Overview
The article underscores the critical importance of mastering clinical trial oversight in Ecuador to secure successful research outcomes in the face of evolving regulatory frameworks. It highlights that:
- A thorough understanding of new regulations
- The implementation of effective vendor management
- Enhanced patient engagement
- The strategic use of technology
are essential for ensuring compliance and bolstering the integrity of clinical trials. These elements collectively foster trust and collaboration within the research community, paving the way for more effective clinical research practices.
Introduction
Ecuador stands on the brink of a transformative shift in its clinical trials landscape, with new regulations poised to take effect on June 30, 2025. This pivotal change, encapsulated in Ministerial Agreement No. 00069-2024, aims to enhance the approval and oversight processes, ensuring that clinical research aligns with international ethical standards.
As the country prepares for these developments, clinical research professionals must navigate a complex web of guidelines designed to improve the rigor and transparency of trials.
With an emphasis on ethical considerations and comprehensive training programs, the new framework promises to elevate the quality of clinical research, ultimately benefiting public health.
As Ecuador embraces this regulatory evolution, it sets the stage for a more robust and trustworthy clinical trials environment, fostering collaboration and innovation in the region.
Understand Regulatory Frameworks for Clinical Trials in Ecuador
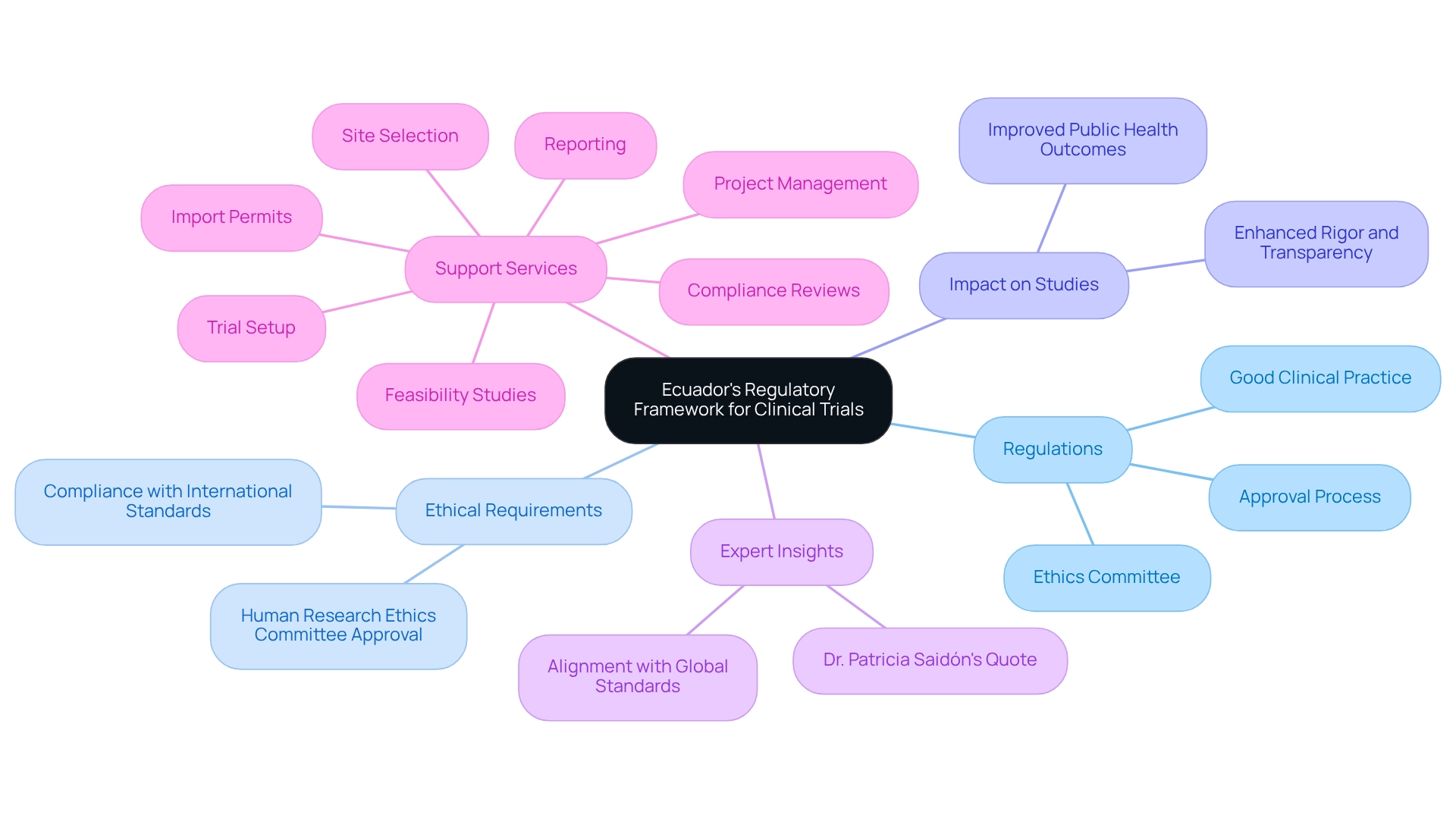
The regulatory environment for medical studies is undergoing significant transformation in Ecuador, particularly in terms of clinical trial oversight in Ecuador, with new rules set to take effect on June 30, 2025. Established under Ministerial Agreement No. 00069-2024, these regulations outline comprehensive guidelines for the approval, authorization, execution, oversight, and control of research studies. It is imperative for clinical research professionals to familiarize themselves with the requirements for clinical trial oversight in Ecuador to ensure compliance and bolster the