Introduction
In the realm of medical devices, navigating the intricate landscape of regulatory approval is paramount for manufacturers aiming to ensure safety and efficacy. Premarket Approval (PMA) stands as a cornerstone of this process, particularly for high-risk devices that demand rigorous scrutiny before they can reach the market. This comprehensive pathway, governed by the U.S. Food and Drug Administration (FDA), not only safeguards public health but also enhances the credibility and market positioning of manufacturers.
As the PMA process evolves, understanding its critical components—from clinical trials to post-approval compliance—becomes essential for any organization seeking to thrive in the competitive Medtech industry. This article delves into the multifaceted PMA journey, offering insights into its significance, the application process, and best practices for successful navigation.
Understanding Premarket Approval (PMA): Definition and Importance
Premarket Approval (PMA) represents a critical regulatory pathway established by the U.S. Food and Drug Administration (FDA) specifically for high-risk medical instruments. This stringent procedure requires that manufacturers provide extensive clinical information to prove the safety and effectiveness of their products prior to market entry. Understanding the intricacies of PMA is essential, as it plays a vital role in safeguarding patient health and ensuring public safety by permitting only those products that comply with rigorous standards.
A well-executed premarket approval application not only enhances a manufacturer’s credibility but also significantly influences its market positioning and viability. The significance of PMA is highlighted by the views of FDA officials, including Lauren K. Roth, who states, 'The PMA procedure is critical for maintaining device safety standards.' Additionally, a recent survey indicated that 33 of 59 participants (55.9%) think a three-fourths majority should be necessary for recommending approval, emphasizing the perceived importance of this procedure.
In 2024, the PMA procedure is poised for potential enhancements, driven by collaborative efforts from key stakeholders aimed at refining the panel system and the Federal Advisory Committee Act (FACA). Additionally, recent trends indicate stability in De Novo Classifications with 24 approvals through June, while Panel Track approvals have declined by about 6.6% year-over-year, with only 13 approvals in the first half of 2024. As the landscape evolves, manufacturers need to recognize that the success of their premarket approval application can be a determining factor in their operational success and market acceptance.
Leveraging accelerated medical equipment clinical study services in Latin America by bioaccess®, which boasts over 20 years of experience in Medtech and a customized approach to clinical trial management, including:
