Introduction
Design controls are an essential framework in the development of medical devices, ensuring that products consistently meet user needs and comply with stringent regulatory requirements. This structured approach involves a series of meticulously documented procedures that guide the design process, from initial concept to final product. By integrating user-centered design principles, which focus on the diverse needs of all stakeholders including patients, clinicians, and support staff, manufacturers can create devices that are both safe and effective.
The significance of design controls is underscored by their ability to bridge the gap between user needs and verifiable design inputs. As highlighted by industry experts, the goal is not only to design devices that meet technical specifications but also to address real-world problems effectively. This requires an in-depth understanding of user behaviors, needs, and the broader healthcare ecosystem.
The process involves iterative design, usability testing, and comprehensive stakeholder analysis to ensure that all elements work together seamlessly.
Moreover, the evolving regulatory landscape poses additional challenges, with increasing demands for compliance and efficiency. Industry surveys reveal that gaining market approval and ensuring regulatory compliance are top priorities for medical device companies, underscoring the critical role of design controls. By adopting best practices in design documentation, collaboration, and training, manufacturers can navigate these complexities and maintain high standards of quality and safety in their products.
What Are Design Controls for Medical Devices?
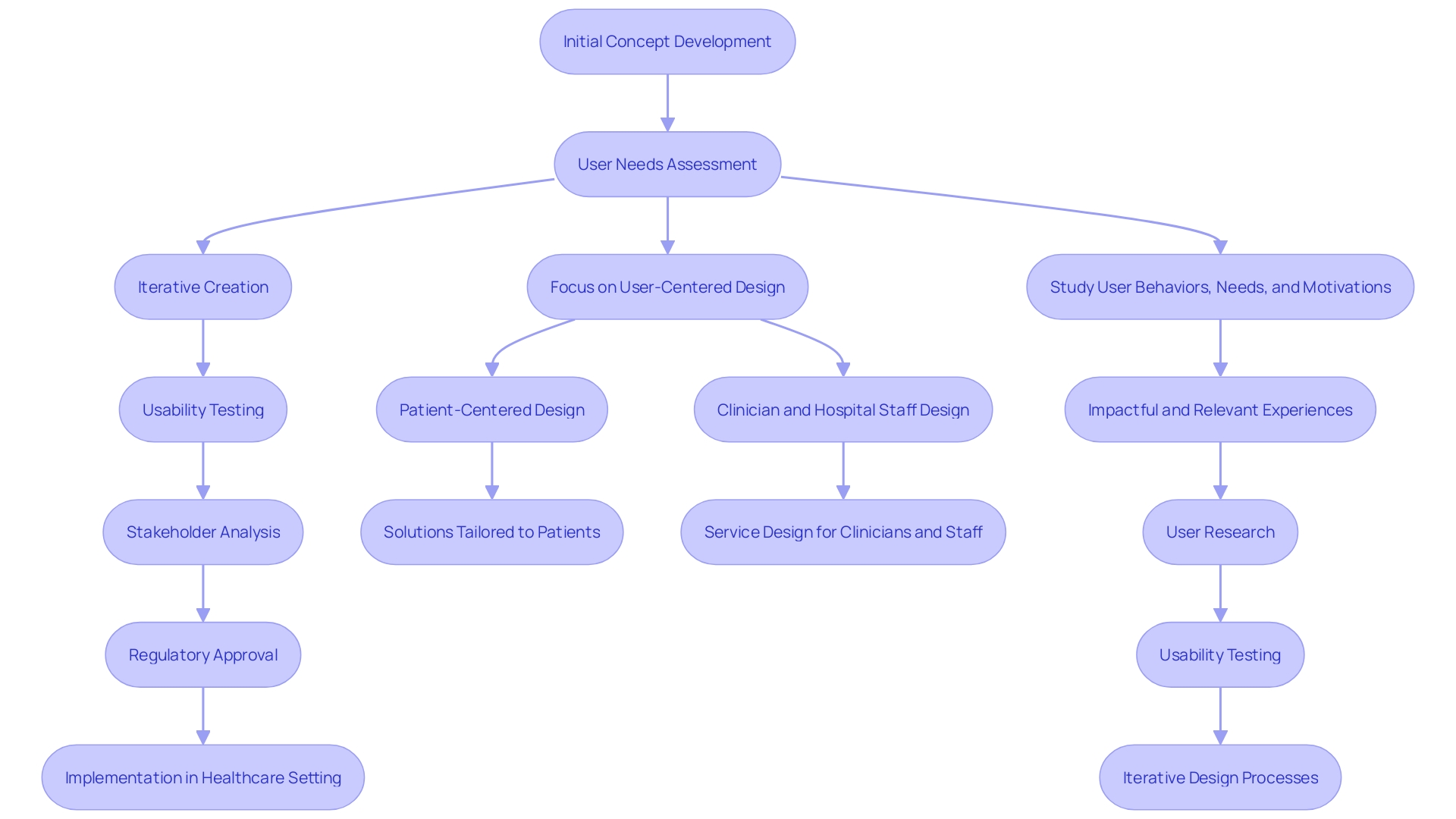
'Design controls are an essential framework in the development of medical instruments, ensuring that products consistently meet user needs and comply with stringent regulatory requirements.'. This structured method involves a series of meticulously documented procedures that guide the creation process, from initial concept to final product. By incorporating user-focused principles, which prioritize the varied requirements of all parties involved including patients, clinicians, and support personnel, manufacturers can produce products that are both safe and effective.
