Overview
This article presents a comprehensive, step-by-step approach for designing post-market studies in Argentina, underscoring the critical importance of understanding the regulatory frameworks and ethical standards established by ANMAT. It offers detailed guidance on:
- Defining study objectives
- Implementing effective recruitment strategies
- Monitoring compliance
By illustrating how adherence to these protocols significantly enhances the integrity and success of clinical research in the region, the article positions itself as an essential resource for professionals navigating this complex landscape.
Introduction
Navigating the intricate landscape of post-market studies in Argentina necessitates a comprehensive understanding of the regulatory framework established by ANMAT, the authority overseeing clinical trials. With regulations that prioritize ethical practices and participant safety, it is crucial for companies to remain vigilant in their compliance efforts.
As this landscape evolves, so too do the requirements, making it imperative for organizations to swiftly adapt their quality management systems. This article explores the essential components of effective post-market studies, including:
- The importance of defining clear objectives
- Designing robust protocols
- Implementing strategic recruitment and data collection methods
By leveraging expert insights and adhering to regulatory standards, companies can enhance their market entry strategies and ensure the integrity of their research endeavors.
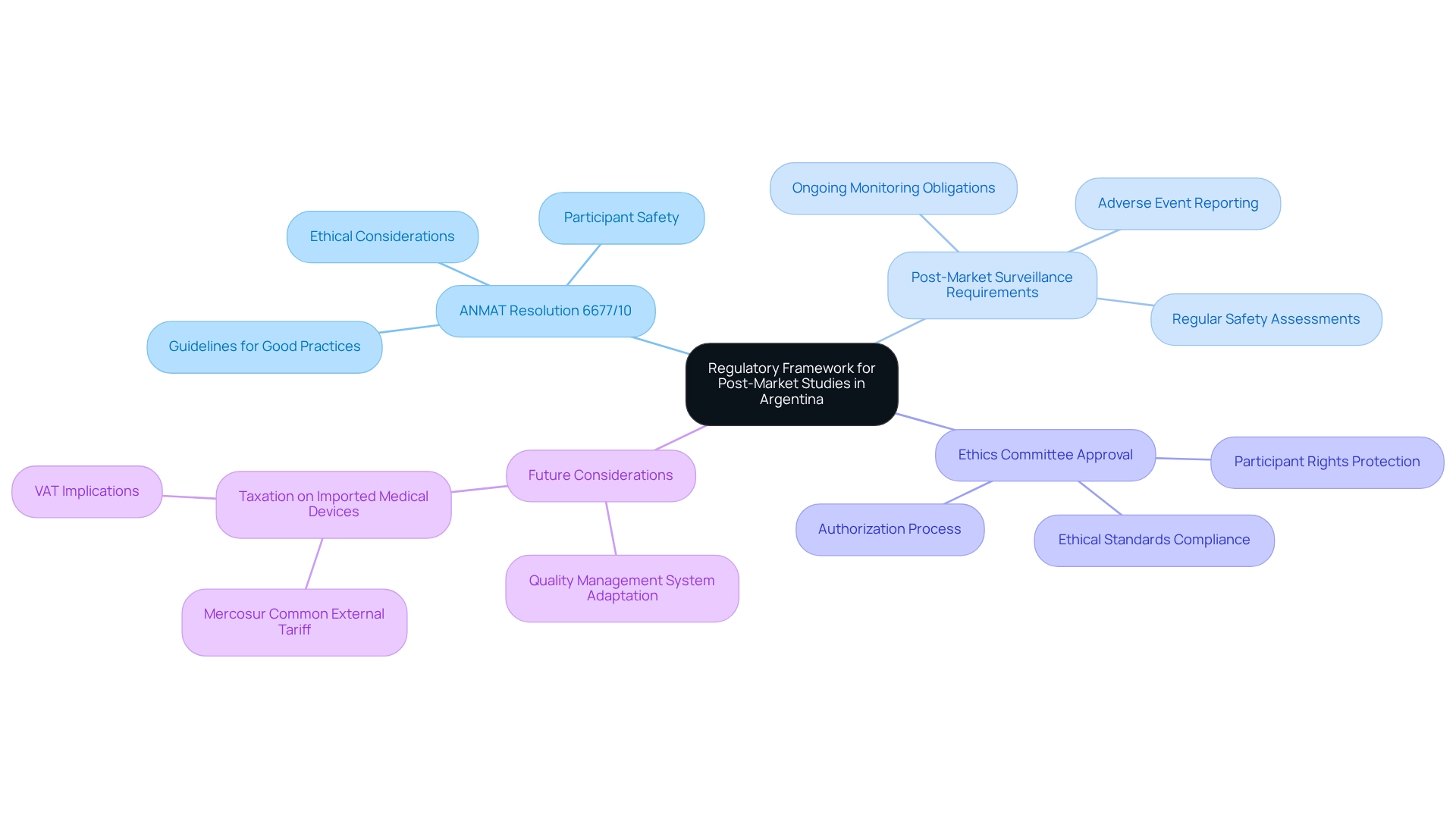
Understand the Regulatory Framework for Post-Market Studies in Argentina
Understanding the regulatory framework established by ANMAT, the primary authority overseeing trials, is essential for designing post-market studies in Argentina. Key regulations include:
- ANMAT Resolution 6677/10: This resolution outlines the guidelines for good practices in trials, highlighting ethical considerations and the critical importance of participant safety. Adhering to these guidelines is vital for preserving the integrity of the study and safeguarding participant welfare. As Malay Singh observes, "The document addresses the fundamentals of medical research and trials," underscoring the foundational significance of such regulations.
