Overview
Integrating real-world evidence (RWE) into Paraguayan clinical trials is essential for enhancing the relevance and applicability of research findings to local health issues. By leveraging RWE, researchers can align study methodologies with actual patient demographics and treatment practices. This alignment not only improves the accuracy and reliability of trial outcomes but also addresses the regulatory and infrastructural challenges unique to Paraguay. The importance of this integration cannot be overstated, as it directly impacts the effectiveness of clinical research in addressing local health concerns.
Introduction
In the evolving landscape of clinical research, real-world evidence (RWE) stands as a pivotal component, offering insights that extend beyond the confines of traditional clinical trials. This article explores the significance of RWE, particularly in regions such as Paraguay, where unique challenges in conducting clinical trials demand innovative approaches.
By leveraging data from electronic health records, patient registries, and other real-world sources, researchers can enhance the relevance and applicability of their findings, ensuring they accurately reflect the true patient experience.
As the demand for effective healthcare solutions intensifies, understanding how to integrate RWE into trial design and methodology becomes essential. This integration paves the way for improved patient outcomes and more informed healthcare decisions.
Understand Real-World Evidence and Its Importance in Clinical Trials
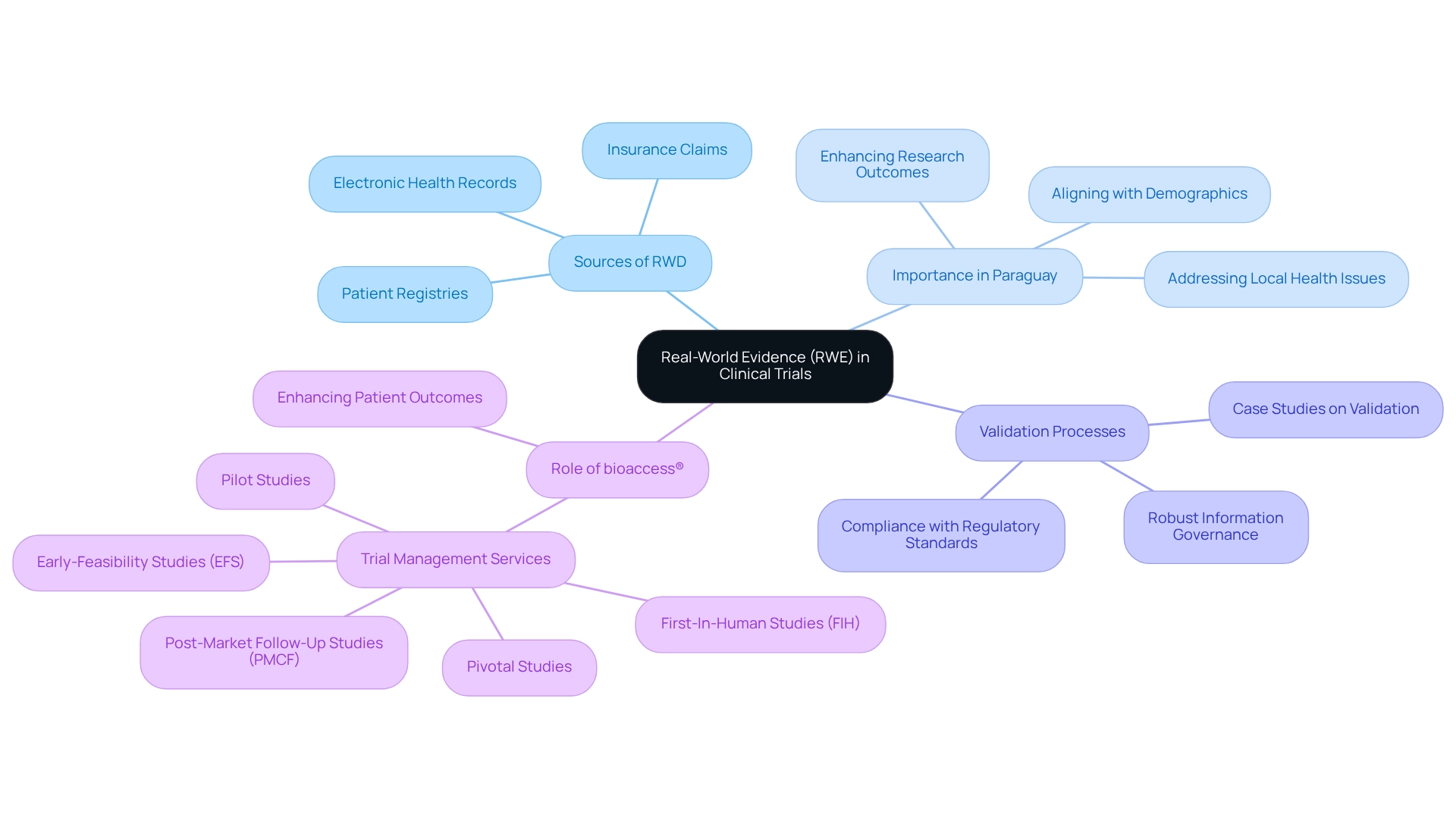
Real-world evidence (RWE) encompasses insights derived from real-world data (RWD), which includes information collected beyond standard medical studies, such as electronic health records, patient registries, and insurance claims.