integrity of their studies. Key components of this new framework include mandatory approval from the Human Research Ethics Committee and strict adherence to Good Clinical Practice (GCP) standards. The anticipated implementation of these regulations is expected to enhance clinical trial oversight in Ecuador, improving the rigor and transparency of medical studies and ultimately yielding better public health outcomes. To facilitate the effective adoption of these guidelines, training programs focusing on ethical and regulatory aspects are being initiated.
As Dr. Patricia Saidón, who oversees research studies at the Ministry of Health, emphasizes, 'the new regulation is vital for carrying out thorough, transparent, and significant studies on the health and welfare of populations.' This statement underscores the importance of the new regulations in elevating the quality of healthcare research.
Furthermore, the introduction of the Portal of Medical Studies of the Americas at the end of 2024 signifies broader efforts to enhance research in the region, aligning with Ecuador's new regulatory framework. The case study titled 'Ecuador's New Regulations on Research Studies' exemplifies the practical application of these guidelines, which are expected to improve the rigor, transparency, and impact of research within the country, particularly emphasizing the importance of clinical trial oversight in Ecuador. Experts in the field note that these new regulations will not only streamline the research process but also align Ecuador's practices with global ethical standards. Such alignment is crucial for fostering trust and collaboration among stakeholders in the research community. Staying informed about these evolving regulations is essential for mitigating risks and ensuring the successful execution of research studies in Ecuador. Additionally, bioaccess offers comprehensive clinical trial management services, including:
- Feasibility studies
- Site selection
- Compliance reviews
- Trial setup
- Import permits
- Project management
- Reporting
These services can significantly assist researchers in navigating the new regulatory landscape, ensuring compliance and enhancing the quality of clinical trials.
