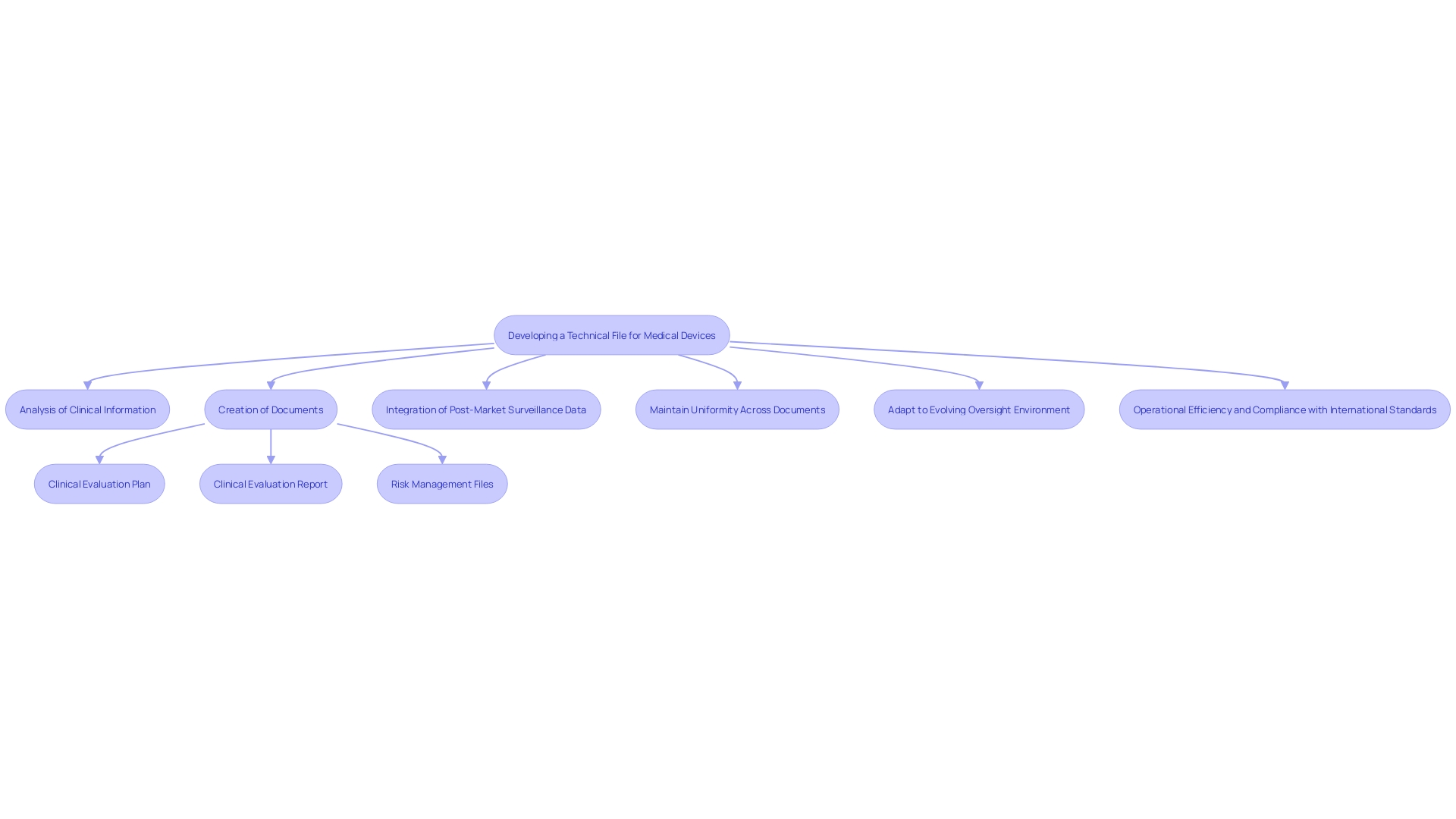
General Safety and Performance Requirements
Your technical file must comprehensively demonstrate that the medical device adheres to the General Safety and Performance Requirements (GSPR) outlined in the MDR. This includes presenting detailed evidence of safety, effectiveness, and compliance with relevant standards. The documentation should encompass a meticulous analysis and coherent presentation of clinical information. It is vital to guarantee uniformity across different documents, such as the clinical evaluation plan, clinical evaluation report, and risk management files, to prevent discrepancies and misalignment in information gathering and analysis strategies. The inclusion of post-market surveillance data, analyzed with the same rigor as primary data, significantly enhances the credibility of the safety and efficacy claims. Moreover, the oversight environment is continually evolving, necessitating an efficient approach to capturing, managing, and sharing information. By adopting a proactive stance and ensuring that all information is readily retrievable and reusable, medical equipment suppliers can streamline compliance content management, thus optimizing operational efficiency and ensuring adherence to international standards, such as ISO 13485 and FDA regulations.
