Introduction
The implementation of a Quality Management System (QMS) is crucial for medical device manufacturers. It is a comprehensive and structured system that ensures medical devices meet regulatory standards and deliver on safety, efficacy, and quality. In this article, we will explore the key components of a QMS, the benefits of implementing one, the regulatory requirements, and best practices for achieving excellence.
We will also discuss the role of technology in QMS and how it is revolutionizing the medical device industry. Join us on this journey of understanding the importance of QMS in delivering high-quality medical devices and ensuring patient safety.
What is a Quality Management System (QMS) for Medical Devices?
The establishment of a Quality Management System (QMS) is vital for manufacturers of healthcare equipment. It is a comprehensive and structured system of policies, processes, and procedures that guide the manufacturing, control, and distribution of medical devices. This ensures they meet the required regulatory standards and deliver on safety and efficacy. The QMS is not a static entity; it is a dynamic framework that aids organizations in setting objectives, monitoring performance, and fostering an environment of continuous improvement.
Quality Assurance (QA) extends beyond mere testing; it is a fundamental aspect of the entire development process. This concept is eloquently described by James Whittaker in "How Google Tests Software," where he states, "Quality is not equal to test. Quality is achieved by putting development and testing into a blender and mixing them until one is indistinguishable from the other." This philosophy is actively practiced at Clover for their Clover Assistant application, which supports doctors in making clinical decisions. Their Software Development Life Cycle (SDLC) is structured with gates at each stage to preemptively detect and fix defects and maintain a user-centric design.
Industry leaders, like Jennifer Mascioli-Tudor from Greenlight Guru, emphasize that agility and adaptability are key to an effective QMS. She asserts that a successful quality and regulatory professional must possess robust project management skills, as managing a QMS is not just about the product design but encompasses all ancillary elements contributing to the final product.
Moreover, with more than two million varieties of healthcare equipment in the worldwide market, as estimated by the World Health Organization (WHO), the importance of handling clinical data in accordance with regulations cannot be emphasized enough. Good clinical data management ensures safety for trial participants and allows for the accurate analysis of data, which is a cornerstone of the EU Medical Device Regulation (EU MDR).
Modifications in the design of healthcare equipment, whether in response to feedback from customers, changes in materials, or adjustments in manufacturing, must be carefully documented, and in certain situations, require new regulatory submissions. Understanding the impact of these changes on a gadget's form, fit, and function is essential to maintain compliance and ensure gadget safety and performance.
As the healthcare equipment industry continues to grow, facilities like UL Solutions' laboratory in Rochester Hills, Michigan, expand their testing capabilities to meet increasing demand. Their dedication to enhancing the safety, security, usability, and interoperability of healthcare instruments is proof of the industry's commitment to excellence.
In summary, a well-established quality management system is not only a regulatory necessity; it is the foundation for delivering high-quality products that meet consumer expectations and contribute to sustainable business success. It is a journey of quality that every manufacturer must embark on to ensure that their products not only meet the current standards but also adapt to the evolving landscape of technology.
Key Components of a Quality Management System for Medical Devices
The implementation of a Management System (QMS) for medical devices is a multifaceted process designed to ensure device excellence and safety. A thorough QMS is based on a Quality Policy that expresses the organization's commitment to excellence and establishes its objectives. This is supported by a Manual of Excellence, which outlines the organization's management policies, procedures, and roles.
Effective Document Control is crucial, ensuring the creation, review, approval, and upkeep of quality-related documents and records are systematic and regulated. Training and Competency initiatives are vital, confirming that staff have the necessary training and skills to execute their responsibilities proficiently.
Risk Management procedures play a pivotal role by identifying, evaluating, and curtailing risks in the device's design, manufacturing, and utilization. Supplier Management is equally important, involving the careful selection, evaluation, and supervision of suppliers to ensure the high standard and dependability of components and materials procured.
Corrective and Preventive Actions are established to pinpoint, investigate, and rectify nonconformities, as well as to prevent their recurrence. Monitoring and Measurement are employed to assess the performance of processes, products, and the QMS itself. Additionally, Management Review sessions are conducted regularly by top management to evaluate the QMS' effectiveness and pinpoint improvement areas. Lastly, Continuous Improvement processes are instituted to identify enhancement opportunities and to bolster the QMS's effectiveness and efficiency.