With over 20 years of expertise in the Medtech field, bioaccess® underscores the importance of RWE in research studies, particularly in 2025, as it provides a comprehensive understanding of treatment efficacy and safety within routine healthcare environments. This relevance is especially pronounced in Paraguay, where the medical research framework may not align with the real-world evidence for Paraguayan trials found in more advanced regions.
By leveraging real-world evidence for Paraguayan trials, researchers can more effectively address local health issues, ensuring that studies accurately reflect the demographics and treatment practices of the actual patient population. The profound impact of real-world evidence for Paraguayan trials on research outcomes is significant. It enhances the relevance of findings by aligning baseline demographics and disease characteristics, which is crucial for precise statistical analysis.
Furthermore, implementing robust information governance frameworks supports regulatory compliance and ensures the reliability of the evidence generated. Recent case studies emphasize that validating RWE tools and processes is essential as the utilization of RWE for regulated purposes continues to grow. This validation, grounded in established guidelines, guarantees that the evidence produced is both reliable and compliant with regulatory standards.
bioaccess®, with its extensive trial management services—including Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Follow-Up Studies (PMCF)—is ideally positioned to facilitate this validation process.
Expert opinions highlight that incorporating RWE into research not only enhances patient outcomes but also aligns with the evolving landscape of medical technology. As Heather Colvin notes, while the role of AI in medical research is expanding, the focus remains on closed-loop AI systems that enhance data analysis and patient care.
The advantages of RWE in medical research are clear: it fosters a deeper understanding of treatment effects, ultimately leading to improved patient outcomes and more effective healthcare solutions.
Identify Challenges in Conducting Clinical Trials in Paraguay
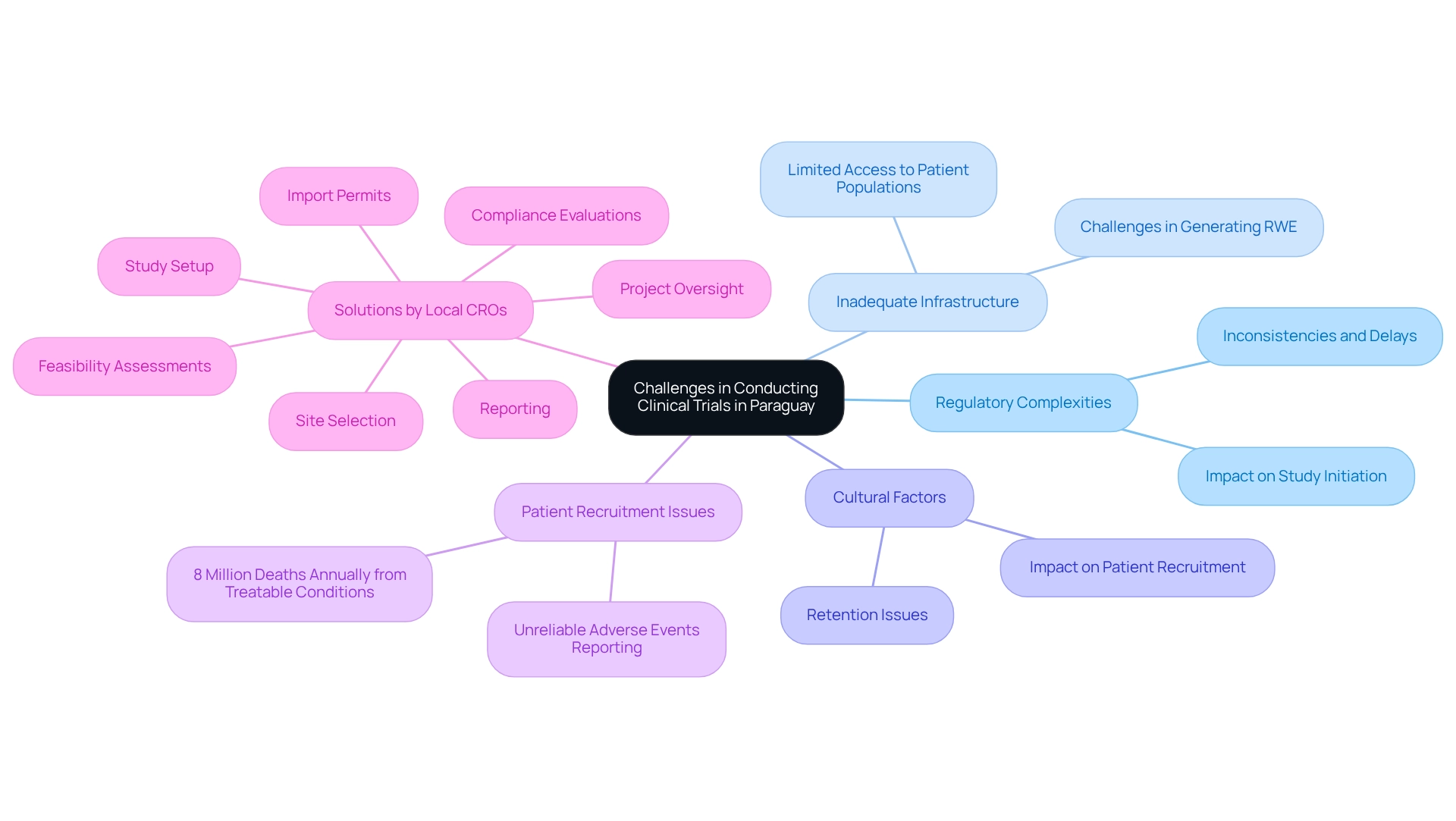