Technical Documentation Structure and Content
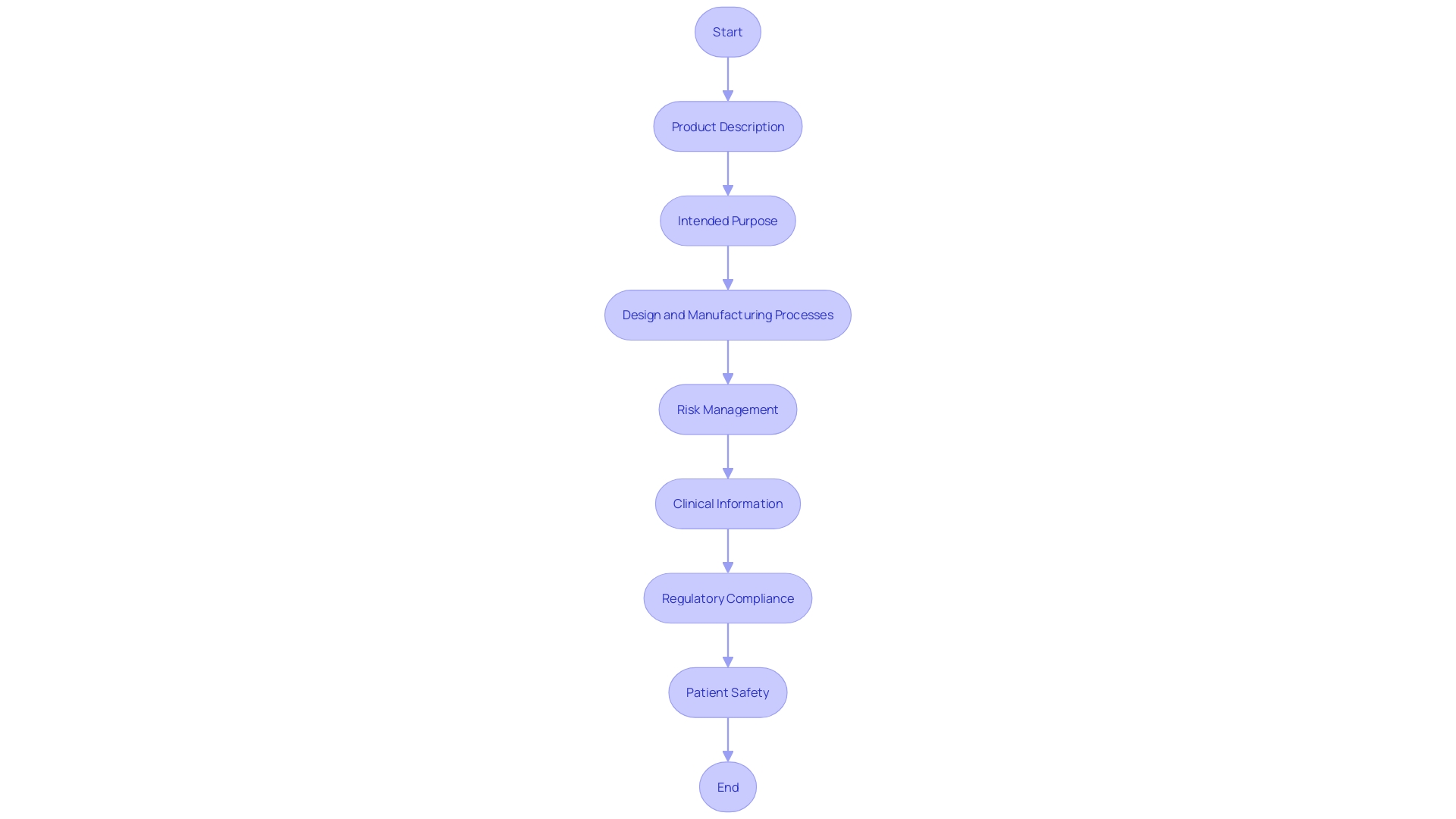
To ensure an efficient review by regulatory bodies, it is crucial to organize your technical file with a clear and structured approach. Start with a section outlining the product description, which should include comprehensive information about its medical purpose, intended users, and target populations. This enhances understanding and sets a solid foundation for subsequent sections.
