Introduction
In the highly regulated medical device industry, compliance with stringent standards is crucial to ensure product safety, effectiveness, and quality. Regulatory bodies such as the FDA in the United States and the EMA in Europe provide comprehensive guidelines that manufacturers must adhere to, with the FDA’s Quality System (QS) regulation being a prime example. Non-compliance can lead to severe repercussions including hefty financial penalties, product recalls, and tarnished reputations, emphasizing the need for a robust electronic Quality Management System (eQMS).
A well-implemented eQMS ensures adherence to regulatory requirements across all processes, from design to post-market surveillance, thereby enhancing patient safety and fostering industry advancement. This article delves into the essential components of an eQMS, the benefits it offers, the step-by-step implementation process, and the critical regulatory considerations to keep in mind, providing a comprehensive guide for medical device manufacturers aiming to achieve and maintain high standards of quality and compliance.
Understanding the Importance of Compliance
Adhering to regulatory standards is essential in the medical equipment sector to guarantee that products are safe, effective, and of the highest quality. Regulatory bodies such as the FDA in the United States and the EMA in Europe provide stringent guidelines that must be strictly followed. For instance, the FDA’s Quality System (QS) regulation outlines essential quality elements that manufacturers must implement, though it allows some flexibility in the specific methods used. This leeway enables manufacturers to tailor procedures to their unique processes and devices, ensuring a comprehensive approach to quality management.
Non-compliance can result in severe consequences, including substantial financial penalties, product recalls, and significant damage to a company’s reputation. This highlights the necessity of establishing an effective electronic Quality Management System. A digital quality management system ensures that all processes, from design through post-market surveillance, adhere to regulatory requirements. The EU's Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR) further highlight the significance of a strong eQMS by presenting a detailed framework for conformity assessments, which encompasses reviews of a manufacturer’s technical documentation and quality assurance system.
Furthermore, risk oversight is a vital element of the Quality Control System Regulation (QMSR). By incorporating risk oversight principles into design, manufacturing, and post-market monitoring, producers can proactively detect and reduce potential hazards. 'This approach not only guarantees adherence but also improves patient safety and aids in the overall progress of the medical equipment industry.'. Adhering to standards like ISO 14971, which provides a thorough framework for risk management, is essential for continuously improving these processes.
Post-market surveillance and monitoring are equally crucial. Manufacturers must establish procedures for monitoring and analyzing post-market information, including complaints and adverse events. This continuous watchfulness aids in recognizing possible hazards and taking suitable measures to lessen them, guaranteeing the ongoing safety and efficacy of medical instruments. Ultimately, a well-executed electronic quality management system aids in the creation and accessibility of high-quality products that comply with regulatory standards and provide optimal patient results.
Key Components of an eQMS for Medical Devices
An effective eQMS for medical devices includes several critical components: document control, training oversight, design control, risk assessment, supplier and equipment oversight, and post-market surveillance. Each of these elements plays a significant role in maintaining adherence and ensuring quality throughout the product lifecycle.
Document control ensures that all documentation is managed and versioned appropriately, facilitating streamlined regulatory submissions and audits. According to RQM+ CEO John Potthoff, leveraging AI in document handling can significantly accelerate preparation processes, thus reducing time-to-market and enhancing compliance confidence.
Training oversight is essential for tracking and maintaining employee competencies. This component guarantees that staff are well-versed in the latest standards and regulations, thereby minimizing errors and improving overall efficiency. Continuous monitoring and iterative improvements in training programs ensure that the QMS evolves in tandem with the business, delivering sustained value.
Design control and risk oversight are crucial for the safe and effective advancement of medical instruments. 'Following guidelines like ISO 14971, which offers a thorough structure for risk oversight, improves patient safety and aids in the progress of the medical equipment sector.'. Highlighting risk oversight is essential in guaranteeing the safety and effectiveness of medical devices.
Supplier and equipment management ensure that all external partners and tools meet stringent quality standards. 'This not only involves the initial choice of suppliers but also continuous evaluations to ensure adherence to international regulations like ISO 13485 and IEC 62304.'. A well-validated eQMS can greatly assist in showing adherence during audits.
Finally, post-market surveillance is essential for monitoring performance once it is in use. Procedures must be established for analyzing post-market information, including complaints and adverse events. This data is crucial for identifying potential risks and implementing necessary actions to mitigate them. As mentioned, understanding how medical professionals utilize your product in the field can provide essential insights for future development and regulatory adherence.
By combining these elements, a quality management system not only guarantees adherence to regulations but also promotes the creation of high-quality medical devices that satisfy the changing needs of the sector and provide the best patient results.
