Overview
Successfully conducting multi-site trials in Mexico necessitates a thorough understanding of the regulatory framework, meticulous logistical planning, and effective recruitment strategies aimed at enhancing participant diversity and data quality. This article underscores that robust communication and collaboration among sites, coupled with strict adherence to ethical standards and comprehensive training, are critical components for improving the likelihood of successful outcomes in clinical research.
Introduction
In the realm of clinical research, the complexity and nuances of multi-site trials present both challenges and opportunities for researchers aiming to enhance the quality and applicability of their findings. These trials, which span various locations, offer a unique chance to tap into diverse participant demographics, significantly enriching the data collected.
Particularly in regions like Mexico, where demographic diversity is vast, the advantages of multi-site trials become even more pronounced. By broadening participant recruitment and improving statistical power, researchers can mitigate the risks associated with single-site studies, ultimately driving more robust and generalizable results.
This article delves into the essential components of successful multi-site trials, exploring:
- Regulatory frameworks
- Logistical considerations
- Effective recruitment strategies
- The critical role of training and communication in fostering collaboration across sites
Each element is vital for ensuring that trials not only meet regulatory standards but also uphold ethical considerations and participant safety, paving the way for advancements in medical technology and improved healthcare outcomes.
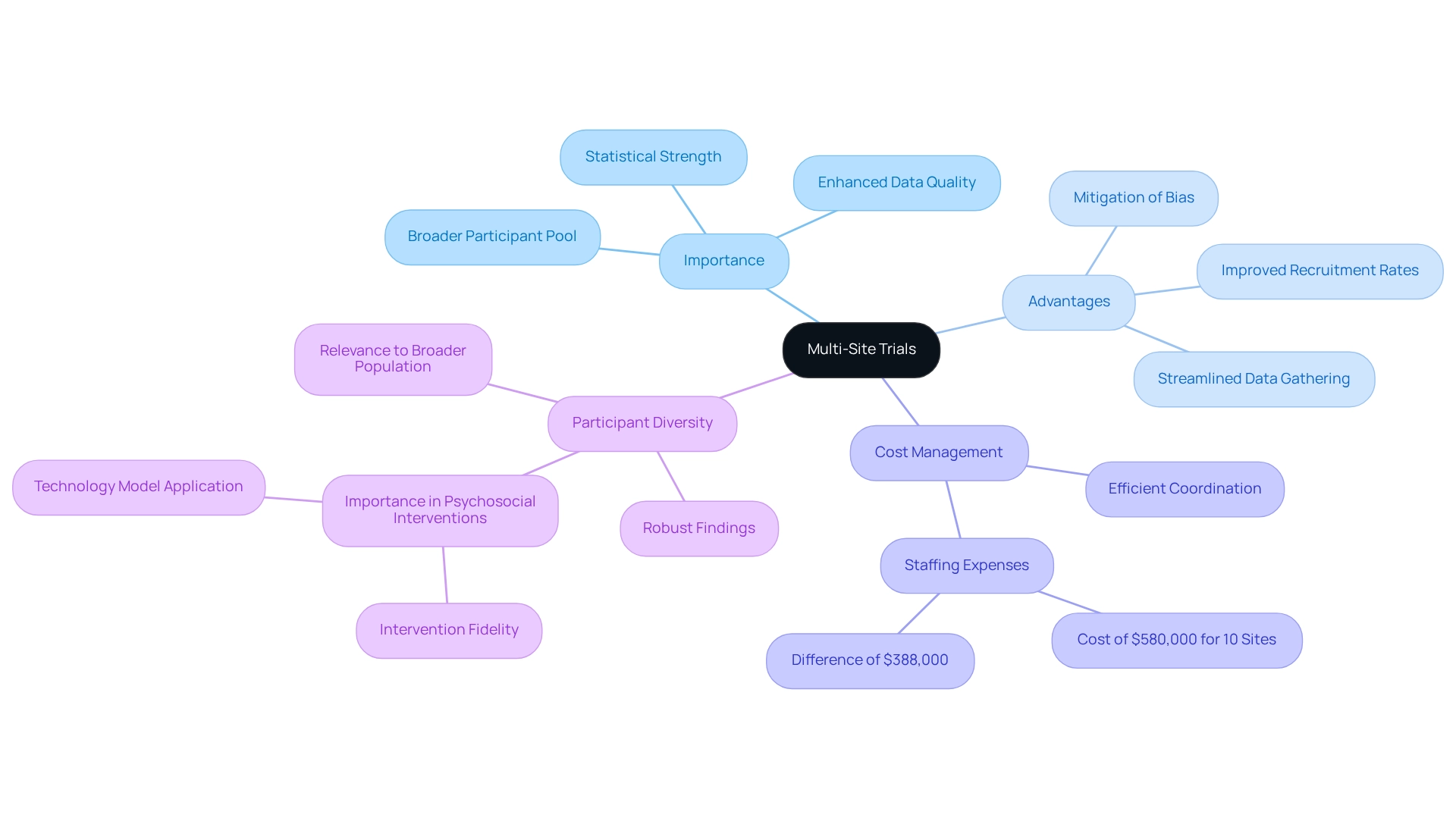
Understanding Multi-Site Trials: Definition and Importance
Multi-site studies are pivotal in conducting clinical research across diverse locations, significantly broadening the participant pool and enhancing demographic diversity. This approach holds particular importance in Mexico, where the rich tapestry of demographics can yield more generalizable results. The significance of multi-location studies is underscored by their ability to bolster statistical strength, elevate participant recruitment rates, and streamline data gathering processes.