Next, allocate a section for the intended purpose of the equipment. This should clearly communicate the performance of the apparatus and any reasoning behind its operation, contributing to overall transparency and explainability. 'These aspects are essential as they ensure that information affecting hazards and patient outcomes is effectively communicated.'.
The design and manufacturing processes should be thoroughly documented in another section. Emphasizing these processes not only aids in adhering to regulations but also promotes a clear comprehension of how the equipment fits into the healthcare workflow, outlining its inputs and outputs.
Risk management is another critical component. This section must provide a detailed analysis of potential risks associated with the device and the measures implemented to mitigate them. Transparency in this area is paramount, as it directly influences patient safety and regulatory approval.
Lastly, include a section dedicated to clinical information. This should encompass all relevant clinical trials, performance data, and any other supporting evidence that demonstrates the device's efficacy and safety. Including these elements guarantees a strong and thorough technical file that aligns with the latest compliance standards and industry best practices.
Best Practices for Technical File Maintenance and Updates
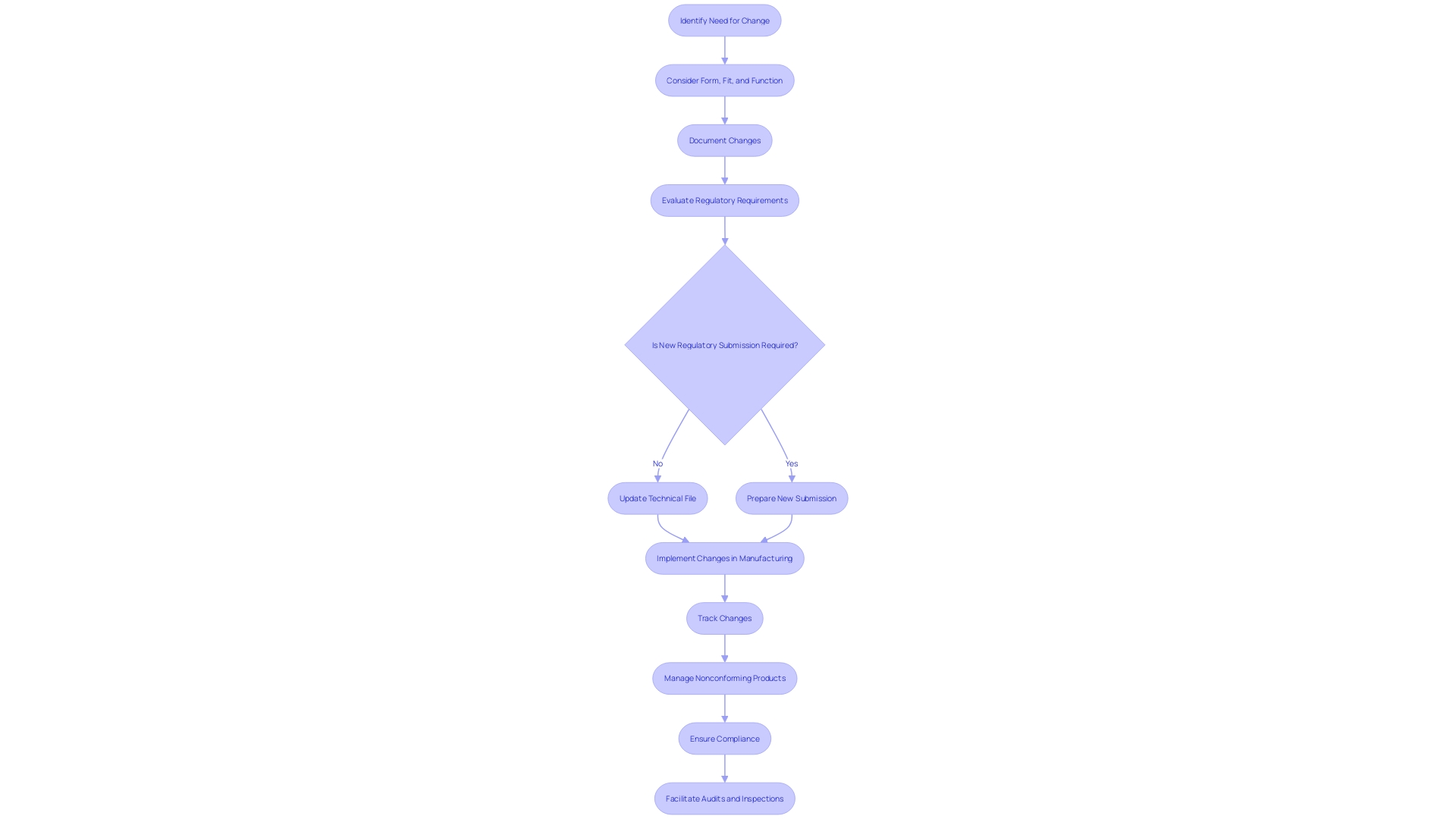
Consistently refreshing your technical file is crucial to document any alterations in design, manufacturing processes, or compliance requirements. This includes addressing customer feedback, changes in materials, or modifications in manufacturing processes. Each of these changes necessitates thorough documentation and may require new regulatory submissions, in line with the latest regulatory initiatives.
Implementing a robust document control system is crucial. This system should ensure that all versions of documents are tracked and easily accessible. For example, managing nonconforming products at the serial number level using manual methods presents a significant chance for potential errors, such as determining which units are still in the warehouse or on the factory floor. A well-maintained document control system can mitigate these risks by keeping accurate records of all changes and updates.