In the spirit of integrating excellence into the development process, as emphasized by James Whittaker, the QMS is not a standalone element but an integral part of product development, ensuring that testing and quality are inseparable. This philosophy is exemplified in applications like the Clover Assistant, which supports clinical decision-making, reflecting the proactive integration of excellence from the earliest stages of the Software Development Life Cycle (SDLC).
Moreover, the agility and adaptability of a QMS, as highlighted by Jennifer Mascioli-Tudor, underline the necessity of strong project management capabilities within the quality and regulatory realm. This inclusive strategy guarantees that all additional components contributing to the finalized healthcare equipment are considered, spanning from design to post-market clinical data gathering, as observed with tools like Greenlight Guru Clinical.
In today's digitally-driven environment, real digitalization is about understanding crucial data and using it to guide business and compliance objectives. This intelligent digitization allows more than just attaining desired results; it guarantees the attainment of essential objectives, unraveling the intricacies of healthcare technology, and promoting progress in the sector.
Benefits of Implementing a Quality Management System for Medical Devices
A Quality Management System (QMS) in the realm of healthcare instruments is a organized system of procedures and processes covering all aspects of design, manufacturing, supplier management, risk management, complaint handling, clinical data management, and more. It is the backbone that ensures a product's safety, efficacy, and quality, while also maintaining compliance with regulatory requirements such as those set by the FDA and the European Union's Medical Device Regulation (MDR).
An effective QMS provides a multitude of benefits. It establishes the foundation for consistent manufacturing of top-notch healthcare equipment, which not just fulfills regulatory criteria but also surpasses customer anticipations. This is crucial in an industry where the health and safety of patients depend on the reliability and safety of the equipment they use. With a QMS in place, manufacturers can navigate the complexities of the medical device market with greater confidence and agility, responding to changes with the necessary documentation and without sacrificing compliance. This adaptability is essential, as highlighted by Jennifer Mascioli-Tudor, who emphasizes the significance of project management skills in regulatory and compliance professions.
In the digital era, a digital QMS can integrate with existing systems and technologies, enhancing efficiency and data transparency. As recommended by MedTech Intelligence, establishing distinct goals and developing a phased implementation plan are strategic measures to guarantee seamless integration of digital systems. This approach aligns with the need for a focused adoption of new technologies, without being swayed by the hype surrounding them. It ensures that digital enhancements truly serve the organization's vision and business objectives.
Moreover, a QMS is not merely a compliance obligation; it is a strategic asset that fosters a culture of continuous improvement. This commitment to excellence is what gives organizations a competitive advantage in the industry. According to Mary Joyce from UL Solutions, the capacity to effectively carry out testing of healthcare equipment is evidence of a company's commitment to enhancing the safety and excellence of its products. In Michigan, for instance, there is a flourishing technology sector for healthcare equipment, supported by a skilled workforce and manufacturing capabilities, which enables such rigorous testing and quality assurance processes.
In the end, a successful QMS is a process towards greatness, enabling organizations to provide products that guarantee patient safety and contentment, which are the foundations of sustainable achievement in the healthcare equipment industry.