Conducting medical studies in Paraguay presents a range of challenges, particularly in navigating regulatory complexities, inadequate infrastructure, and cultural factors that can significantly impact patient recruitment and retention. While organizations such as DINAVISA have made strides in streamlining processes, inconsistencies and delays frequently obstruct the initiation of studies. The variability within the healthcare system further complicates access to diverse patient populations, which is essential for generating meaningful real-world evidence (RWE). The urgency of effective medical research in these regions is underscored by the alarming statistic that approximately 8 million individuals die annually from treatable conditions due to inadequate healthcare in low- and middle-income countries. Moreover, the process of reporting and attributing adverse events in Paraguay has proven to be less reliable than anticipated, complicating study management and potentially leading to underreporting or misattribution of adverse events.
Local Contract Research Organizations (CROs) are pivotal in addressing these challenges by facilitating connections between researchers and hospitals, thereby enhancing the efficacy of medical studies. For instance, bioaccess® offers comprehensive study management services, including:
- Feasibility assessments
- Site selection
- Compliance evaluations
- Study setup
- Import permits
- Project oversight
- Reporting
With over 20 years of experience, bioaccess® manages a variety of studies, such as Early-Feasibility, First-In-Human, Pilot, Pivotal, and Post-Market Clinical Follow-Up Studies. This expertise was exemplified when enVVeno successfully conducted a first-in-human study in Paraguay with its CoreoGraft device, demonstrating the capacity of local CROs to optimize processes and improve outcomes.
Recognizing these challenges is essential for researchers aiming to design effective studies that incorporate RWE. By tailoring their approaches to the local context and leveraging the extensive research management services provided by bioaccess®, researchers can more effectively navigate the regulatory landscape and enhance patient engagement, ultimately fostering the advancement of medical technologies in Paraguay.