- Post-Market Surveillance Requirements: Companies are required to meet stringent obligations for the ongoing monitoring of medical devices after approval. This includes timely reporting of adverse events and conducting regular safety assessments to ensure continued adherence to safety standards. bioaccess® specializes in delivering comprehensive clinical trial management services, including Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), and Post-Market Clinical Follow-Up Studies (PMCF), with a strong focus on post-market study design for Argentina, ensuring meticulous handling of all aspects of post-market surveillance.
- Ethics Committee Approval: Securing authorization from a recognized ethics committee is a prerequisite before commencing any research. This step guarantees that the research aligns with ethical standards and protects participant rights throughout the research process. With over 20 years of experience in Medtech, bioaccess® is adept at efficiently navigating these requirements.
As we look ahead to 2025, the regulatory framework continues to evolve, with ANMAT stressing the necessity for companies to adapt their quality management systems within 120 days of new regulations taking effect. This timeframe is crucial for ensuring compliance and upholding the credibility of the research. Additionally, awareness of the taxation on imported medical devices, which falls under the Mercosur Common External Tariff ranging from 0% to 16%, can significantly influence the cost structure for medical device companies.
bioaccess® can assist clients in navigating these financial implications, facilitating a smoother market entry strategy.
Familiarity with these regulations will empower you to craft a compliant and ethically sound post-market study design for Argentina, paving the way for effective market entry and sustained success. Leveraging the expertise of bioaccess® can further enhance your ability to manage these complexities, ensuring a streamlined approach to post-market study design for Argentina.
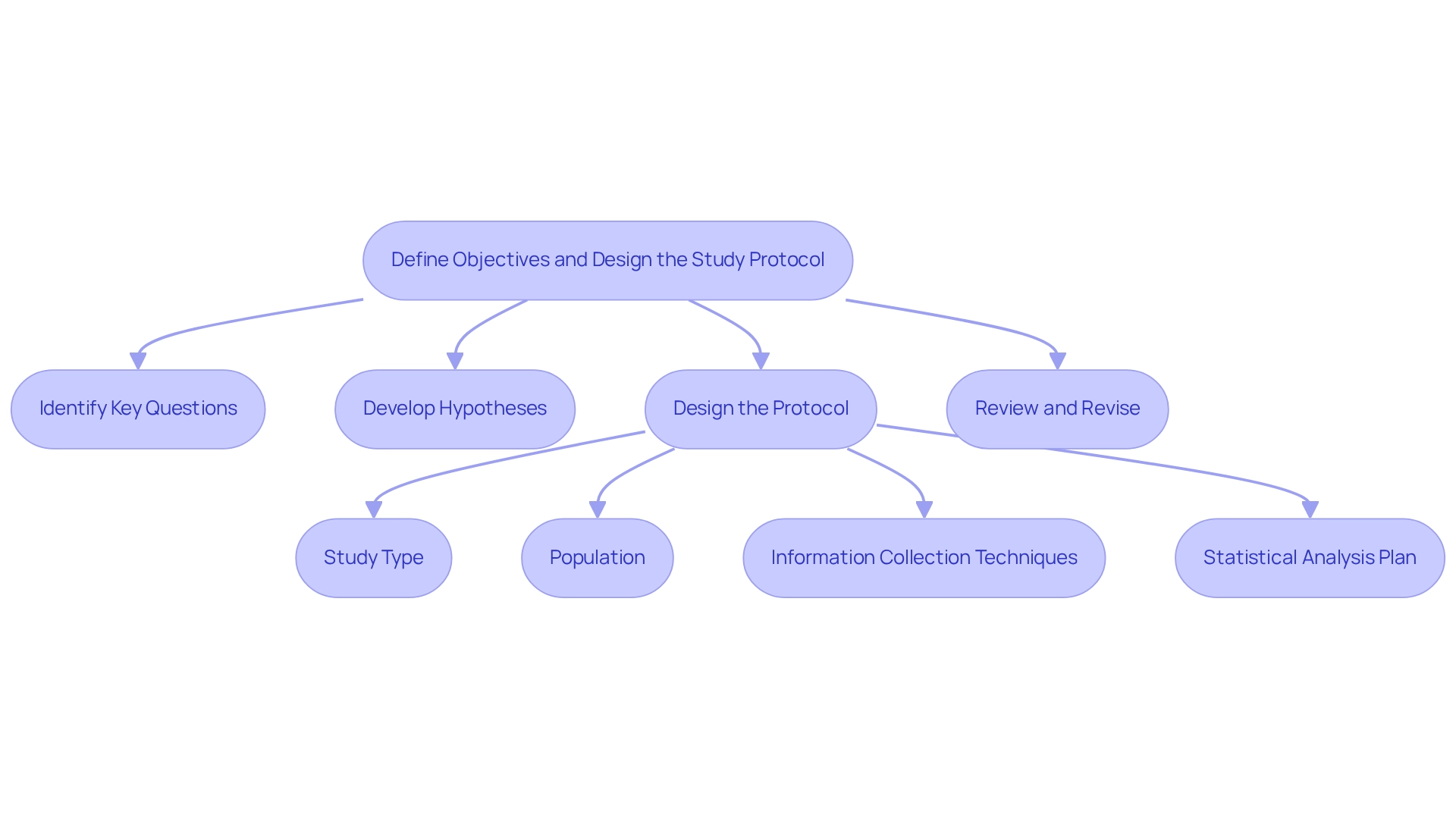
Define Objectives and Design the Study Protocol
To effectively articulate the primary objectives of your post-market study, it is crucial to follow these essential steps:
-
Identify Key Questions: Determine the specific outcomes you wish to measure, such as device reliability, effectiveness, or user satisfaction. Notably, a survey revealed that 11% of manufacturers identified new safety concerns through their post-market study design for Argentina, underscoring the importance of thorough inquiry.