- Early-Feasibility Studies
- First-In-Human Studies
- Pilot Studies
- Pivotal Studies
- Post-Market Clinical Follow-Up Studies
Along with the expertise of professionals like Katherine Ruiz in Regulatory Affairs for medical equipment and in vitro diagnostics, can significantly streamline the process of the premarket approval application and enhance the likelihood of approval.

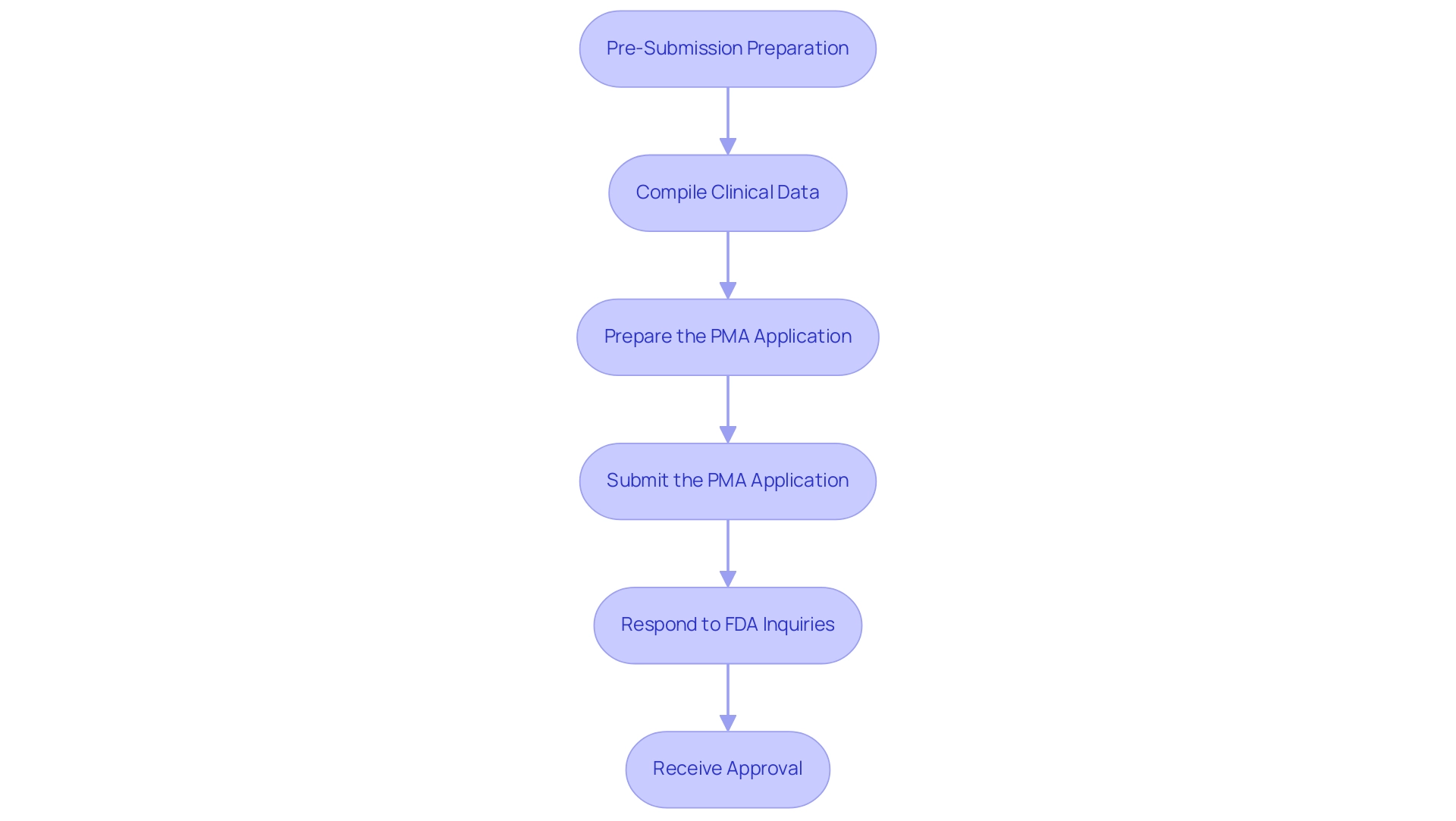
Step-by-Step Guide to the PMA Application Process
- Pre-Submission Preparation: Begin by conducting comprehensive research to familiarize yourself with the specific requirements of the premarket approval application relevant to your product. Gather all required documentation, which should include detailed descriptions of the equipment, proposed indications for use, and any pre-existing clinical data to support your application.
Compile Clinical Data: Leverage the expertise and flexibility of bioaccess® to design and execute robust clinical trials aimed at collecting information on your product's safety and efficacy. This includes Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), and Pivotal Studies, all while ensuring compliance with Good Clinical Practice (GCP) standards and meticulous documentation to facilitate the review process.
- Prepare the PMA Application: Fill out the PMA application form thoroughly, ensuring accuracy across all sections. This includes providing summaries of your clinical data, comprehensive manufacturing details, and labeling information that adheres to regulatory standards for your premarket approval application.
Submit the premarket approval application: Once the premarket approval application is complete, submit it to the FDA along with the requisite fees. Organize all documents systematically to ensure they are easily accessible during the review.
- Respond to FDA Inquiries: Stay prepared to address any inquiries or requests for additional information from the FDA throughout their review period. Providing timely and comprehensive responses is vital for maintaining momentum in the premarket approval application process.
Receive Approval: After a thorough review, the FDA will issue an approval letter, granting you the ability to market your device in the U.S. As a reference, the average approval time for Class III devices is approximately 274 days, highlighting the critical nature of thorough preparation. Furthermore, in the first half of 2023, the average approval time for panel-track supplements decreased to 304 days, reflecting a 27% improvement from the previous year. This evolving efficiency within the premarket approval application landscape underscores the necessity for manufacturers to be proactive and prepared.