Implement Effective Vendor Management and Oversight Strategies
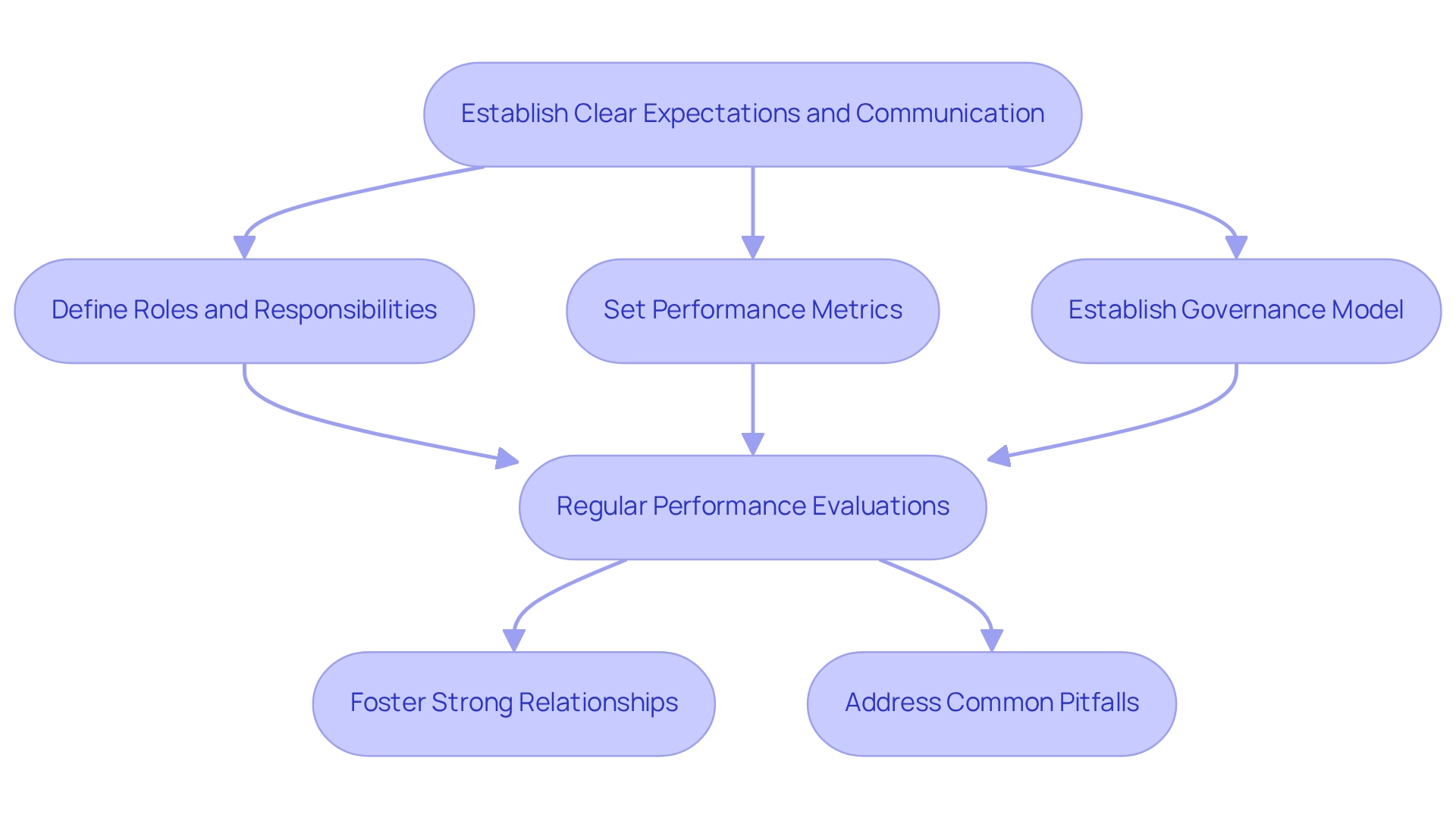
Implementing effective vendor management strategies begins with establishing clear expectations and communication channels from the outset. This entails defining roles, responsibilities, and performance metrics for each supplier involved in the research study. A governance model may be established for communication links at both project and organizational levels, ensuring that all parties remain aligned and informed throughout the process.