Integrate Real-World Evidence into Trial Design and Methodology
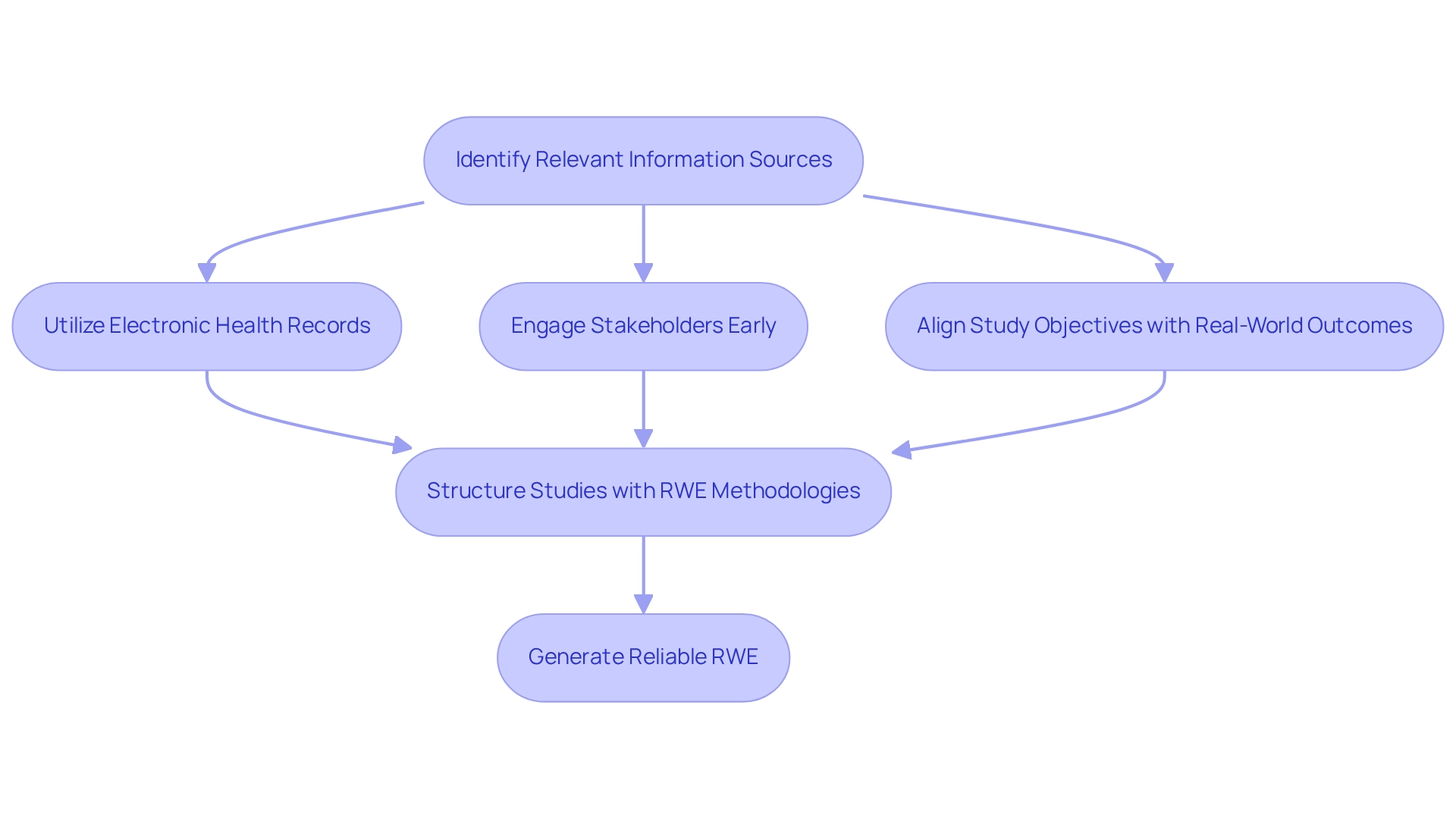
To effectively incorporate real-world evidence (RWE) into clinical trial design, researchers must first identify relevant information sources that accurately reflect the characteristics and treatment patterns of the target population. This necessitates the utilization of electronic health records, patient registries, and community health information.
The commitment and investment in real-world data (RWD) from stakeholders are essential for realizing the full potential of RWE in medical research. Subsequently, studies should be structured to include RWE methodologies, such as pragmatic experiments or hybrid designs that combine traditional randomized controlled studies with observational data.
Involving stakeholders—patients and healthcare providers—early in the design process is vital to ensure that the study addresses real-world needs and challenges. As noted by the PMDA, "Data that is electronically generated and stored by medical institutions" can serve as a valuable resource in this context.
By aligning study objectives with real-world outcomes, researchers can significantly enhance the relevance and applicability of their findings. The increasing focus on RWE is underscored by its capacity to address the varied requirements of regulators, payers, physicians, patients, and the industry, establishing it as a crucial element of contemporary research in medicine.