'The importance of controls related to creation is emphasized by their capability to connect user needs and verifiable inputs for creation.'. As emphasized by industry specialists, the objective is not just to create devices that fulfill technical requirements but also to tackle real-world issues efficiently. This requires an in-depth understanding of user behaviors, needs, and the broader healthcare ecosystem. The process includes iterative creation, usability testing, and comprehensive stakeholder analysis to ensure that all elements function together seamlessly.
Furthermore, the changing legal environment presents extra difficulties, with rising expectations for adherence and efficiency. Industry surveys indicate that obtaining market approval and ensuring regulatory adherence are primary focuses for healthcare product firms, highlighting the essential function of control measures. By adopting best practices in documentation, collaboration, and training, manufacturers can navigate these complexities and maintain high standards of quality and safety in their products.
Purpose and Benefits of Design Controls
'Creation controls are essential in mitigating the risk of creation flaws and ensuring the quality of medical devices from conception to deployment in healthcare settings.'. These controls are not only about enhancing product reliability but also about attaining strict compliance with standards such as ISO 13485 and FDA regulations. The integration of Human-Factors Engineering (HFE) and Usability Engineering (UE) into the development process is crucial. This user-centered approach ensures that the needs of all stakeholders, including patients, clinicians, and hospital staff, are addressed, leading to better product usability and patient outcomes.
A considerable advantage of efficient control measures is the promotion of smoother compliance submissions and post-market monitoring. 'With the increasing complexity of compliance requirements, such as those involving e-labeling and the Fast Healthcare Interoperability Resources (FHIR) standard, maintaining adherence is more challenging than ever.'. Nevertheless, planning controls assist in simplifying these procedures, facilitating navigation through the regulatory environment.
Furthermore, implementing development controls enhances communication among team members by providing a structured framework for progress. This collaborative approach ensures that everyone involved, from engineers to healthcare providers, is aligned on the product's goals and requirements. 'In the context of contemporary healthcare instruments, which frequently merge physical and digital components, this alignment is crucial for producing high-quality, safe, and effective products.'.
The emphasis on user-oriented creation, including the specific needs of various user groups, is illustrated in practical applications. For instance, designing for healthcare at the intersection of digital and physical experiences has transformed surgical procedures from purely hardware-based to digitally augmented processes. This evolution highlights the significance of regulatory measures in adjusting to technological progress and improving the overall quality of healthcare instruments.