Interacting with knowledgeable regulatory advisors, like Katherine Ruiz, who focuses on Regulatory Affairs for Medical Devices and In Vitro Diagnostics in Colombia, is essential in this endeavor. For instance, the case study titled 'Ensuring Compliance with FDA’s Premarket Approval Application Requirements' illustrates how a team with former FDA personnel can effectively guide manufacturers through the premarket approval application journey, ensuring compliance and enhancing the likelihood of timely approval.
As Tom Rish aptly noted, assembling the necessary data, insights, and design requirements will likely take several rounds of internal revisions, underscoring the importance of meticulous preparation in navigating the PMA landscape. With more than 20 years of experience in Medtech, the bioaccess® team provides the high expertise and flexibility necessary to navigate the complexities of the PMA procedure, including compliance reviews and project management, ensuring a comprehensive approach to clinical trial management.
Navigating Challenges: Timelines, Costs, and Best Practices
The process for the premarket approval application can be both lengthy and resource-intensive, with the FDA aiming for a review period of approximately six months for applications. However, actual review times can extend beyond this target, often reaching 180 days or more, particularly for intricate products that necessitate extensive clinical data. Expenses related to the premarket approval application can vary greatly, ranging from tens of thousands to several million dollars, influenced by various factors such as device complexity and the necessary clinical studies.
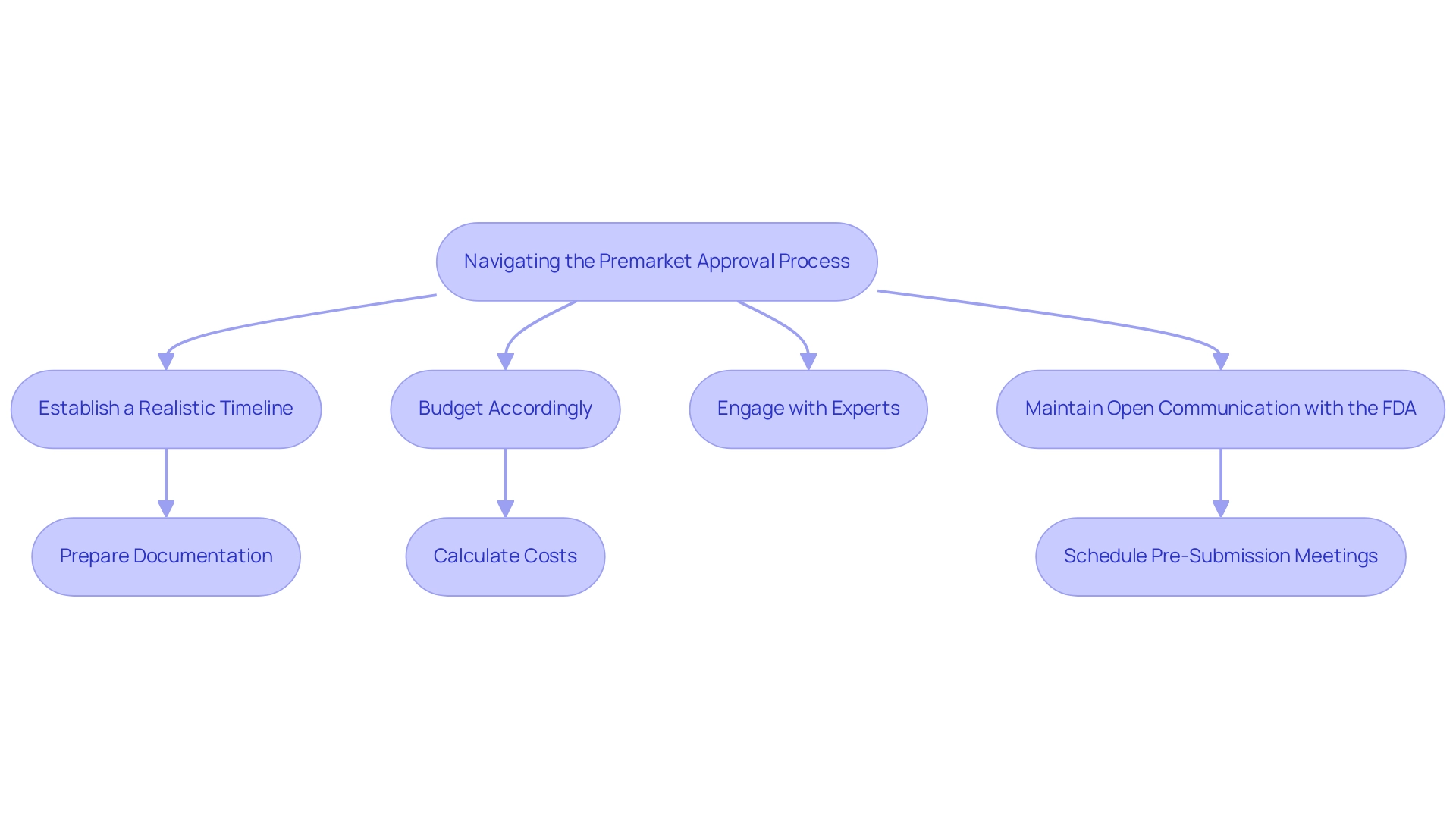
To effectively navigate the challenges of the PMA process, consider the following best practices:
- Establish a Realistic Timeline: Begin the process early to provide sufficient time for each phase, ensuring that all necessary documentation and data are in place before submission.