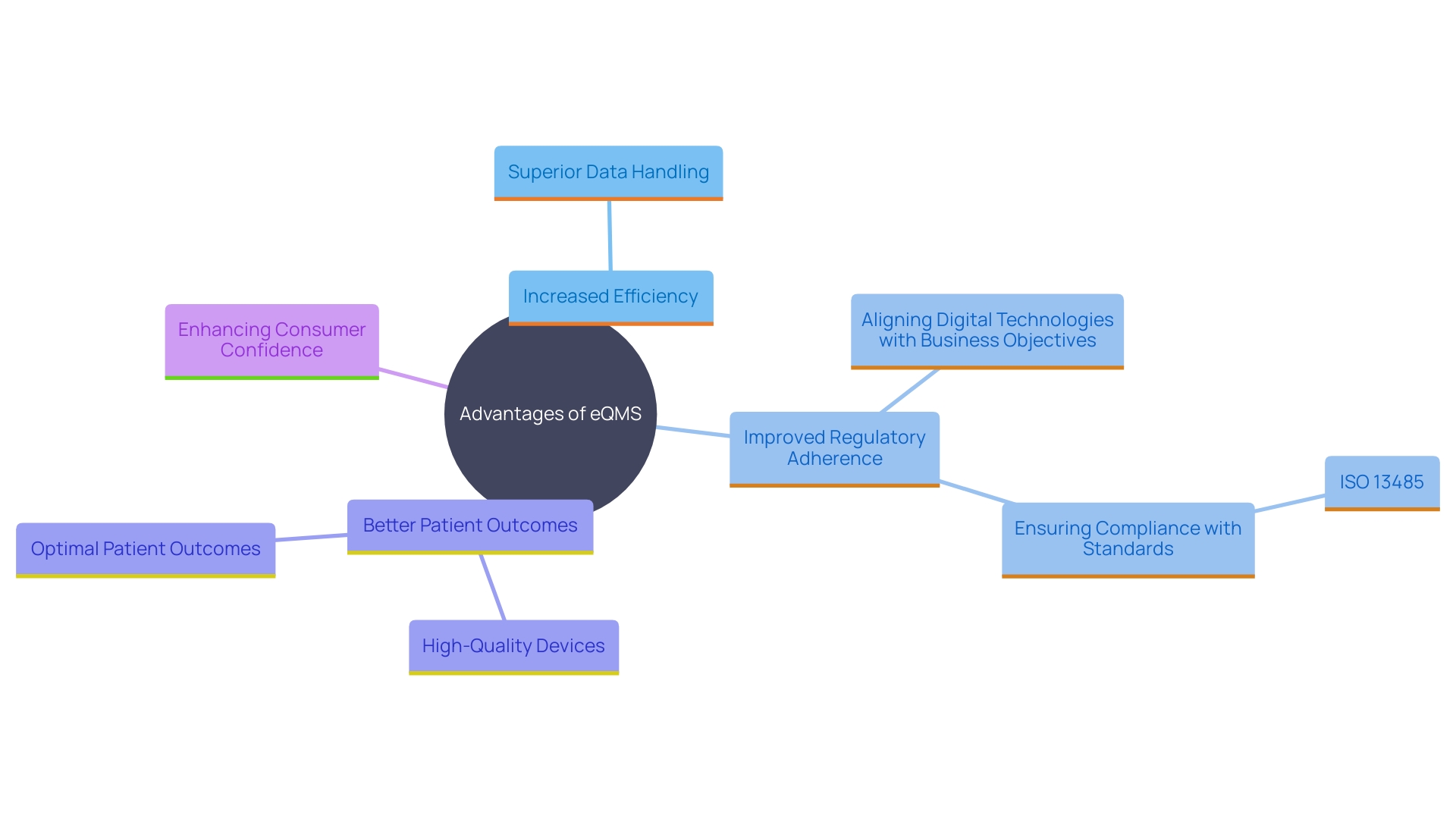
Benefits of Implementing an eQMS
Adopting an electronic Quality Management System provides various advantages, such as increased efficiency, superior data handling, and improved adherence to regulations. By digitizing quality processes, organizations can streamline workflows, reduce errors, and ensure real-time access to critical information. According to industry experts, a well-implemented QMS can lead to better patient outcomes and a stronger market reputation. The system aids in building trust with customers and regulatory bodies by demonstrating a commitment to safety and quality.
Additionally, a quality management system can provide valuable insights through data analysis, aiding in decision-making and continuous improvement efforts. For instance, Conrad emphasizes the importance of aligning digital technologies with business objectives to ensure long-term success. This alignment ensures that the company can proactively identify and mitigate potential risks, leading to the development of high-quality devices that meet regulatory requirements.