Design Control Process: Key Elements
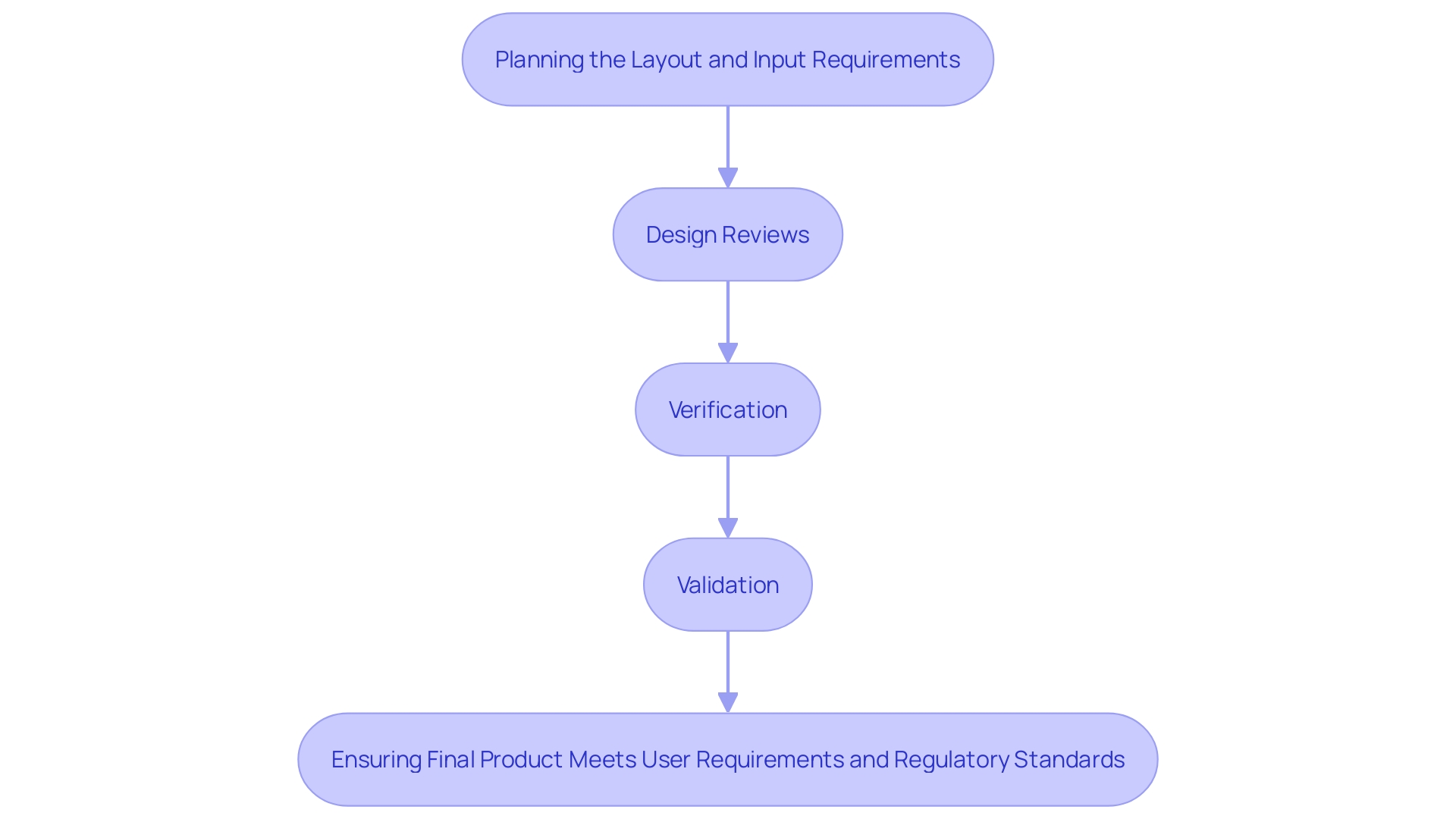
The control process involves several interconnected components that cover the lifecycle of a medical device, ensuring that the final product satisfies both user requirements and regulatory standards. These elements encompass planning of the layout, which establishes the direction and scope of the project, and input requirements, which outline the specific needs and expectations of all users, including patients, clinicians, and support staff. Output specifications then convert these requirements into practical features.
Design reviews are conducted at critical points to assess progress and make necessary adjustments, ensuring alignment with regulatory guidelines. Verification activities confirm that the outputs meet the input requirements, while validation activities ensure that the final product performs as intended in real-world conditions. This holistic approach integrates user-centered concepts, emphasizing the importance of understanding the behaviors, needs, and motivations of all stakeholders involved in patient care.