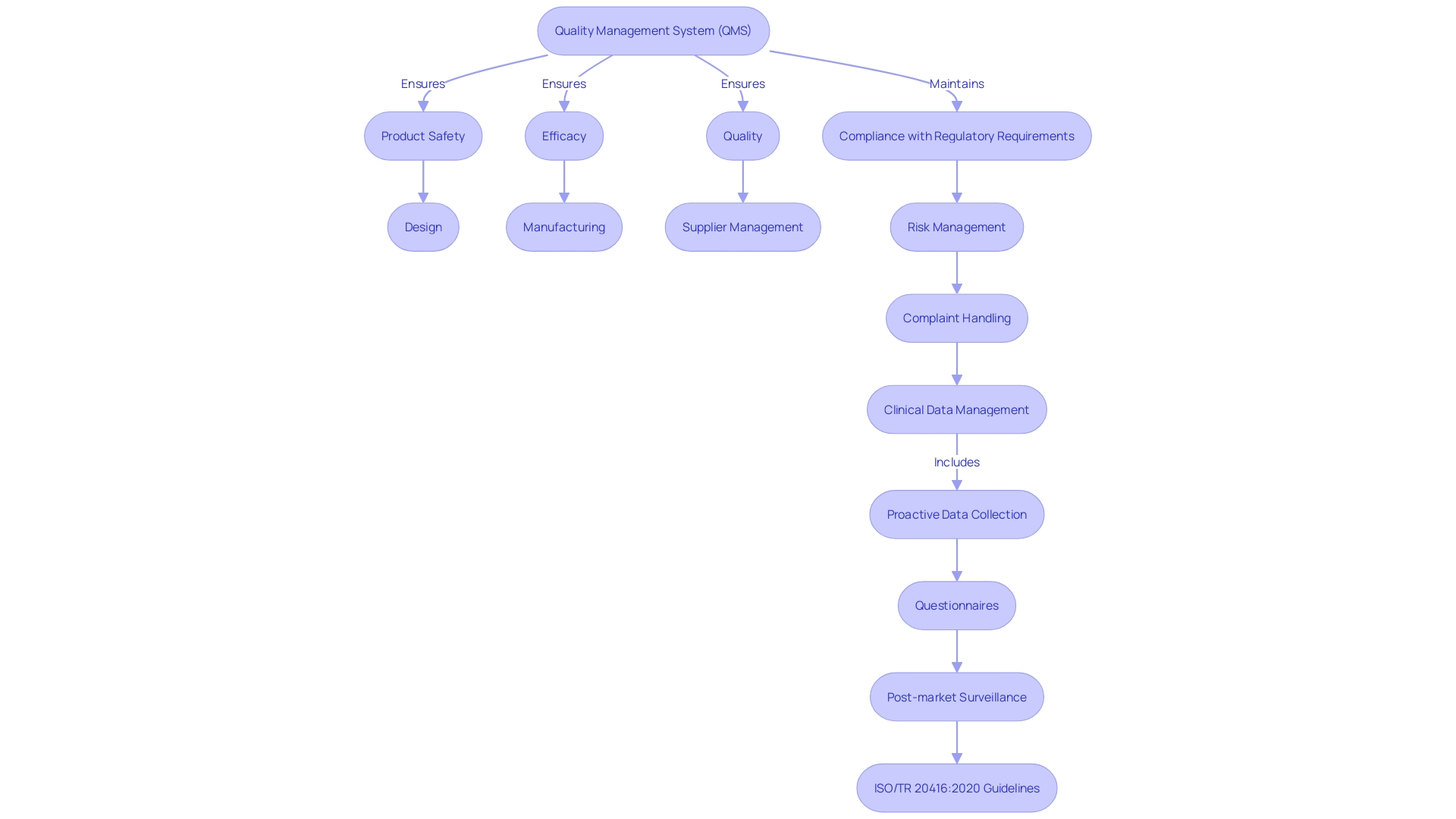
Regulatory Requirements for a Quality Management System for Medical Devices
Quality Management Systems (QMS) are crucial for ensuring the safety, effectiveness, and compliance of healthcare equipment. The regulatory requirements for a QMS vary by country or region, but notable frameworks include the FDA's Quality System Regulation (QSR), ISO 13485, and the European Union Medical Device Regulation (MDR). The FDA's QSR, also referred to as 21 CFR Part 820, imposes regulations for manufacturers of healthcare instruments in the United States. ISO 13485 is a worldwide standard, widely acknowledged for establishing QMS requirements for healthcare equipment. The MDR offers an all-encompassing set of regulations for the creation, production, and distribution of healthcare instruments within the European Union.
In the ever-evolving realm of regulation for healthcare equipment, it's obvious that adopting automation and shifting from traditional, paper-based validation procedures is not just endorsed by governing entities like the FDA but is becoming indispensable. The shift towards automated systems is evident in the insights shared at the Medtech Summit, which highlights the need for the industry to develop more robust and efficient processes.
To navigate this complex regulatory environment, professionals must possess strong project management skills, as noted by Jennifer Mascioli-Tudor from Greenlight Guru. A QMS is not only concerned with the product design; it's a comprehensive framework that integrates every aspect contributing to the ultimate medical equipment. This encompasses an emphasis on risk management, which is essential for the creation of high-quality instruments that meet regulatory requirements and guarantee patient safety.