Regular performance evaluations are crucial to ensure that vendors meet established Key Performance Indicators (KPIs). Strong relationships, fostered through open communication and collaboration, can significantly enhance vendor performance and accountability, particularly in the sphere of clinical trial oversight in Ecuador, where the trend of outsourcing testing and services has surged due to the increasing complexity and demand for accelerated research.
Firms such as bioaccess®, with over 20 years of expertise in Medtech, are strategically positioned to navigate this evolving landscape, providing expedited medical device research services that encompass:
- Early-Feasibility Studies
- First-In-Human Studies
- Pilot Studies
- Pivotal Studies
- Post-Market Follow-Up Studies
Their extensive knowledge ensures that clinical trial oversight in Ecuador facilitates efficient conduct of medical studies, maximizing research funding and accelerating innovation. These platforms ensure that all suppliers adhere to regulatory standards and maintain high-quality criteria, ultimately contributing to the success of research studies.
As Dr. Christoph Ortland states, 'Let our team assist you in choosing the right Third-party Vendors for your study,' underscoring the critical role of selecting appropriate vendors in achieving trial success.
It is also essential to acknowledge the impact of performance metrics on vendor oversight. Establishing clear performance metrics not only holds vendors accountable but also drives continuous improvement in their services. Additionally, being aware of common pitfalls in vendor management, such as insufficient communication or vague expectations, can assist research directors in avoiding missteps and refining their vendor management strategies.
Enhance Patient Engagement and Recruitment Strategies
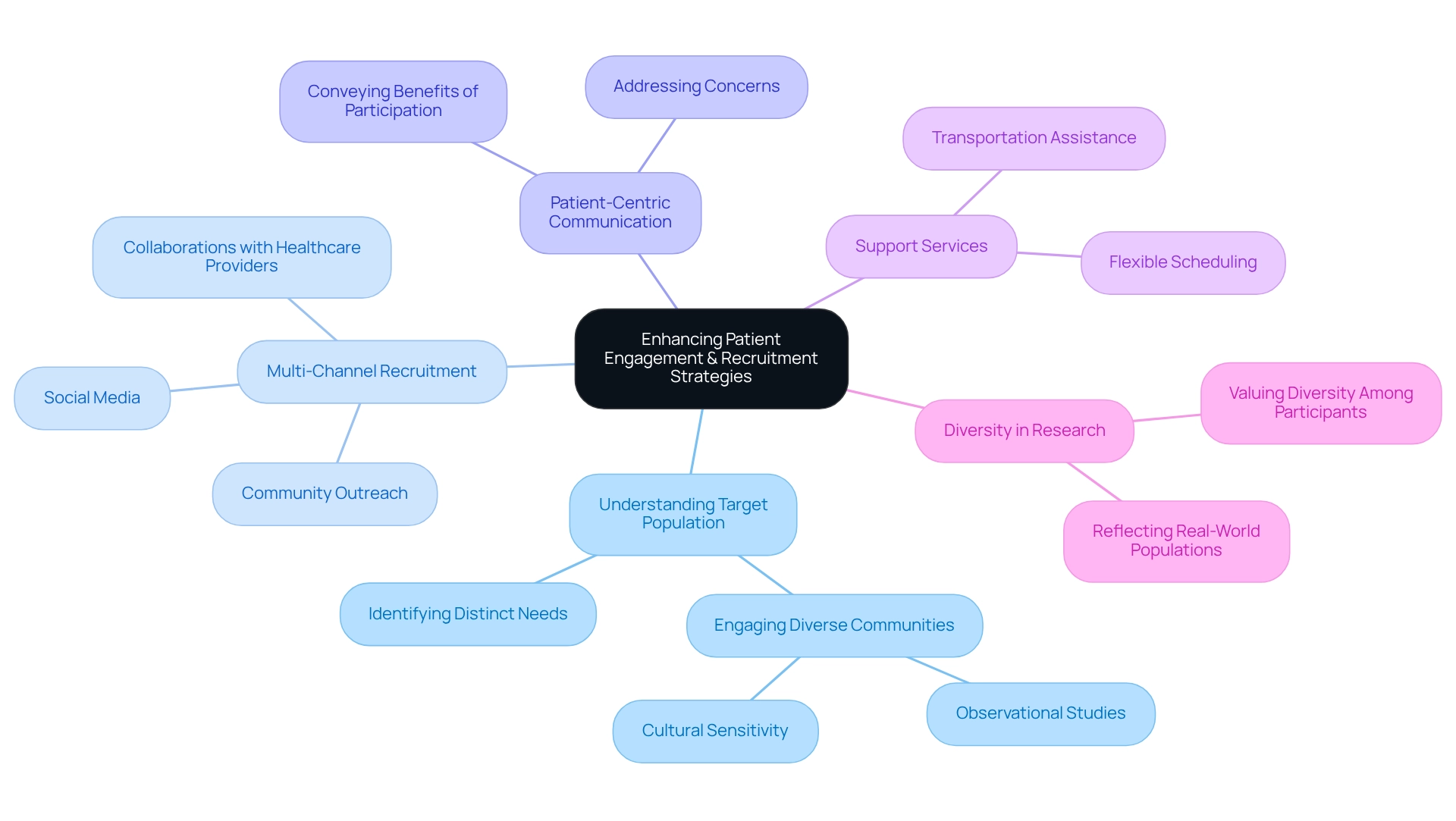