Recent surveys reveal that nearly half of respondents lack confidence in the manufacturing process and quality of their medications. This sentiment underscores the critical role of an eQMS in preventing recalls and boosting consumer confidence. Moreover, the integration of digital quality management systems helps in adhering to standards such as ISO 13485, which is crucial for maintaining high standards of quality and safety.
Ultimately, these efficiencies translate into faster time-to-market for medical products, allowing companies to respond promptly to market needs. Continuous monitoring and iterative improvements ensure that the QMS evolves in tandem with the business, delivering sustained value over time. By having a robust QMS, manufacturers can identify potential issues early, take corrective actions, and continuously improve their processes.
Step-by-Step Implementation Process
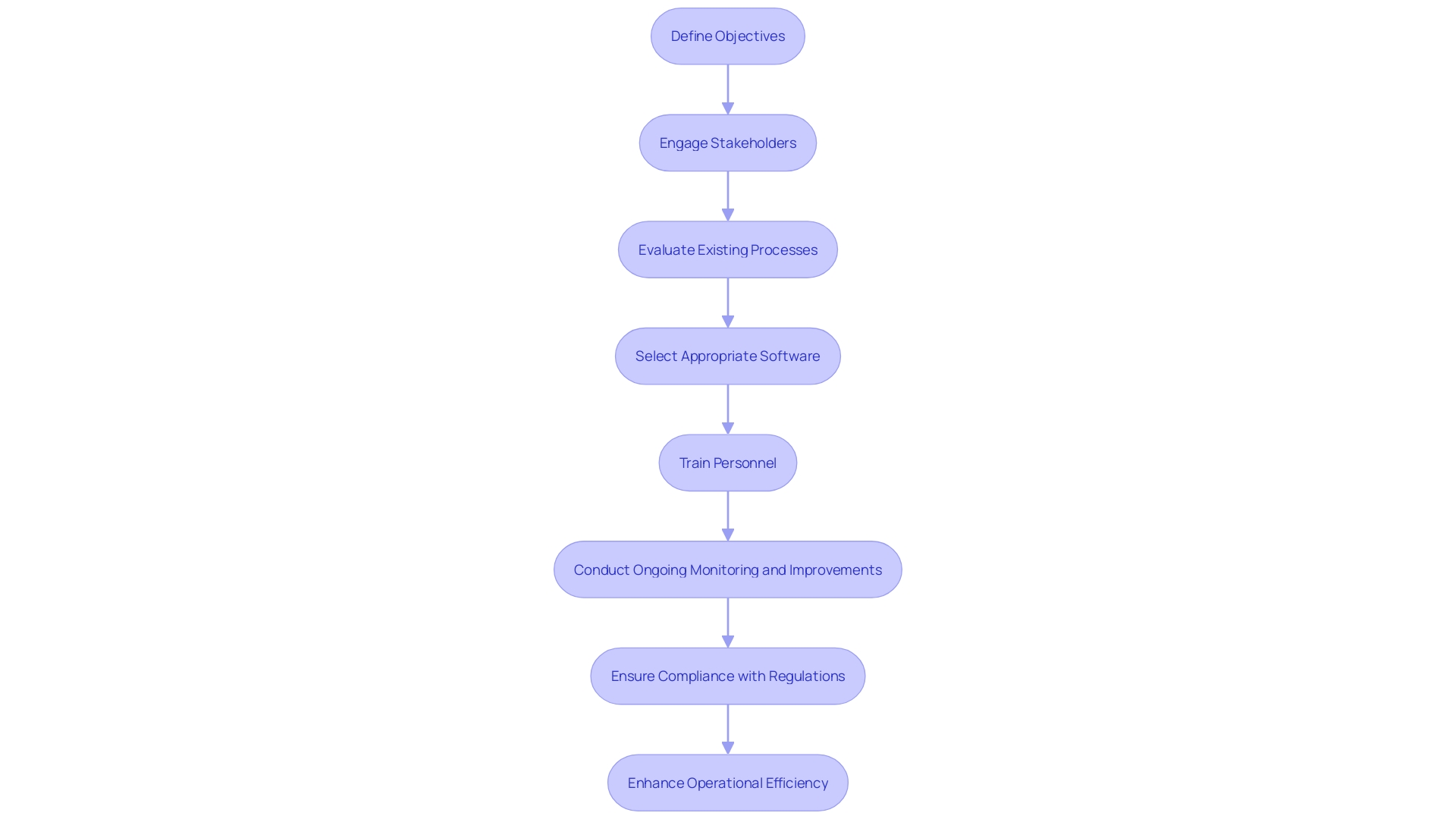
'Implementing an electronic Quality Management System in medical device projects requires a well-structured approach to ensure its efficiency and compliance with stringent regulations.'. The initial step involves defining clear objectives for the system, aligned with both operational goals and regulatory requirements. Engaging stakeholders, including quality and product development teams, from the outset is crucial.