By leveraging multiple sites, researchers can effectively mitigate the risks associated with single-site studies, such as limited participant diversity and inherent biases.
bioaccess® excels in comprehensive clinical study management services, encompassing Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies (PMCF). With over 20 years of experience in Medtech, bioaccess® possesses the expertise and tailored approach necessary to navigate the complexities of multi-site studies, ensuring effective coordination and adherence across various locations.
For instance, a recent analysis revealed that staffing expenses for non-Calculator studies could soar to $580,000 for ten sites from the investigators' meeting to the commencement of recruitment, highlighting the financial implications of study management. This statistic emphasizes the need for efficient coordination in multi-location studies to manage costs effectively. Moreover, regression analysis has shown a strong correlation between the logarithm of test duration and forecasting outcomes, showcasing the efficiency improvements achievable through multi-site coordination.
As Harry Glorikian, MBA, notes, "The capacity to carry out studies across various locations not only enhances data quality but also ensures that the results are relevant to a broader population." The benefits of conducting multi-site trials in Mexico extend beyond logistical advantages; they also enrich participant diversity, which is crucial for the validity of medical research. Diverse populations can yield more robust findings, reflecting a wider range of responses to interventions.
This relevance is particularly pronounced in the realm of psychosocial interventions, where consistency in delivery—termed intervention fidelity—can significantly influence research outcomes. A case study on this subject illustrates how employing a Technology Model to ensure intervention fidelity can enhance the quality of nursing research and promote evidence-based healthcare practices.
In conclusion, multi-site studies not only enhance the efficiency and effectiveness of medical research but also contribute to the overall integrity and relevance of study findings. With bioaccess®'s expertise in managing multi-site trials in Mexico, these studies emerge as a vital component of contemporary research strategies, ultimately fostering local economic growth and improving healthcare.
Navigating the Regulatory Framework for Clinical Trials in Mexico
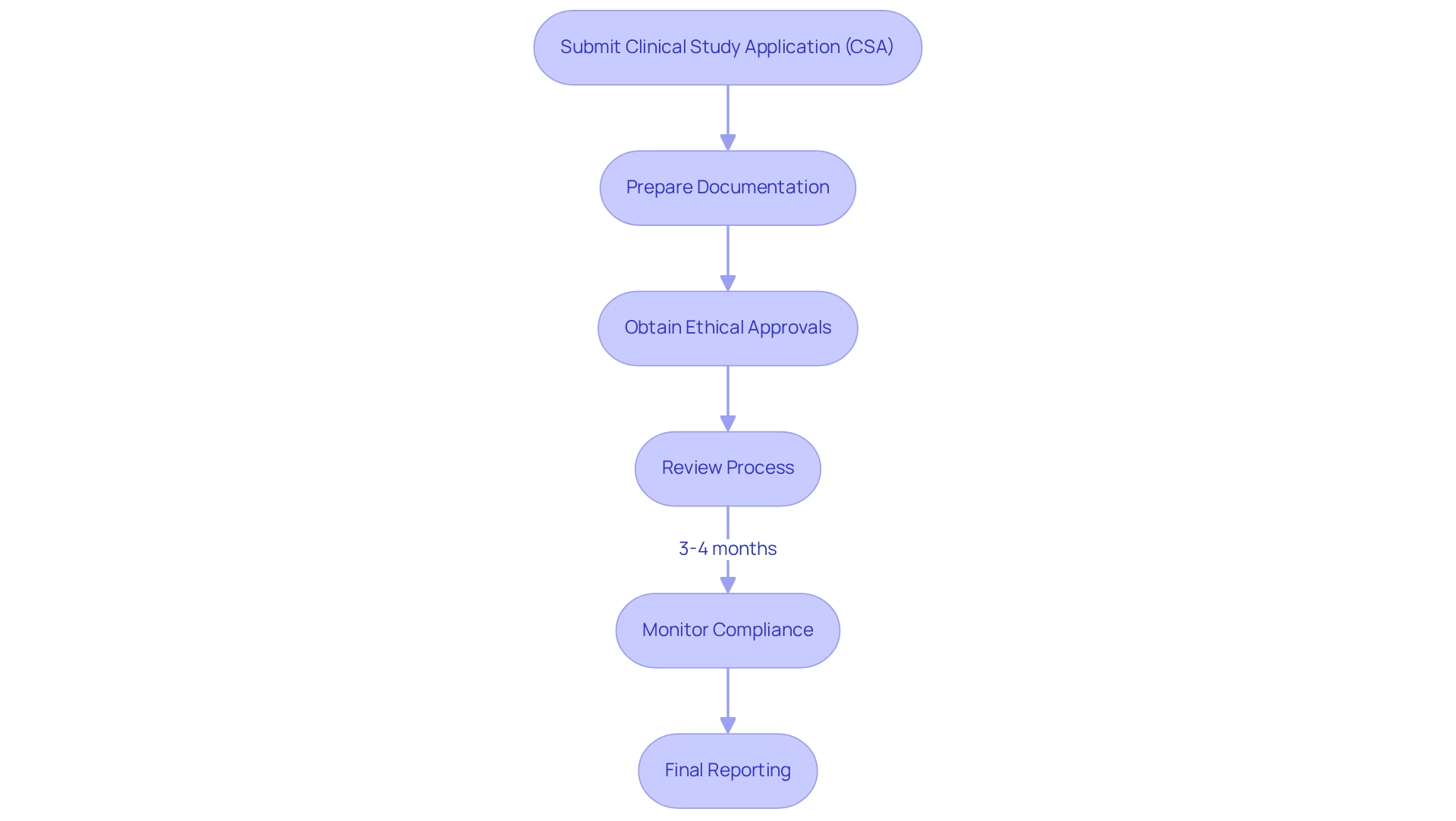