Moreover, the increasing use of voluntary consensus standards, developed by Standards Development Organizations (SDOs) following principles of transparency and balance of representation, is a testament to the industry's commitment to regulatory quality. These standards are part of a rigorous conformity assessment process that validates whether products or systems meet the specified requirements.
As the industry prepares for the future, improving efficiency in regulatory and safety document preparation is a key priority. Industry experts are continuously seeking ways to streamline these processes to meet standards efficiently, thus minimizing delays and errors while dealing with the impact of increasing regulations. The healthcare equipment industry is actively adjusting to the evolving landscape, as demonstrated by the latest perspectives and pragmatic methods reported by industry experts.
Implementing and Maintaining a Quality Management System for Medical Devices
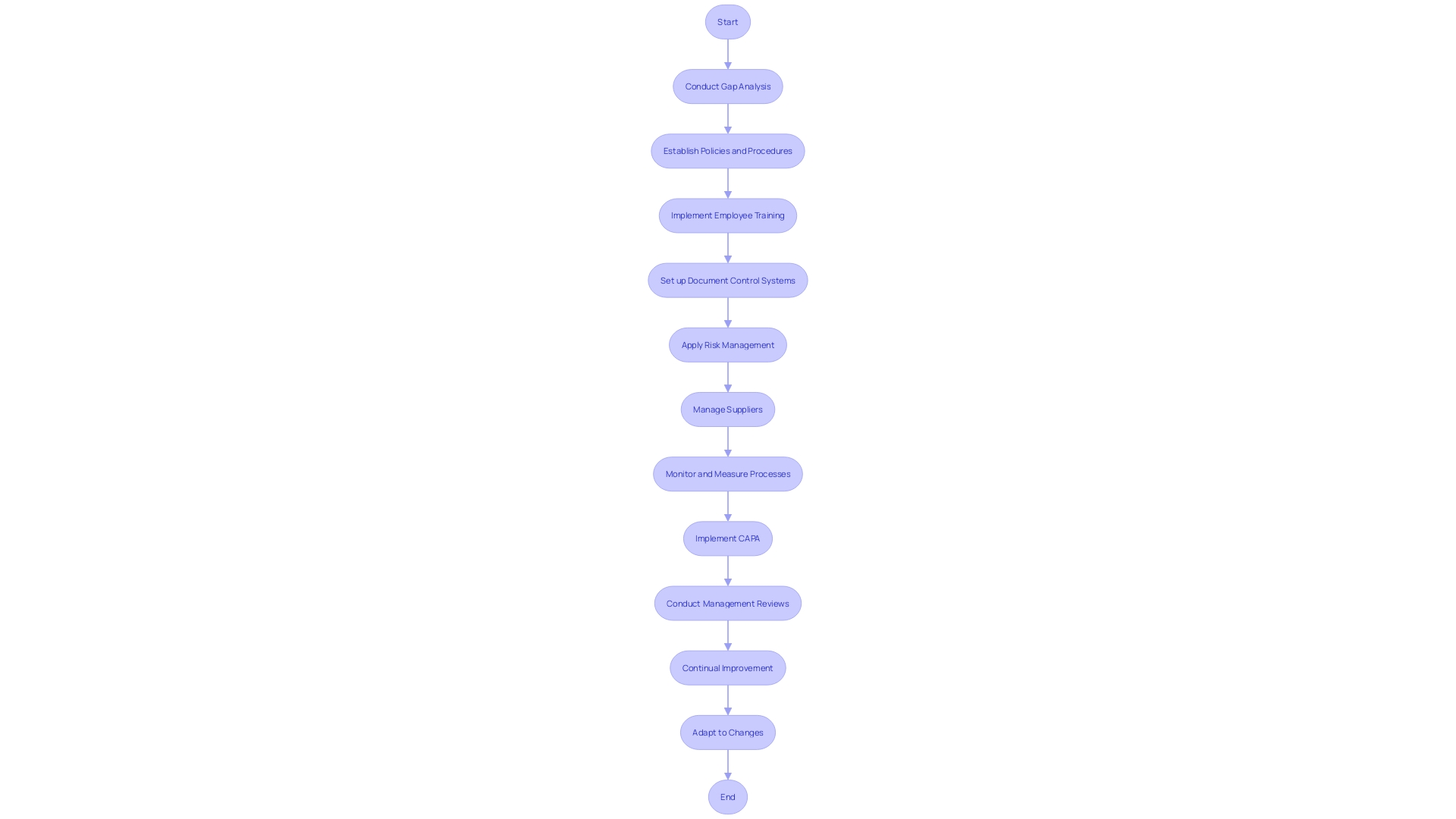
Quality Management Systems (QMS) are crucial for medical manufacturers to ensure products meet regulatory standards and are safe for patient use. A robust QMS should start with a comprehensive gap analysis to understand current practices versus regulatory requirements, such as ISO 13485 or FDA QSR. Establishment of clear policies and procedures is crucial to define management approaches that align with these frameworks.