Furthermore, the generation of reliable and trustworthy RWE is critical as its use for regulated purposes continues to grow, reinforcing the credibility of the evidence being utilized. Insights from case studies, such as 'Evaluating Treatment Benefits-Risks in Rare Diseases,' emphasize the methodological difficulties encountered in proving treatment advantages in research settings, particularly in intricate situations.
By incorporating these elements, researchers can better navigate the integration of RWE into clinical trial design.
Utilize Tools and Resources for Effective RWE Collection and Analysis
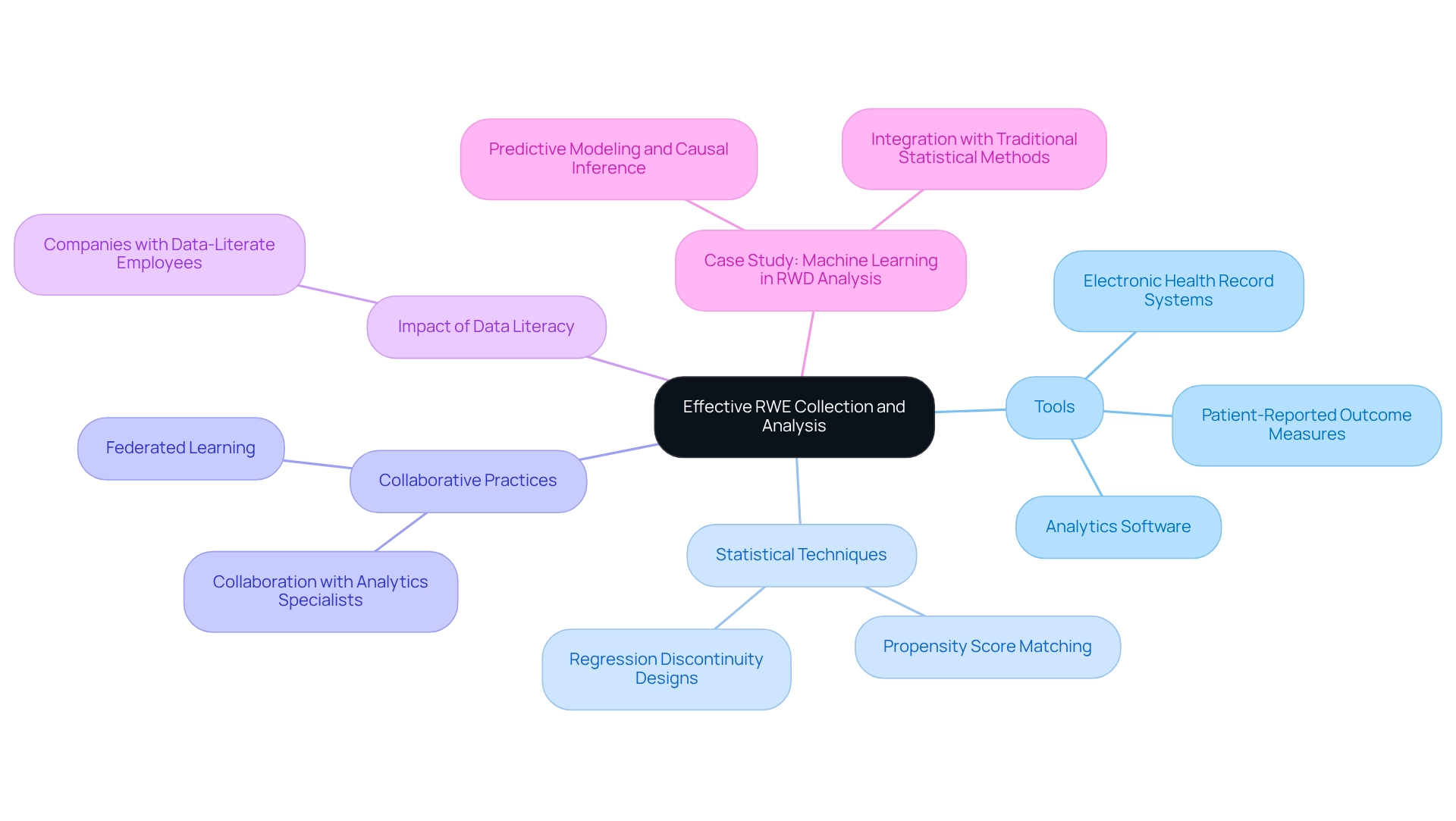
To effectively gather and examine real-world evidence (RWE), researchers can utilize a range of specialized tools and platforms designed for real-world information management. Essential resources include:
- Electronic health record systems
- Patient-reported outcome measures
- Sophisticated analytics software capable of handling extensive datasets
The demand for information specialists is increasing at an impressive rate of 36%, underscoring the significance of analytics expertise in RWE analysis. Employing statistical techniques intended for RWE analysis, such as:
- Propensity score matching
- Regression discontinuity designs
is crucial for addressing potential biases inherent in observational information. Collaborating with analytics specialists can significantly enhance the thoroughness of the examination, ensuring that the results are both robust and trustworthy. As Hilary Mason, a scientist and founder of Fast Forward Labs, noted, "When utilized in the right context, this knowledge can create a significant impact—not only on businesses but on society as a whole."