In Mexico, the Federal Commission for Protection Against Sanitary Risk (COFEPRIS) serves as the regulatory authority overseeing multi-site trials and monitoring research studies. To initiate a medical study, researchers must submit a Clinical Study Application (CSA) that encompasses comprehensive study protocols, informed consent forms, and ethical approvals from local ethics committees, particularly vital for multi-site trials. A thorough understanding of the General Health Law and the Health Research Secondary Regulations is essential for compliance, especially given that the approval process is generally efficient, with timelines averaging between three to four months.
To facilitate a smooth review process, meticulous preparation of all documentation is imperative. This preparation includes not only the CTA but also a clear outline of the informed consent process, which must explicitly detail any compensation for health damages directly attributable to the research. This requirement is critical for both compliance and participant safety.
Final reports for multi-site trials in Mexico must provide a comprehensive description of results, including a summary, introduction, discussion, and relevant exhibits, highlighting the extensive documentation required.
A notable case study illustrates the importance of effectively navigating regulatory frameworks. In emergency situations where prior consent cannot be obtained, regulations allow for the use of investigational drugs under specific conditions. In such instances, the ethics committee must be informed, and consent should be sought as soon as possible following the emergency.
This provision emphasizes the commitment to participant safety while adhering to ethical standards in multi-site trials, enabling necessary medical interventions during critical situations. As of 2025, COFEPRIS continues to evolve its regulations regarding multi-site trials, making it crucial for researchers to remain informed about any changes that may affect the Clinical Trial Application process. With over 20 years of experience in Medtech, bioaccess® offers a comprehensive approach to research management, encompassing feasibility evaluations, study set-up, initiation, investigator selection, regulatory adherence, and reporting on study status and adverse occurrences. Their voluntary information bank is vital for safeguarding research and financial investments, mitigating risks associated with compulsory disclosures that could provoke inquiries overseas and adversely affect the medical technology landscape.
By leveraging expert insights and grasping the nuances of COFEPRIS regulations, researchers can significantly enhance their chances of successful multi-site trials in Mexico. Furthermore, bioaccess® ensures information security and transparency while supporting regulatory compliance, reinforcing their commitment to client trust and information protection. For any inquiries or concerns regarding data protection, clients are encouraged to contact bioaccess®'s Grievance Officer at info@bioaccessla.com.