- Budget Accordingly: Account for all potential expenses, including clinical study costs, application fees, and any additional testing mandated by the FDA.
- Engage with Experts: Collaborate with regulatory consultants or legal advisors who specialize in PMA submissions. Their expertise can simplify the application procedure and enhance the quality of your submissions.
- Maintain Open Communication with the FDA: Utilize pre-submission meetings to clarify expectations and requirements, which can help mitigate delays and misunderstandings.
For those seeking assistance, bioaccess® provides extensive clinical study management services, including feasibility assessments, site selection, compliance evaluations, and study setup. With over 20 years of Medtech experience, bioaccess® specializes in managing Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies (PMCF). Their adaptability and specialized expertise guarantee that each test is steered towards success, addressing the distinct challenges of the premarket approval application procedure.
A notable example of successful PMA navigation is ZuriMED Technologies AG, which recently received FDA clearance for its FiberLocker® System. This case underscores the importance of effectively managing the premarket approval application timeline and indicates that following best practices can lead to favorable outcomes. As noted by Sebastian Rodriguez-Elizalde, M.D., "Intellijoint Surgical welcomes Sebastian Rodriguez-Elizalde, M.D. to the Scientific Advisory Board," emphasizing the value of expert guidance in navigating regulatory challenges.
Furthermore, the latest statistics indicate that the costs related to the premarket approval application for 2024 have continued to evolve, reflecting ongoing trends in the industry. As an additional insight, average approval times for panel-track supplements reported in the first half of 2023 show a decline to 304 days, reflecting a 27% improvement from 2022 levels. Such statistics suggest ongoing enhancements in the FDA's efficiency in processing applications, a trend that can further aid in planning and execution.