This proactive approach not only helps maintain compliance but also facilitates smoother audits and inspections. As highlighted by industry experts, improving efficiency in regulatory and safety document preparation is a key priority. Such efficiency ensures that your operations are not just compliant but also streamlined, thereby enhancing overall productivity and safety in the medical device sector.
Conclusion
Navigating the complexities of the Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR) is imperative for ensuring compliance and operational efficiency in the medical device industry. These regulations establish rigorous standards for safety, performance, classification, and market access, necessitating a comprehensive understanding of the documentation requirements involved. The emphasis on detailed clinical data analysis and robust post-market surveillance practices underscores the importance of maintaining high safety and efficacy standards.
The technical file serves as a cornerstone for demonstrating compliance, encompassing vital components such as design and manufacturing details, risk management files, and clinical evaluation reports. Each element must be meticulously documented to ensure alignment with regulatory requirements, particularly as the classification of medical devices dictates the degree of oversight necessary. The evolving regulatory landscape, including updates from agencies like the MHRA and the European Commission, highlights the need for continuous adaptation and proactive strategies in regulatory document preparation.
Best practices for maintaining and updating technical files are crucial to navigate this dynamic environment effectively. Regular updates reflecting changes in design, materials, and regulatory expectations are essential for compliance and operational integrity. Implementing a robust document control system can mitigate risks associated with nonconforming products and enhance overall efficiency.
By adopting these strategies, medical device professionals can not only maintain market access but also contribute to the advancement of patient safety and care in a rapidly evolving industry.