Employee training is crucial for awareness and understanding of the QMS, highlighting their role in its effectiveness. Document control systems are necessary for managing QMS-related documents, ensuring they are up-to-date and available. Risk management processes help identify and mitigate risks associated with equipment design, manufacturing, and use. Supplier management ensures that suppliers adhere to the required standards, maintaining the integrity of the supply chain.
Monitoring and measurement of the QMS, products, and processes help identify improvement areas. Corrective and Preventive Actions (CAPA) are processes that address nonconformities and prevent their recurrence. Regular management reviews assess QMS effectiveness, identify improvement areas, and allocate resources. Continual improvement should be a cultural goal, encouraging employees to seek and implement enhancements to the QMS.
Adjusting to changes, whether caused by customer feedback, material changes, or manufacturing processes, requires careful consideration of the form, fit, and function of the product, and may require new documentation or regulatory submissions. Utilizing digital QMS solutions, as mentioned by Jennifer Mascioli-Tudor, can offer agility and adaptability, with project management skills being essential for regulatory and compliance professionals.
With the industry for medical equipment flourishing in regions such as Michigan, as mentioned by UL Solutions vice-president Mary Joyce, manufacturers have access to cutting-edge testing facilities to ensure compliance and safety of the equipment. The Compliance Group's subscription package offers tools for streamlined compliant design control and risk management, reflecting the shift towards integrated digital quality management systems. With the World Health Organization estimating two million distinct healthcare equipment on the market, effective Quality Management System implementation is more than a regulatory requirement; it's a commitment to patient safety and product excellence.
Role of Technology in Quality Management Systems for Medical Devices
Advancing technologies in Quality Management Systems (QMS) are transforming the healthcare equipment industry. They facilitate a methodical approach to ensuring equipment meets the utmost standards and adheres to regulatory requirements, ultimately securing the well-being of patients.
- Electronic Document Management Systems (EDMS): EDMS are transformative in creating, storing, and managing electronic documents. They ensure critical control over document versions and provide seamless access and traceability, an essential aspect as per the European Union Medical Device Regulation (EU MDR) which stipulates the safeguarding of reliable and robust clinical data.
Automating the training workflow, these systems are not just about compliance with training requirements but also about ensuring the competency of personnel involved in the lifecycle of healthcare instruments, which is crucial for maintaining instrument effectiveness and efficacy.
- Risk Management Software: This software is indispensable for identifying, evaluating, and mitigating risks. It aligns with the mission of safeguarding subjects in clinical investigations as outlined by the EU MDR, ensuring the well-being of healthcare instruments on the market.
Supplier Relationship Management Systems play a crucial role in maintaining a strong supply chain by handling supplier selection, evaluation, and monitoring, thereby ensuring the quality and compliance of the components used in healthcare equipment.
- Audit Management Systems: These systems enhance the audit process efficiency by automating scheduling, execution, and tracking, in line with the necessity for thorough documentation and potential regulatory submissions for device amendments.
Incorporating technology like the Internet of Things (IoT) and Big Data analytics also plays a crucial role in research and patient care by enabling real-time health monitoring and advanced diagnostics. The healthcare technology market's growth, driven by research and development, along with the evolving digital landscape, calls for smart digitalization that understands the importance of data. As industry experts suggest, 'Real digitalization starts with understanding what data is important and how to break your work product into that data. Smart digitalization acts as an enabler to get you not just where you want to go, but where you need to go.'
The impact of these technologies on QMS is significant, offering improved efficiency, enhanced data accuracy, and a reduced administrative burden, which aligns with the mission of Medical Device How to demystify medical devices and guide industry professionals towards achieving compliance and excellence in their operations.