The Role of Clinical Trials in Securing PMA Approval
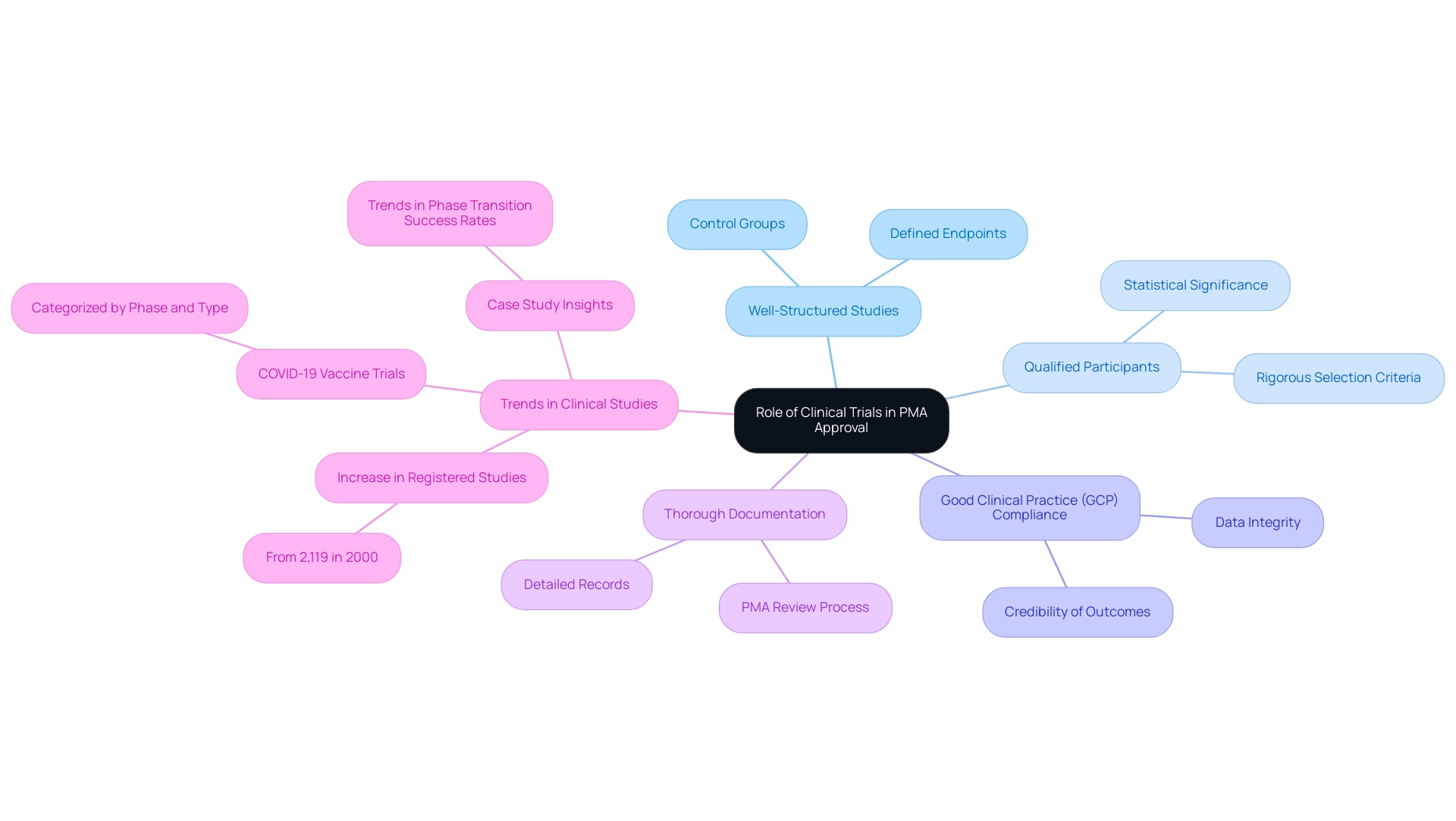
Clinical studies are crucial to the premarket approval application process, serving as the basis for demonstrating the safety and effectiveness of medical devices. The FDA mandates robust clinical evidence, which encompasses several critical elements:
- Designing Well-Structured Studies: Effective studies must be meticulously crafted to meet regulatory standards, incorporating appropriate control groups and clearly defined endpoints to ensure comprehensive evaluation.
- Recruiting Qualified Participants: The selection criteria for participants should be rigorously defined to ensure that the data collected is relevant and statistically significant.
- Adhering to Good Clinical Practice (GCP) Standards: Compliance with GCP is vital to safeguard the integrity of the data, thereby reinforcing the credibility of the outcomes.
- Documenting Results Thoroughly: Keeping detailed records of all study activities is crucial, as thorough documentation will play a pivotal role during the PMA review process.
Research indicates that successful clinical studies can markedly increase the likelihood of obtaining a premarket approval application. For instance, a study titled Trends in Phase Transition Success Rates Over Time highlights a notable correlation between improved drug licensing practices and the successful application of biomarkers, suggesting that enhanced methodologies can lead to greater productivity in drug development. This is especially pertinent considering the substantial rise in registered clinical studies, which expanded from merely 2,119 in 2000 to a considerably higher figure today, reflecting a strong development in the field.