Logistical Considerations for Multi-Site Trials: Planning and Coordination
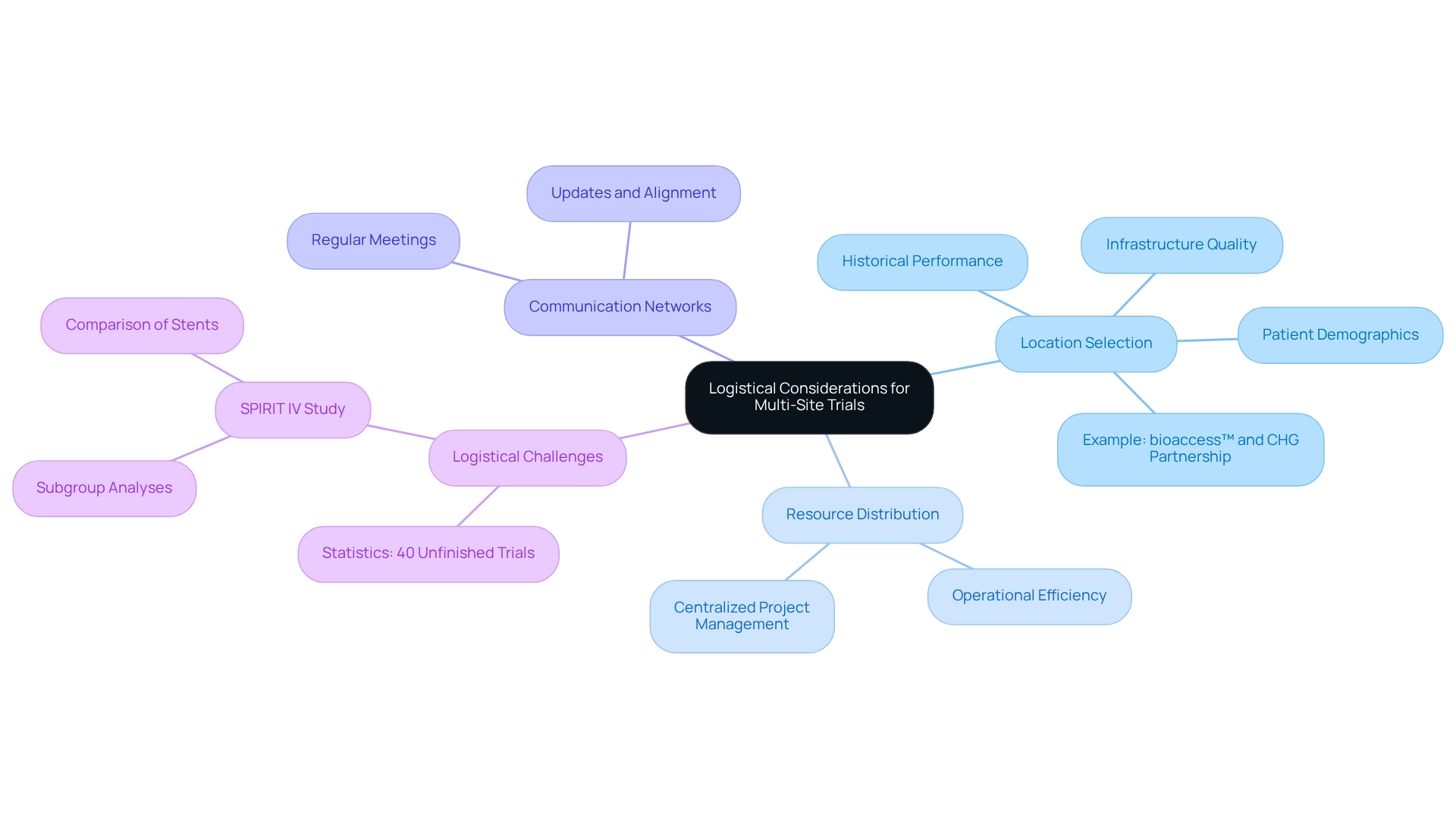
Careful planning and coordination are essential for executing successful multi-site trials in Mexico. Key logistical factors include location selection, resource distribution, and the establishment of robust communication networks among participating sites. Researchers must evaluate potential locations by considering various elements, such as infrastructure quality, patient demographics, and historical performance in medical studies.
For instance, the partnership between bioaccess™ and Caribbean Health Group (CHG), announced on March 29, 2019, aims to position Barranquilla as a premier site for medical studies in Latin America, with support from Colombia's Minister of Health. This initiative underscores the importance of effective location selection and logistical planning in enhancing the attractiveness of research locations.
A centralized project management system can significantly boost operational efficiency by streamlining processes, tracking progress, and ensuring consistent adherence to protocols across all sites. Regular meetings and updates are crucial for maintaining alignment among teams and promptly addressing any emerging challenges. The comprehensive clinical study management services provided by bioaccess™, including feasibility assessments, site selection, compliance evaluations, study setup, ethics committee approval, import permits, project management, and reporting on serious and non-serious adverse events, further illustrate the critical components necessary for successful execution.
The importance of effective site selection and logistical planning is highlighted by the SPIRIT IV study, which compared everolimus-eluting stents with paclitaxel-eluting stents, focusing on subgroup analyses to assess treatment efficacy in diabetic versus non-diabetic patients. This study demonstrated how logistical challenges can influence outcomes, raising questions about regulatory decisions based on subgroup findings. Furthermore, understanding the logistical difficulties encountered in multi-site trials in Mexico is paramount; statistics indicate that 40% of National Cancer Institute-sponsored studies remain unfinished, emphasizing the necessity of meticulous logistical preparation.
By implementing effective site selection strategies and proactively addressing logistical hurdles, researchers can significantly enhance the likelihood of success in their studies. As noted by E. Daly-DeJoy and colleagues, the contributions of various experts can markedly improve the quality of clinical research reviews, reinforcing the need for comprehensive planning and collaboration. Additionally, collaborations such as that of bioaccess™ and GlobalCare Clinical Trials, which have achieved over a 50% reduction in recruitment time and 95% retention rates, exemplify the positive impact of teamwork on study efficiency and outcomes.