Enhancing patient engagement begins with a comprehensive understanding of the target population and their distinct needs. Implementing a multi-channel recruitment strategy—leveraging social media, community outreach, and collaborations with healthcare providers—significantly boosts awareness and participation in research studies. Notably, the partnership between bioaccess™ and Caribbean Health Group, announced on March 29, 2019, aims to position Barranquilla as a leading hub for medical research in Latin America, with support from Colombia's Minister of Health. This initiative exemplifies a commitment to improving recruitment efforts and the overall clinical research landscape in the region. Research indicates that engaging diverse communities in observational studies can pave the way for increased research participation, ensuring the safety and efficacy of medical interventions.
Utilizing patient-centric communication strategies that clearly convey the benefits of participation while addressing potential concerns fosters trust and promotes enrollment. Essential support services, such as transportation assistance and flexible scheduling, are crucial for improving retention rates throughout the study. Moreover, involving patients as partners in the research process not only enriches their experience but also results in more meaningful outcomes and heightened satisfaction levels. A recent survey revealed that a majority of respondents value diversity among research participants and personnel, highlighting the necessity for evaluations to accurately reflect the populations they aim to serve. Furthermore, GlobalCare Clinical Trials' collaboration with bioaccess™ has achieved over a 50% reduction in recruitment time and an impressive 95% retention rate, demonstrating effective strategies in practice. As Red Adair astutely noted, "If you think it’s expensive to hire a professional, wait until you hire an amateur," underscoring the critical importance of investing in effective patient engagement strategies. Insights from the case examination 'Patient Engagement Trends in Medical Research' underscore that representing real-world populations in research is vital for ensuring the safety and effectiveness of medications across all demographics.