Furthermore, the recent increase in COVID-19 treatment vaccine experiments, classified by phase and type as of June 2022, further demonstrates the essential role of clinical evaluations in the premarket approval application process. As the landscape of clinical studies evolves, it is imperative for clinical researchers to design studies that not only adhere to FDA requirements but also reflect the latest advancements in medical research. Bioaccess® stands out as a vetted CRO and consulting partner for U.S. medical device companies in Colombia, leveraging over 20 years of experience in Medtech to offer comprehensive clinical study management services, including Early-Feasibility, First-In-Human, Pilot, Pivotal, and Post-Market Follow-Up Studies.
They offer a tailored method to guide firms toward acquisition, ensuring that all study designs are effective and compliant. Andrew W. Lo aptly summarizes the importance of this endeavor, stating,
The views and opinions expressed in this article are those of the authors only, and do not necessarily represent the views and opinions of any institution or agency, any of their affiliates or employees, or any of the individuals acknowledged above.
By employing effective trial designs and fostering a culture of meticulous documentation and compliance, the pathway to achieving a premarket approval application becomes significantly more attainable.
Post-Approval Compliance: Ensuring Continued Market Access
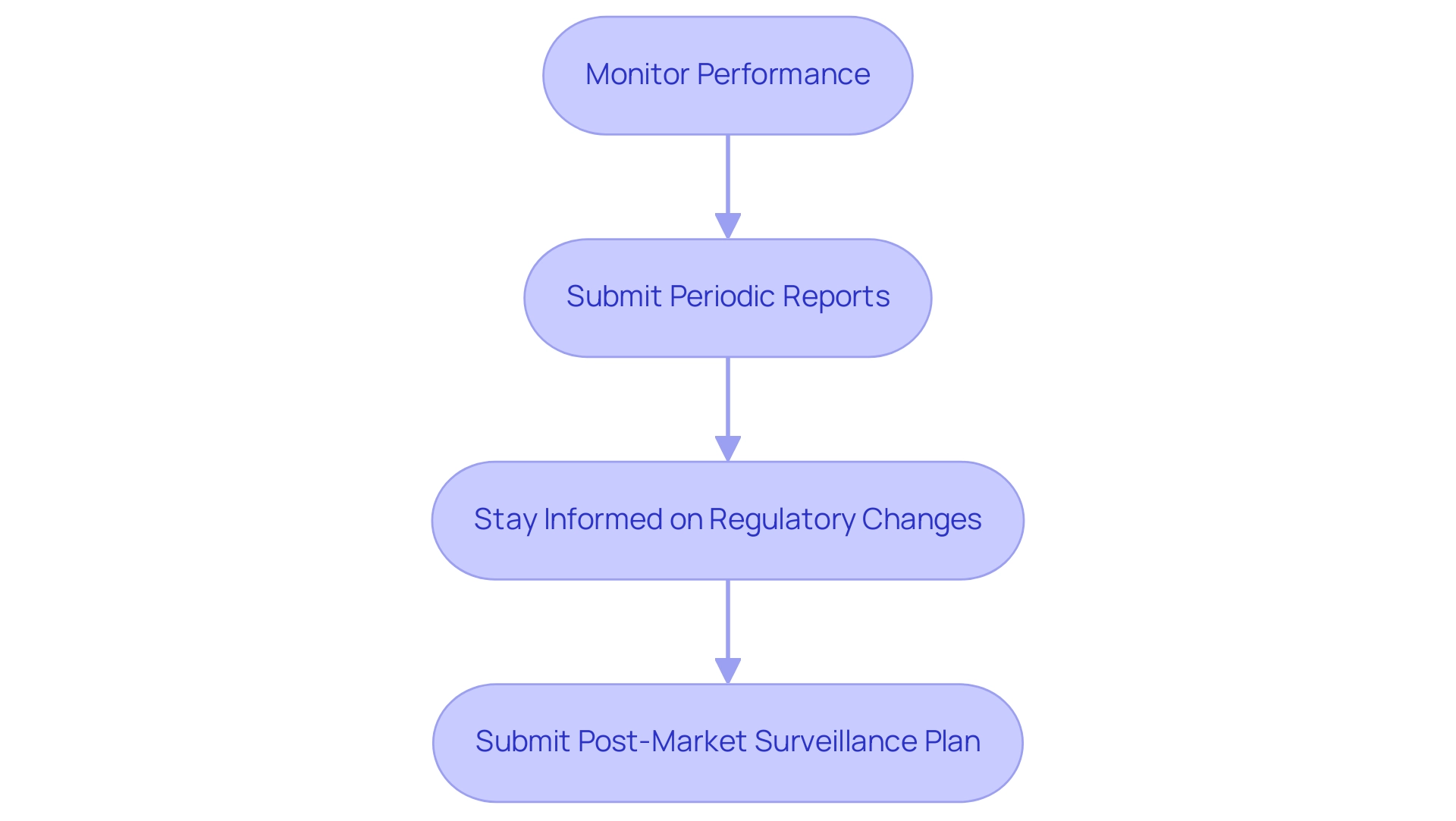
Following the receipt of the premarket approval application (PMA), manufacturers are faced with critical post-approval compliance requirements that are essential for maintaining market access and ensuring patient safety. In 2020, there were numerous types of medical equipment present in general hospitals across Japan, highlighting the vast market landscape that manufacturers must navigate. Key obligations include:
-
Monitor Performance: It is imperative for manufacturers to conduct regular assessments of the performance of the product in the marketplace. This includes a proactive approach to identifying and reporting any adverse events to the FDA, ensuring that potential issues are addressed swiftly.
-
Submit Periodic Reports: Depending on the equipment and its associated risks, manufacturers may be mandated to provide periodic reports that detail the safety and effectiveness of the product, drawing on real-world data to support their findings. Continuous adherence to the quality standards established during the premarket approval application procedure is vital. This entails regular audits of manufacturing processes to ensure that they conform to regulatory expectations and maintain the integrity of the product.
-
Stay Informed on Regulatory Changes: The regulatory landscape is dynamic, and manufacturers must remain vigilant to any changes that could impact their post-approval obligations. Adapting to new regulations in a timely manner is crucial for sustained compliance.