Furthermore, integrating machine learning techniques, as demonstrated in the case study titled "Machine Learning in RWD Analysis," can bolster the ability to generate regulatory-grade real-world evidence and improve healthcare outcomes. Innovative approaches like federated learning enable local devices to collaboratively learn without sharing training data, thereby reducing privacy risks. By leveraging these tools and resources, researchers can unlock the full potential of real-world evidence for Paraguayan trials in their clinical studies, ultimately contributing to enhanced healthcare outcomes.
Conclusion
Real-world evidence (RWE) is revolutionizing clinical research, especially in regions like Paraguay, where traditional trial methodologies encounter substantial challenges. By leveraging data from electronic health records, patient registries, and other real-world sources, researchers can obtain profound insights into treatment effectiveness and safety, ensuring that findings resonate with the actual patient experience. The integration of RWE not only amplifies the relevance of clinical trials but also aligns with the urgent need for effective healthcare solutions tailored to local demographics.
Navigating the complexities of conducting clinical trials in Paraguay demands an understanding of the regulatory landscape and cultural factors that influence patient recruitment and retention. Local Contract Research Organizations (CROs) are instrumental in overcoming these hurdles, facilitating connections that streamline trial processes and enhance outcomes. By customizing approaches to the unique context of Paraguay and utilizing the expertise of organizations like bioaccess®, researchers can optimize patient engagement and propel medical technology development.
As the demand for reliable evidence escalates, the significance of incorporating RWE into clinical trial design and methodology cannot be overstated. By concentrating on real-world data sources and employing innovative analytical tools, researchers can ensure that their studies address real-world needs, ultimately leading to improved healthcare outcomes. The future of clinical research resides in the successful fusion of traditional methodologies with real-world insights, paving the way for a healthcare landscape that is increasingly responsive to the needs of patients and providers alike.
Frequently Asked Questions
What is real-world evidence (RWE)?
Real-world evidence (RWE) encompasses insights derived from real-world data (RWD), which includes information collected beyond standard medical studies, such as electronic health records, patient registries, and insurance claims.
Why is RWE important in medical research?
RWE provides a comprehensive understanding of treatment efficacy and safety within routine healthcare environments, enhancing the relevance of research findings, especially in specific regions like Paraguay.
How does RWE benefit Paraguayan trials?
By leveraging RWE, researchers can effectively address local health issues and ensure that studies reflect the demographics and treatment practices of the actual patient population in Paraguay.
What impact does RWE have on research outcomes?
RWE enhances the relevance of findings by aligning baseline demographics and disease characteristics, which is crucial for precise statistical analysis.
What role does information governance play in RWE?
Implementing robust information governance frameworks supports regulatory compliance and ensures the reliability of the evidence generated from RWE.
Why is it essential to validate RWE tools and processes?
Validating RWE tools and processes is essential as the utilization of RWE for regulated purposes continues to grow, ensuring that the evidence produced is reliable and compliant with regulatory standards.
How does bioaccess® contribute to RWE validation?
bioaccess® offers extensive trial management services and is ideally positioned to facilitate the validation process of RWE tools and methodologies.
What are the advantages of incorporating RWE into medical research?
Incorporating RWE enhances patient outcomes and aligns with the evolving landscape of medical technology, leading to improved understanding of treatment effects and more effective healthcare solutions.
What is the role of AI in relation to RWE?
While the role of AI in medical research is expanding, the focus remains on closed-loop AI systems that enhance data analysis and patient care in conjunction with RWE.