Effective Recruitment Strategies for Multi-Site Trials
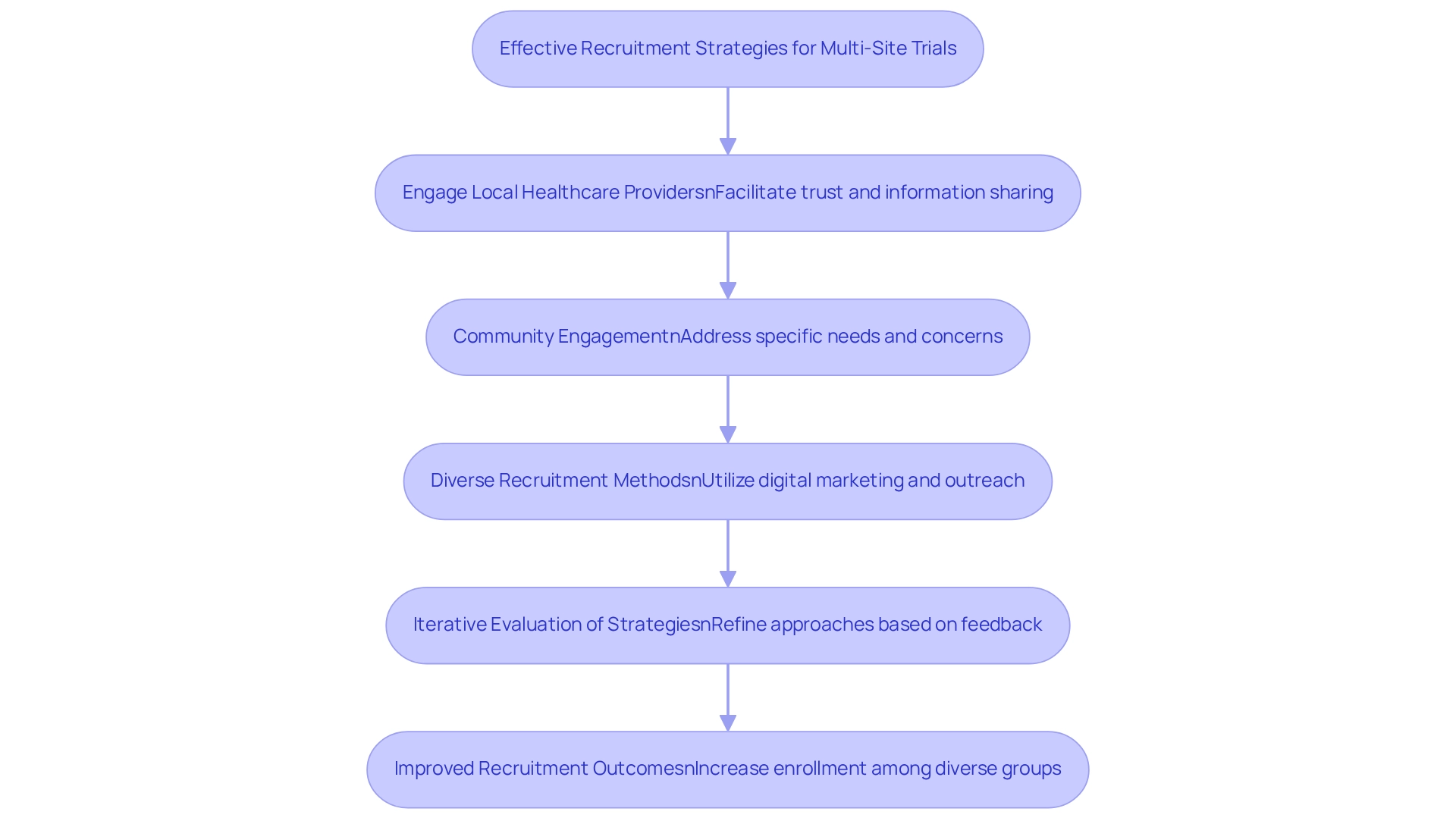
Recruiting participants for multi-site trials in Mexico necessitates a meticulously designed and intricate strategy. Engaging local healthcare providers is essential, as they serve as trusted sources of information for potential participants. Establishing robust connections with these providers not only facilitates recruitment but also fosters a sense of community trust in the research process.
Enhancing recruitment efforts is significantly bolstered by community engagement. By actively involving local populations and addressing their specific needs and concerns, researchers can cultivate a more inviting environment for participation in multi-site trials. This approach has been demonstrated to substantially improve recruitment outcomes, as evidenced by studies that underscore the effectiveness of tailored outreach initiatives.
Grier highlighted the challenges of ensuring that recruitment panels accurately reflect the true diversity of the local population regarding ethnicity and socioeconomic status, emphasizing the necessity of addressing these issues in recruitment strategies.