Moreover, manufacturers are required to submit a post-market surveillance plan to the FDA, detailing how they will meet these regulatory requirements. By diligently fulfilling these post-approval compliance requirements, manufacturers not only secure their market position but also play a pivotal role in safeguarding patient safety. As emphasized by industry expert Katie Hobbins, maintaining a strong compliance posture is essential for long-term success in the medical equipment sector. For instance, the case of INSIGHTEC EXABLATE, which received marketing authorization under submission number P150038 on July 11, 2016, underscores the significance of effective monitoring post-approval. The ongoing oversight of device performance ensures that manufacturers can swiftly respond to any emerging safety concerns, thereby reinforcing their commitment to both regulatory adherence and patient welfare.
Conclusion
Navigating the Premarket Approval (PMA) process is essential for manufacturers of high-risk medical devices, as it plays a pivotal role in ensuring both safety and efficacy. Understanding the intricacies of this regulatory pathway—from the initial submission of clinical data to the critical post-approval compliance requirements—empowers manufacturers to strategically position themselves in a competitive market. The significance of a well-executed PMA application cannot be overstated; it not only enhances the credibility of the manufacturer but also fosters trust in the medical devices that ultimately benefit patient health.
The journey through the PMA process involves meticulous preparation, clear communication with regulatory bodies, and a commitment to quality throughout clinical trials. By adhering to best practices, including engaging with experienced regulatory consultants and maintaining open lines of communication with the FDA, manufacturers can effectively navigate the complexities of the approval process. Furthermore, robust clinical trials are paramount in demonstrating the safety and effectiveness of devices, underscoring the necessity of a structured approach to trial design and participant recruitment.
Post-approval compliance remains equally critical, as it ensures that devices continue to meet safety standards and perform effectively in the market. Manufacturers must proactively monitor device performance, submit periodic reports, and stay informed about regulatory changes to maintain their market access. By prioritizing compliance and fostering a culture of accountability, manufacturers not only safeguard patient welfare but also secure their long-term success in the medical device industry. The evolving landscape of PMA highlights the importance of diligence and adaptability, reinforcing that a thorough understanding of regulatory processes is key to thriving in this dynamic field.