Best Practices for Achieving Excellence in Quality Management Systems for Medical Devices
In the realm of medical device manufacturing, achieving a superior Quality Management System (QMS) is not a one-time endeavor but a continuous journey that involves rigorous adherence to best practices. To ensure a robust QMS that drives continuous improvement, the following practices are paramount:
-
Top Management Engagement: Leaders must not only endorse quality initiatives but actively engage in the QMS, setting transparent objectives that guide the organization’s quality direction.
-
Process-Oriented Approach: Embrace a systematic view where processes are interconnected. Comprehending and improving these interrelations is crucial to enhancing overall performance.
-
Comprehensive Employee Involvement: Engage employees at all levels in QMS activities to foster a culture of shared responsibility and proactive contribution to standards.
-
KPIs for QMS: Establish clear performance indicators to monitor the QMS's efficacy, facilitating targeted improvements and enabling consistent progress tracking.
-
Ongoing Skill Enhancement: Invest in continuous training programs that empower employees with the latest knowledge and skills pertinent to maintaining excellence.
-
Risk Management Integration: Incorporate risk assessment within the QMS to proactively identify and address potential issues throughout the product lifecycle, as highlighted by the QMSR's emphasis on safety and product efficacy.
-
Customer-Centric Approach: Understand and fulfill customer needs while incorporating their feedback as a driver for continuous QMS enhancement.
-
Strategic External Partnerships: Forge collaborations with key industry players, including regulatory bodies and associations, to stay abreast of legislative changes and emerging quality trends.
-
Regular Auditing: Perform systematic internal audits to assess the QMS's effectiveness and pinpoint areas for improvement.
The QMSR's acknowledgement of risk management highlights the significance of incorporating such principles into the manufacturing of healthcare equipment. According to the World Health Organization (WHO) estimation, the market accommodates two million categories of healthcare tools, each interacting with numerous patients. Thus, meticulous risk assessment during design, production, and post-market phases is essential for safeguarding patient safety and achieving regulatory compliance. Furthermore, as the qualification of healthcare equipment becomes more intricate because of regulatory and confidentiality obstacles, complying with Good Manufacturing Practice (GMP) and standards such as ISO 13485 and FDA regulations becomes crucial.
The subscription package offered by Compliance Group for startups in the field of healthcare equipment exemplifies how industry-leading technologies and practices can streamline design control and risk management, reflecting a commitment to excellence that resonates across the sector.
Considering the information provided in Greenlight Guru's MedTech Industry Report, the capability of a regulatory professional to efficiently handle projects is vital. This viewpoint aligns with the necessity for agility and adaptability in building a QMS that not only concentrates on the device but also on the ancillary aspects contributing to the end product. Therefore, maintaining a QMS that is well-equipped to meet future quality goals is the hallmark of a high-performing organization in the medical technology field.
Conclusion
In conclusion, a well-established Quality Management System (QMS) is crucial for medical device manufacturers. A comprehensive QMS ensures devices meet regulatory standards, deliver on safety and efficacy, and exceed customer expectations. It encompasses key components such as a Quality Policy, Quality Manual, Document Control, Training and Competency, Risk Management, Supplier Management, Corrective and Preventive Actions, Monitoring and Measurement, Management Review, and Continuous Improvement.
Implementing a QMS brings benefits such as consistent production of high-quality devices, confidence in navigating the market, and the ability to adapt without sacrificing compliance. Digitalization plays a role through technologies like Electronic Document Management Systems, Training Management Systems, Risk Management Software, Supplier Relationship Management Systems, and Audit Management Systems.
Regulatory requirements for a QMS vary, with notable frameworks including the FDA's Quality System Regulation, ISO 13485, and the EU Medical Device Regulation. Strong project management skills are essential, as a QMS involves all aspects of device development.
Best practices for achieving excellence in QMS include top management engagement, a process-oriented approach, inclusive employee participation, key performance indicators, ongoing skill enhancement, risk management integration, a customer-centric approach, strategic external partnerships, and regular auditing. These practices ensure continuous improvement and adherence to quality standards.
In summary, a well-implemented and maintained QMS is essential for medical device manufacturers. By embracing technology, adhering to regulations, and following best practices, organizations can deliver high-quality devices that prioritize safety and satisfaction. This leads to long-term success in the industry.