Employing diverse recruitment methods, such as digital marketing campaigns and outreach to patient advocacy groups, can further amplify these efforts. Such strategies enable researchers to reach a broader audience and ensure that recruitment messages resonate with various demographic groups. For instance, regular evaluations of recruitment strategies, as illustrated in the case study titled "Evaluating The Effectiveness Of Recruitment Strategies," reveal which methods yield the best results based on participant feedback and enrollment metrics.
This iterative process empowers researchers to continuously refine their approaches, leading to improved recruitment outcomes in future studies.
Statistics indicate that effective recruitment strategies can dramatically influence participation rates in multi-site trials. For example, trials that incorporate community engagement initiatives frequently observe a notable increase in enrollment, particularly among underrepresented groups. The partnership between bioaccess™ and Caribbean Health Group aims to position Barranquilla as a leading site for clinical research in Latin America, supported by Colombia's Minister of Health.
This initiative not only enhances recruitment efforts but also contributes to local economic growth through job creation and healthcare improvement. A literature search limited to articles published between 1995 and 2021 underscores the ongoing relevance of these findings. By providing transparent details regarding the study's advantages and addressing potential concerns, researchers can further promote enrollment and ensure a diverse participant group that reflects the local population's demographics.
This is vital not only for ethical considerations but also for the overall success of the study. Furthermore, bioaccess™ offers comprehensive management services for studies, including feasibility assessments, site selection, compliance evaluations, setup, import permits, project management, and reporting, which are critical to the success of these initiatives.
Training and Communication: Key to Successful Multi-Site Collaboration
Training serves as a cornerstone of successful multi-site clinical studies, ensuring that research staff across various locations are well-versed in study protocols and ethical considerations. Initial training sessions, complemented by ongoing support, are vital for maintaining high compliance standards. Statistics reveal that only 31% of participants completed all tasks in a mobile app-based study, underscoring the need for effective training methods to enhance engagement and adherence.
To foster collaboration, regular communication is essential. Utilizing video conferences, newsletters, and shared digital platforms keeps all team members informed about study progress and emerging challenges. This approach not only facilitates the exchange of information but also strengthens team cohesion.
Establishing a culture of open communication encourages team members to share insights and proactively address issues, which is crucial for the success of multi-site trials. Case studies have shown that effective communication methods, such as structured training programs and regular updates, significantly improve collaboration among research sites. For instance, a study on the challenges encountered by research professionals highlighted that even under optimal conditions, participants struggled to complete training modules. This highlights a gap between training formats and the requirements of research professionals in healthcare, stressing the significance of customizing training to suit the particular context of multi-site studies.
As one attendee noted, "this course should be made available to anyone using/collecting data rather than just statisticians," underscoring the need for inclusive training approaches. Expert insights suggest that successful collaboration strategies include clear communication protocols and the use of technology to bridge geographical gaps. By emphasizing training and effective communication, research teams can improve their operational efficiency and ensure the successful execution of multi-site clinical studies. Moreover, bioaccess® seeks to expedite medical devices earlier through its knowledge and tailored methods, which can be efficiently utilized in the context of multi-site evaluations, including Early-Feasibility Studies, First-In-Human Studies, and Post-Market Clinical Follow-Up Studies.
Bioaccess® provides customized training programs aimed at addressing the distinct requirements of each study, ensuring that all team members possess the essential skills and knowledge to thrive.
Data Management and Monitoring in Multi-Site Trials
Efficient information management in multi-site trials in Mexico is paramount for upholding the integrity and reliability of clinical research results. Establishing standardized procedures for information collection, entry, and monitoring is crucial. The adoption of electronic information capture (EDC) systems significantly streamlines information management processes, enhancing accuracy and reducing the likelihood of errors.
Recent trends reveal that 73% of pharmaceutical companies have transitioned to cloud-based clinical information management systems, a notable increase from 45% five years ago. As Sarah Lee observes, this shift highlights the industry's recognition of the necessity for agile, scalable solutions.
Regular audits and monitoring visits are vital for identifying discrepancies and ensuring compliance with study protocols. By implementing a centralized information management system, researchers can achieve real-time tracking of information across multiple sites, enabling timely interventions when issues arise. This proactive approach can lead to a reduction in testing expenses by 10-25%, a particularly significant consideration for multi-site trials in Mexico, making it a financially prudent strategy.
Moreover, training personnel on information entry protocols and underscoring the importance of data integrity are essential for maintaining high-quality records. Expert opinions indicate that AI-driven discrepancy detection is increasingly recognized as a critical component for healthcare analytics platforms, with 33% of survey participants deeming it essential. The integration of Artificial Intelligence not only enhances decision-making and patient care through the analysis of complex datasets but also raises ethical considerations, such as algorithmic bias and equitable access. This necessitates collaboration among stakeholders to ensure secure and efficient implementations.
In summary, the effective execution of electronic data capture systems in multi-site trials in Mexico hinges on best practices in data management, continuous staff training, and the adoption of innovative technologies that facilitate effective and precise data handling.