Integrating Risk Management with Design Controls
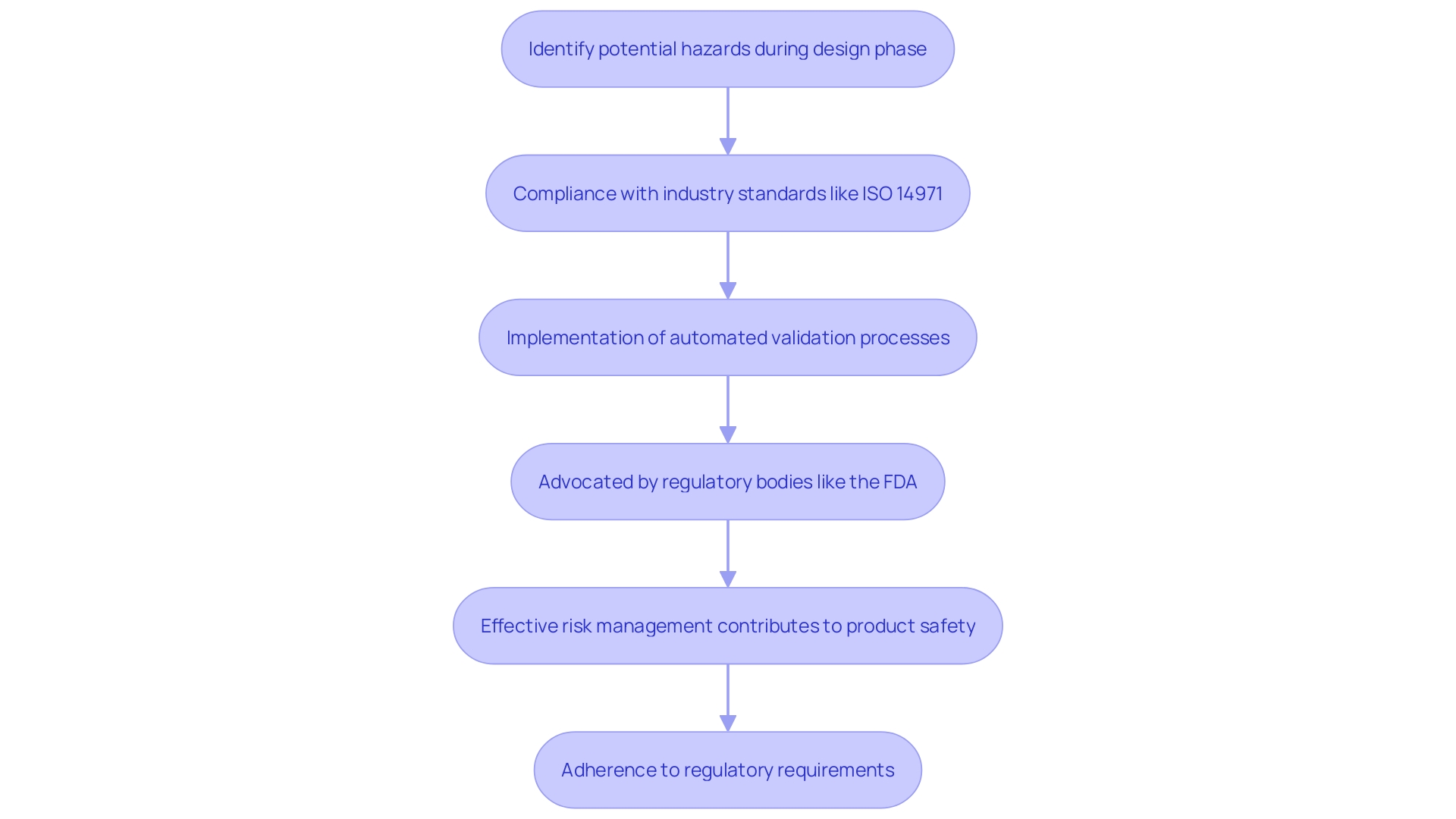
Risk management is essential to the control process for healthcare products, ensuring safety and adherence from the initial phases of development. By embedding risk management principles early in the design phase, manufacturers can proactively identify and mitigate potential hazards. This approach is crucial for meeting stringent industry standards, such as ISO 14971, which emphasizes the importance of risk management in the lifecycle of medical devices.
As the landscape of compliance demands continues to evolve, the need for efficient and effective risk management has never been greater. Regulatory bodies, including the FDA, increasingly advocate for automated validation processes to enhance compliance and efficiency. For instance, the FDA's encouragement towards automated approaches reflects a broader industry trend where digital technologies augment traditional hardware, as seen in the transition from manual surgical tools to digitally integrated systems.