Subsequently, organizations should conduct a thorough evaluation of existing processes to identify areas needing improvement. This involves meticulous planning and gap analysis, ensuring that the chosen quality management system software addresses all identified needs effectively. Advanced analytics can play a pivotal role here, as they help pinpoint improvement opportunities by analyzing data from various stages of the production process.
Choosing a suitable electronic quality management system solution customized to the organization's particular needs is the next essential step. For instance, Greenlight Guru Quality is an enterprise eQMS designed specifically for MedTech companies, guaranteeing adherence to regulations such as FDA's Quality System Regulation (QSR) and the European Union's Medical Device Regulation (MDR).
Once the software is implemented, comprehensive training for all relevant personnel is essential. This guarantees appropriate utilization and observance of set protocols, which is essential for upholding regulations and achieving operational efficiency. Post-implementation, continuous monitoring and iterative improvements should be conducted to measure the system's success. This involves tracking key performance indicators such as defect reduction, compliance rates, and overall efficiency gains.
Moreover, periodic reviews guarantee that the electronic quality management system remains aligned with evolving regulatory standards and business needs. According to a Grand View Research study, over 50% of manufacturers encountered significant non-compliance issues during audits last year, underscoring the importance of ongoing vigilance.
Engaging in post-market surveillance and monitoring is also crucial. This involves analyzing post-market information, including complaints and adverse events, to identify potential risks and take corrective actions. By adopting such a comprehensive approach, organizations can ensure that their electronic quality management system delivers sustained value, enhances market competitiveness, and supports the introduction of innovative products.
Regulatory Considerations and Compliance
Comprehending regulatory factors is essential when establishing an electronic quality management system for medical products. Companies must keep pace with the latest regulations and standards, such as ISO 13485 and the EU Medical Device Regulation (MDR), which has reshaped the legal framework in Europe. For instance, manufacturers must recertify their existing devices to comply with the new MDR requirements during the transition period.
Regular audits and assessments should be integral to the eQMS to ensure ongoing adherence. This includes post-market surveillance and monitoring, where procedures for analyzing post-market information, complaints, and adverse events are established to identify risks and take corrective actions. Interacting with regulatory agencies early in the development process can offer clarity and assistance in fulfilling requirements.
Documenting procedures and maintaining thorough records are essential for demonstrating compliance during inspections. The proposal from the Commission aims to accelerate the launch of parts of EUDAMED by late 2025, enhancing transparency and improving the availability of medical products. This effort is part of a broader initiative to modernize and enhance the EU framework for medical products, ensuring their safety for patients.
By adhering to these standards and continuously improving their risk management processes, manufacturers can enhance patient safety and contribute to the overall advancement of the medical device industry.
Conclusion
Compliance with regulatory standards is essential in the medical device industry for ensuring product safety, effectiveness, and quality. This article emphasizes the importance of adhering to guidelines from regulatory bodies like the FDA and EMA, specifically the FDA’s Quality System (QS) regulation and the EU's Medical Device Regulation (MDR). A robust electronic Quality Management System (eQMS) is critical for achieving compliance and mitigating risks such as financial penalties and product recalls.
Key components of an eQMS—including document control, training management, design control, risk management, supplier and equipment management, and post-market surveillance—play significant roles in maintaining quality throughout the product lifecycle. Each element contributes to a comprehensive approach that enhances patient safety and supports industry advancement.
Implementing an eQMS offers numerous benefits, such as improved efficiency, better data management, and enhanced regulatory compliance. By digitizing quality processes, organizations can streamline workflows, reduce errors, and ultimately achieve better patient outcomes and a stronger market reputation. Continuous monitoring and iterative improvements ensure that the eQMS adapts to changing regulatory requirements and business needs.
A structured implementation process is vital, focusing on clear objectives, stakeholder engagement, and thorough evaluations. Regular audits and ongoing training are crucial for maintaining compliance and operational efficiency. Staying informed about regulatory considerations and engaging with regulatory bodies further supports successful eQMS implementation.
In summary, a well-implemented eQMS not only ensures compliance with stringent standards but also fosters the development of high-quality medical devices, thereby enhancing patient safety and contributing to the overall advancement of the medical device industry.