Ethical Considerations and Patient Safety in Multi-Site Trials
Ethical considerations in multi-site studies are paramount, encompassing the necessity of obtaining informed consent, safeguarding participant confidentiality, and adhering to the ethical guidelines established by regulatory bodies. Researchers must secure suitable ethics committee approvals at all involved locations before the study commences. To guarantee adherence and comprehension, consistent training sessions centered on ethical practices and patient safety protocols should be mandatory for all staff participating in the study.
A robust informed consent procedure is essential, as research indicates that adherence to informed consent can vary significantly across locations, with some studies reporting compliance rates as low as 60%. This highlights the critical need for clear communication and comprehensive training to enhance participants' understanding. Furthermore, establishing a transparent reporting mechanism for adverse events is vital, ensuring that all sites are well-informed of their responsibilities to protect participant welfare effectively.
Incorporating technology can significantly improve recruitment and participation, especially among underrepresented groups. For instance, utilizing electronic techniques and digital health resources can enhance access, as illustrated in the case study 'Utilizing Technology for Recruitment,' which explores the potential of these tools to boost recruitment and involvement in research studies. However, researchers must remain vigilant regarding the digital divide to ensure equitable participation.
Moreover, GDPR mandates strict anonymization methods for personal information in research publications, underscoring the importance of protecting participant confidentiality. bioaccess® is dedicated to ensuring information security and client trust, with a dedicated Grievance Officer available to address any queries or concerns regarding the processing of personal information. You may contact our Grievance Officer at IMH ASSETS CORP (doing business as "bioaccess®"), 1200 Brickell Avenue, Suite 1950 #1034, email: info@bioaccessla.com. This commitment to transparency and adherence to relevant regulations strengthens the ethical framework within which clinical studies operate.
Expert insights, such as those from Eric Kodish, MD, who noted that "efforts to bolster parental understanding and augment parental participation during the ICC are likely to provide a significant improvement in informed consent," further emphasize the importance of informed consent processes. By addressing these ethical issues and factors, including the transformative role of AI and machine learning in enhancing study design and information analysis, researchers can foster a more ethical and efficient multi-site research environment. Furthermore, our Privacy Policy outlines how we manage personal information, ensuring compliance with protection regulations.
Overcoming Challenges in Multi-Site Trials: Solutions and Best Practices
Carrying out multi-site trials in Mexico presents several frequent difficulties, including variability in location performance, communication barriers, and logistical complexities. Variability in location performance can significantly influence study outcomes; differences in experience and resources among locations may lead to inconsistent data collection and patient management. To alleviate these problems, bioaccess provides extensive clinical study management services, including:
- Feasibility assessments
- Location selection
- Evaluation and feedback on study documents to meet national requirements
This ensures that all participating locations are prepared to follow standardized protocols and guidelines for consistency in study execution.
Effective communication is vital for the success of multi-site trials in Mexico. Establishing consistent communication pathways—such as scheduled updates and feedback sessions—can assist in recognizing potential problems early and promote a cooperative atmosphere among locations. The necessity of effective communication between clinicians and statisticians is essential, requiring a mutual understanding of roles and terminology to improve study execution.
Employing technology, like project management software, can further enhance coordination by offering real-time updates and enabling information sharing across locations. Moreover, statistics indicate that increased annual clinic admission rates correlate with better performance at locations, suggesting that clinics with higher patient volumes may possess more experience and success. Establishing a unified support team focused on assisting locations with their particular difficulties can greatly enhance overall study performance.
This team can provide advice on best practices, resolve issues, and ensure that all sites align with the project's objectives. Additionally, bioaccess assists in the import permits and nationalization of investigational devices, which are essential for study execution. As Dr. John Griffin observed, teamwork is essential in research studies.
By utilizing these strategies, researchers can effectively navigate the complexities of multi-site trials in Mexico, thereby enhancing the quality of their clinical research outcomes and driving global health improvement through international collaboration and innovation in Medtech.
Key Takeaways for Conducting Successful Multi-Site Trials in Mexico
Carrying out successful multi-site trials in Mexico necessitates a comprehensive understanding of the regulatory landscape, meticulous logistical preparation, and effective recruitment strategies. A pivotal factor in enhancing the probability of success (POS) is the involvement of non-industry partners, which has been demonstrated to increase POS by 11.3 percentage points. Establishing transparent communication pathways among all stakeholders is essential, as robust cooperation between sponsors and sites significantly enhances study success.
At bioaccess®, we specialize in extensive research management services, including Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Follow-Up Studies (PMCF). With over 20 years of experience in Medtech, our expertise ensures that studies not only adhere to regulatory standards but also uphold the integrity of the research process. Ethical compliance is paramount in clinical research; for instance, implementing informed consent processes that are culturally sensitive and easily comprehensible to participants can bolster ethical compliance and foster participant trust.