To navigate these complexities, professionals must stay abreast of the latest regulatory requirements and industry best practices. Acquiring proficiency in ISO 14971 not only provides individuals with the essential abilities to establish a compliant risk management framework but also meets the significant need for such expertise throughout the healthcare sector. Bijan Elahi, a seasoned expert in safety risk management for healthcare tools, emphasizes the significance of clear and precise risk management practices in his work, highlighting the essential role of thorough and systematic approaches in ensuring product safety and effectiveness.
Including risk management in the control process is not just a regulatory requirement but a basis for providing safe, effective, and dependable health equipment. By doing so, manufacturers can better navigate the challenges posed by the global nature of the medical device industry, ensuring their products meet the highest standards of quality and safety.
Best Practices for Implementing Design Controls
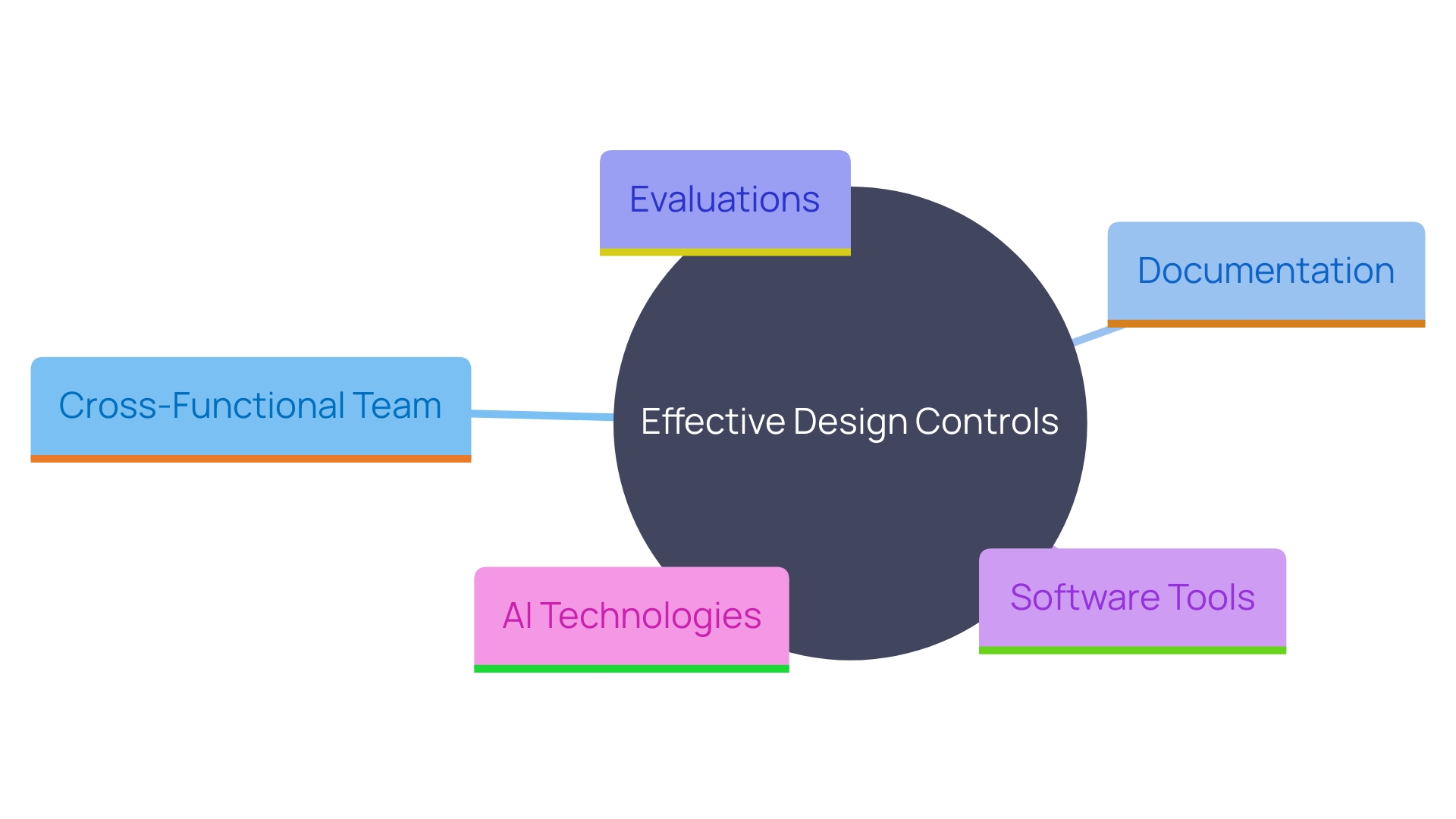
To effectively implement control measures, manufacturers should adopt several best practices. Firstly, establishing a cross-functional team is essential. This team should include stakeholders from various departments such as engineering, quality assurance, Regulatory Affairs, and user experience. By utilizing varied skills, the team can ensure that all elements of the creation and development process are thoroughly considered and addressed.