Leverage Technology for Efficient Data Management and Compliance
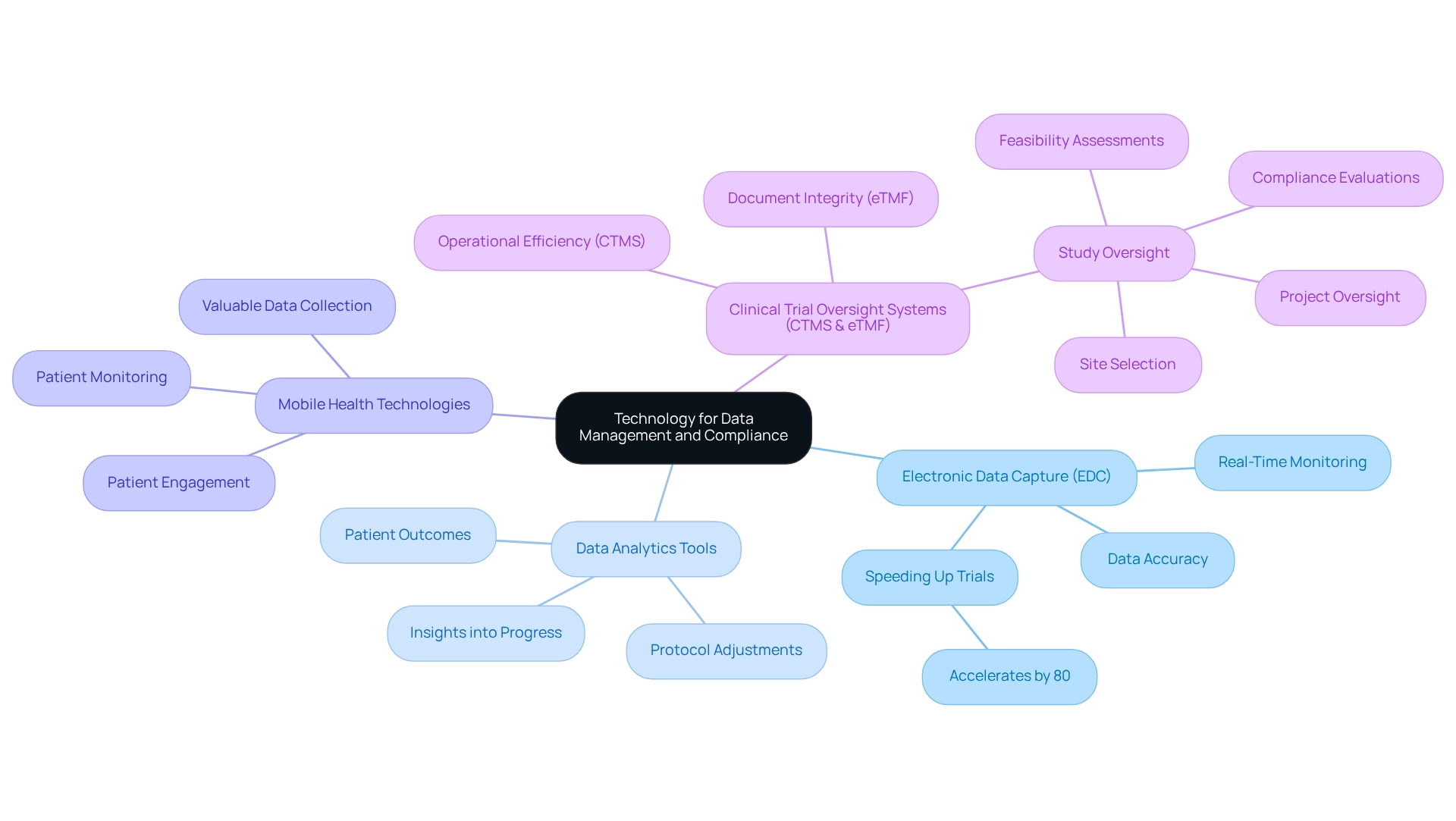
To enhance the efficiency of medical studies, teams should utilize electronic data capture (EDC) systems that allow real-time data entry and monitoring. These systems significantly improve data accuracy and facilitate adherence to regulatory standards. The integration of data analytics tools provides essential insights into progress and patient outcomes, allowing prompt adjustments to research protocols. Furthermore, the use of mobile health technologies and wearables enhances patient monitoring and engagement, yielding valuable data while enriching the overall patient experience. It is essential to ensure that all technological solutions comply with data protection regulations to uphold participant confidentiality and trust.