Moreover, employing uniform data management practices across locations can diminish discrepancies and enhance data quality, which is vital for regulatory submissions and overall study credibility.
Case studies from successful multi-site trials in Mexico underscore the importance of prior study quality and the involvement of collaborators in influencing new study outcomes. For example, logistic regression analyses have indicated that effective communication and strong relationships among study locations correlate positively with successful patient enrollment and registration completion. As highlighted in a statistical examination of clinical studies, the quality of prior studies and the engagement of collaborators greatly impacted the success of new studies.
Conversely, prolonged testing periods have been linked to diminished success rates, emphasizing the necessity for effective management of experiments.
Key takeaways for conducting successful multi-site trials in Mexico include:
- Foster collaboration among sites to enhance communication and streamline processes.
- Prioritize ethical compliance to build trust and ensure participant safety.
- Utilize insights from earlier experiments to guide planning and execution.
- Implement robust recruitment strategies that effectively engage local populations.
As Gaurav Agrawal, a senior partner, asserts, "Although pursuing the right science and advancing the best therapeutic assets are fundamental to R&D productivity, increasing patient enrollment and site engagement will ultimately be critical in achieving this ambition." By proactively addressing these challenges and emphasizing collaboration, researchers can significantly enhance the quality and reliability of their clinical trials, ultimately driving advancements in medical technology and contributing to local economic growth.
Conclusion
Conducting multi-site trials in Mexico presents a unique set of opportunities and challenges that can significantly impact clinical research outcomes. Understanding regulatory frameworks, meticulous logistical planning, effective recruitment strategies, and the critical role of training and communication among sites is essential. By leveraging diverse participant demographics, researchers can enhance the generalizability of their findings while ensuring robust data quality.
The emphasis on ethical considerations and participant safety is paramount. Establishing transparent informed consent processes and maintaining participant confidentiality are crucial for building trust and ensuring compliance. Furthermore, integrating technology in data management and monitoring enhances efficiency, allowing for real-time tracking and timely interventions.
Key takeaways underscore the necessity of fostering collaboration among sites, prioritizing ethical compliance, and leveraging insights from previous trials to inform future research. By proactively addressing these elements, researchers can significantly improve patient enrollment and site engagement, ultimately driving advancements in medical technology and contributing to local economic growth. A commitment to meticulous planning and effective communication will pave the way for successful multi-site trials, yielding results that enhance scientific understanding and improve healthcare outcomes for diverse populations.
Frequently Asked Questions
What are the benefits of multi-site studies in clinical research?
Multi-site studies broaden the participant pool and enhance demographic diversity, which leads to more generalizable results. They also bolster statistical strength, elevate participant recruitment rates, and streamline data gathering processes.
Why is conducting multi-site studies particularly important in Mexico?
Mexico's rich demographic diversity allows for more robust findings that reflect a wider range of responses to interventions, which is crucial for the validity of medical research.
How does bioaccess® support multi-site studies?
Bioaccess® offers comprehensive clinical study management services, including Early-Feasibility Studies, First-In-Human Studies, Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies, leveraging over 20 years of experience in Medtech to ensure effective coordination across various locations.
What are the financial implications of managing multi-site studies?
Staffing expenses for non-Calculator studies can be significant, with costs potentially reaching $580,000 for ten sites from the investigators' meeting to the start of recruitment, highlighting the need for efficient coordination to manage costs effectively.
What regulatory authority oversees multi-site trials in Mexico?
The Federal Commission for Protection Against Sanitary Risk (COFEPRIS) is the regulatory authority that oversees multi-site trials and monitors research studies in Mexico.
What is required to initiate a medical study in Mexico?
Researchers must submit a Clinical Study Application (CSA) that includes comprehensive study protocols, informed consent forms, and ethical approvals from local ethics committees.
What is the average timeline for the approval process of clinical studies in Mexico?
The approval process typically averages between three to four months.
What documentation is necessary for final reports of multi-site trials in Mexico?
Final reports must provide a comprehensive description of results, including a summary, introduction, discussion, and relevant exhibits.
How does bioaccess® ensure compliance with COFEPRIS regulations?
Bioaccess® provides a comprehensive approach to research management, including feasibility evaluations, study setup, investigator selection, regulatory adherence, and reporting on study status and adverse occurrences.
What logistical factors are crucial for successful multi-site trials?
Key logistical factors include location selection, resource distribution, and the establishment of robust communication networks among participating sites.
What is the significance of effective site selection in clinical research?
Effective site selection enhances the attractiveness of research locations and can significantly impact the success and efficiency of clinical studies.
How can logistical challenges affect multi-site trials?
Logistical challenges can influence study outcomes and may lead to unfinished studies, as evidenced by the statistic that 40% of National Cancer Institute-sponsored studies remain incomplete.
What role does collaboration play in enhancing clinical research?
Collaborations among experts can markedly improve the quality of clinical research reviews and streamline processes, leading to better study efficiency and outcomes.