Maintaining clear and comprehensive documentation is another critical practice. Documentation should encompass every stage of the product lifecycle, from initial concept through planning and development to market launch and post-market monitoring. As noted, “Documenting and responding to a nonconformance is a crucial part of a quality management system for medical device manufacturers.” Detailed records help in tracking changes, ensuring compliance, and facilitating audits.
Frequent evaluations of the layout are essential to recognize and address possible problems early in the development process. These reviews should be conducted at predefined milestones and involve all relevant team members. They offer a chance to evaluate progress, confirm choices, and implement necessary modifications.
Utilizing software tools for documentation and project management can significantly streamline the process. Advanced tools can integrate with existing systems, allowing for seamless management of the product lifecycle. As one industry expert mentioned, “What if we create a system that allows you to use any kind of tool you want to use to manage that same lifecycle.” These tools can assist in generating tests, documents, and reports, thereby enhancing efficiency and ensuring adherence to regulatory requirements.
Furthermore, incorporating AI technologies can augment these processes. AI can assist in generating required tests and documents, actively notifying any deviations from established guidelines, and facilitating communication within the team. “We’ve embedded a lot of AI on top of everything. You can talk to it, you can communicate with it.”
In summary, adopting a cross-functional team approach, maintaining meticulous documentation, conducting regular design reviews, and leveraging advanced software and AI tools are critical strategies for implementing effective design controls in medical device manufacturing.
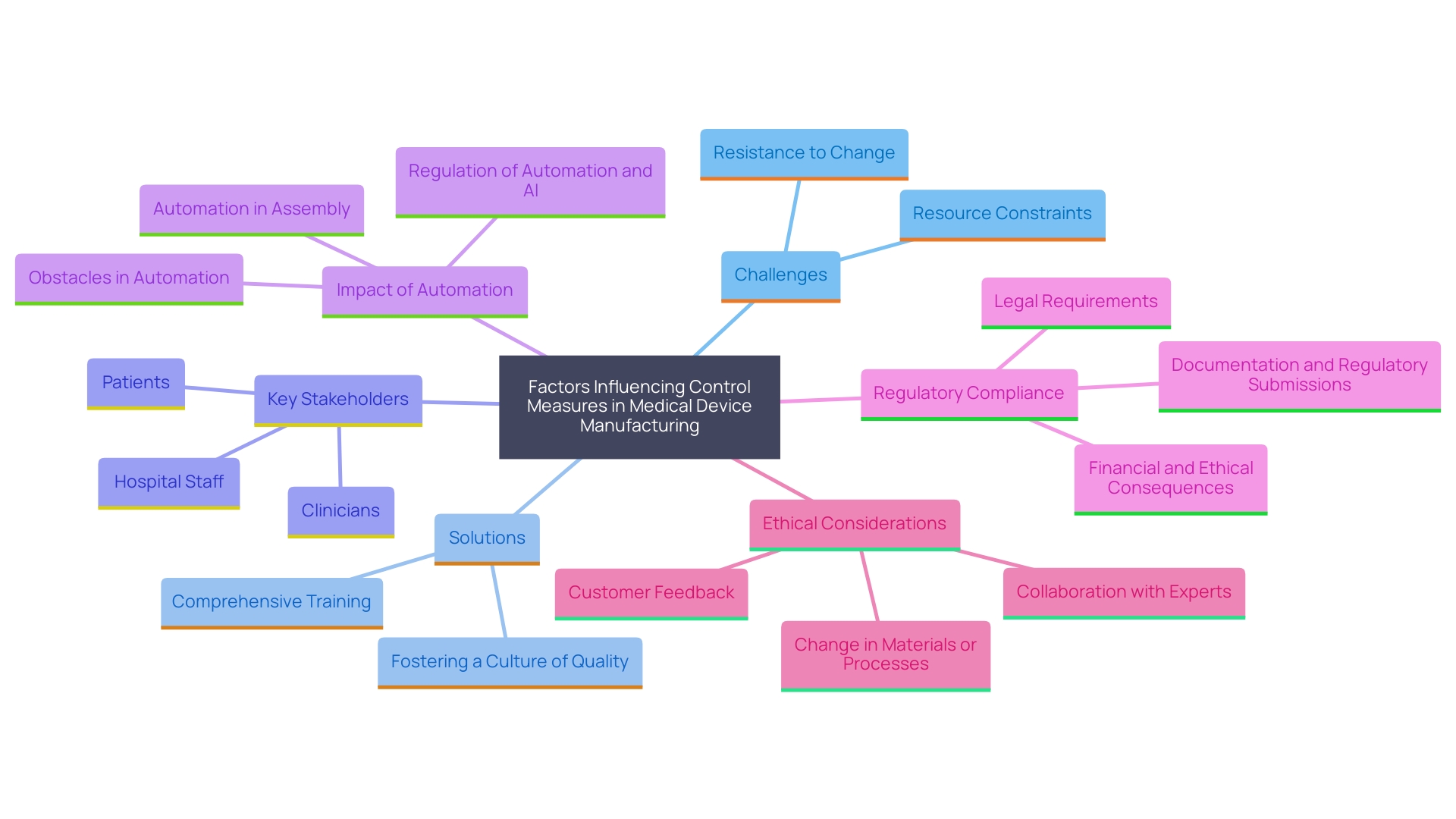
Common Challenges and Solutions
Manufacturers often face significant challenges in implementing control measures, such as resource constraints and resistance to change. To overcome these obstacles, it is essential to provide comprehensive training to team members on the significance of control measures and foster a culture of quality throughout the organization.
Fostering a culture of quality means integrating user-centered design principles that address the unique needs and preferences of all stakeholders, including patients, clinicians, and hospital staff. This comprehensive method guarantees that healthcare instruments are created not only with the patient in focus, but also with regard for the entire medical team that engages with the instrument.