As organizations progressively acknowledge medical data as a valuable resource, the implementation of EDC systems is becoming more widespread, with research showing that such technologies can speed up medical tests by as much as 80 percent in certain situations. Grasping the unique functions of study oversight systems (CTMS) and electronic master files (eTMF) is vital for clinical trial oversight in Ecuador; CTMS emphasizes operational efficiency while eTMF guarantees document integrity. In this context, bioaccess provides extensive services for clinical trial oversight in Ecuador, which include overseeing research processes such as feasibility assessments, site selection, compliance evaluations, setup, import permits, project oversight, and reporting, all of which are crucial for improving research effectiveness.
A case study comparing CTMS and eTMF illustrates how selecting the right tools can enhance trial management effectiveness. By viewing clinical data as a valuable asset, organizations can position themselves for success in the evolving landscape of pharmaceutical research.
Conclusion
Ecuador stands on the brink of a transformative shift in its clinical trials landscape, propelled by new regulations set to take effect on June 30, 2025. These regulations, encapsulated in Ministerial Agreement No. 00069-2024, aim to enhance the approval, oversight, and integrity of clinical trials while aligning with international ethical standards. By mandating approvals and adherence to Good Clinical Practice, this new framework promises to elevate the quality and transparency of clinical research, ultimately benefiting public health.
As clinical research professionals adapt to these evolving guidelines, the significance of effective vendor management and patient engagement strategies becomes paramount. Establishing clear communication channels and performance metrics with vendors, coupled with implementing multi-channel recruitment tactics, will be vital for successful trial execution. Furthermore, leveraging technology for data management and compliance will streamline processes and enhance overall trial efficiency, ensuring that studies are conducted rigorously and ethically.
In conclusion, Ecuador's regulatory evolution marks the dawn of a new era for clinical trials, fostering a more trustworthy and collaborative research environment. By embracing these changes and prioritizing ethical considerations, the clinical research community can significantly enhance public health outcomes and drive innovation in the region. As the landscape continues to evolve, it is essential for stakeholders to remain informed and engaged to navigate this transformative period successfully.
Frequently Asked Questions
What significant changes are happening in the regulatory environment for medical studies in Ecuador?
The regulatory environment for medical studies in Ecuador is undergoing significant transformation, particularly regarding clinical trial oversight, with new regulations set to take effect on June 30, 2025.
What are the key components of the new regulations established under Ministerial Agreement No. 00069-2024?
The key components include mandatory approval from the Human Research Ethics Committee and strict adherence to Good Clinical Practice (GCP) standards for the approval, authorization, execution, oversight, and control of research studies.
How will the new regulations impact clinical trial oversight in Ecuador?
The anticipated implementation of these regulations is expected to enhance clinical trial oversight, improving the rigor and transparency of medical studies, which will ultimately yield better public health outcomes.
What initiatives are being implemented to support the adoption of the new guidelines?
Training programs focusing on ethical and regulatory aspects are being initiated to facilitate the effective adoption of the new guidelines.
What is the significance of the Portal of Medical Studies of the Americas?
The Portal of Medical Studies of the Americas, set to launch at the end of 2024, signifies broader efforts to enhance research in the region and aligns with Ecuador's new regulatory framework.
What practical applications do the new regulations have for research studies in Ecuador?
The new regulations are expected to improve the rigor, transparency, and impact of research, streamlining the research process and aligning Ecuador's practices with global ethical standards.
What services does bioaccess offer to assist researchers with the new regulatory landscape?
Bioaccess offers comprehensive clinical trial management services, including feasibility studies, site selection, compliance reviews, trial setup, import permits, project management, and reporting.
Why is it important for clinical research professionals to stay informed about these evolving regulations?
Staying informed is essential for mitigating risks and ensuring the successful execution of research studies in Ecuador, fostering trust and collaboration among stakeholders in the research community.