The COVID-19 pandemic has accelerated the adoption of automation and digitalization in medical device manufacturing. Automation plays a pivotal role in enhancing efficiency and collaboration throughout the design process. However, the integration of automation technologies must be done in accordance with stringent guidelines, such as ISO 13485 and FDA regulations, to ensure compliance and quality. Failure to meet these requirements can lead to serious financial and ethical consequences.
Utilizing advanced technology platforms and collaborating with the right partners are crucial steps in navigating the complex regulatory landscape. These tools and partnerships help manufacturers gain a comprehensive understanding of the legal environment, enabling the successful implementation of automation technologies while maintaining compliance with all legal standards.
'Good Manufacturing Practice (GMP) frameworks support the qualification process of healthcare devices, ensuring consistent quality and adherence to international standards.'. However, manufacturers must also address the ethical, legal, and social issues that arise in the design and manufacturing process. By considering market incentives, intellectual property, and other shaping factors, manufacturers can evolve and adapt to the ever-changing regulatory landscape, ultimately improving patient outcomes and advancing medical knowledge.
Conclusion
The comprehensive exploration of design controls in medical device development underscores their vital role in ensuring safety, quality, and compliance. By implementing a structured framework that encompasses user-centered design principles, manufacturers can effectively bridge the gap between user needs and regulatory requirements. This approach not only enhances the usability of medical devices but also addresses real-world challenges faced by stakeholders in the healthcare ecosystem.
The integration of risk management throughout the design control process is essential for identifying potential hazards and ensuring compliance with industry standards. By embedding risk management principles from the outset, manufacturers can mitigate risks and deliver products that meet the highest safety and effectiveness standards. The emphasis on collaboration among cross-functional teams further strengthens the design process, facilitating communication and alignment on product goals.
Best practices such as maintaining thorough documentation, conducting regular design reviews, and leveraging advanced technologies are crucial for overcoming common challenges in implementing design controls. By fostering a culture of quality and embracing automation, manufacturers can navigate the complexities of the regulatory landscape while ensuring that their products are both innovative and compliant.
Ultimately, the commitment to rigorous design controls not only enhances the quality and safety of medical devices but also contributes to improved patient outcomes and trust in the healthcare system. As the industry continues to evolve, adherence to these principles will remain a cornerstone of successful medical device development.