-
Develop Hypotheses: Formulate hypotheses aligned with your objectives. For instance, you might hypothesize that your device enhances patient outcomes compared to existing alternatives, which can guide your research focus.
-
Design the Protocol: Create a detailed study design that includes:
- Study Type: Decide whether the study will be observational or interventional.
- Population: Clearly define your target population along with inclusion and exclusion criteria.
- Information Collection Techniques: Specify the methods for information collection, such as surveys or clinical assessments, to ensure comprehensive information gathering.
- Statistical Analysis Plan: Outline how you will examine the information to validate your hypotheses, ensuring robust statistical methods are employed.
-
Review and Revise: Conduct a thorough review of the protocol to ensure it meets regulatory requirements, including those outlined in FDA 21 CFR Part 822, which mandates creating a surveillance plan, data collection, periodic reporting, adverse event reporting, and record keeping. Engaging with colleagues or regulatory experts for feedback can refine your approach and enhance compliance.
Furthermore, it is essential to consider the role of INVIMA in the regulatory landscape, as it supervises medical device compliance in Colombia, ensuring that your research aligns with local regulations.
As Desiree Tarranco, a Quality Assurance Professional, notes, "For medical device companies looking to enhance their PMS activities and ensure compliance with global requirements, bioaccess® is a significant step forward." This emphasizes the essential nature of adherence in the post-market study design for Argentina.
Grasping the benefits and drawbacks of the post-market study design for Argentina, as outlined in the case analysis titled 'Advantages and Limitations of Post-Market Surveillance,' can offer valuable insights. This case analysis outlines the advantages of enhanced patient protection and regulatory adherence, along with the challenges associated with resource intensity and data management.
By following these steps, you will create a solid foundation for the post-market study design for Argentina, ensuring it is scientifically rigorous and aligned with regulatory standards. This structured approach not only enhances patient safety but also navigates the complexities of regulatory compliance, leveraging the expertise of bioaccess® in managing Early-Feasibility, First-In-Human, Pilot, Pivotal, and Post-Market Follow-Up Studies.
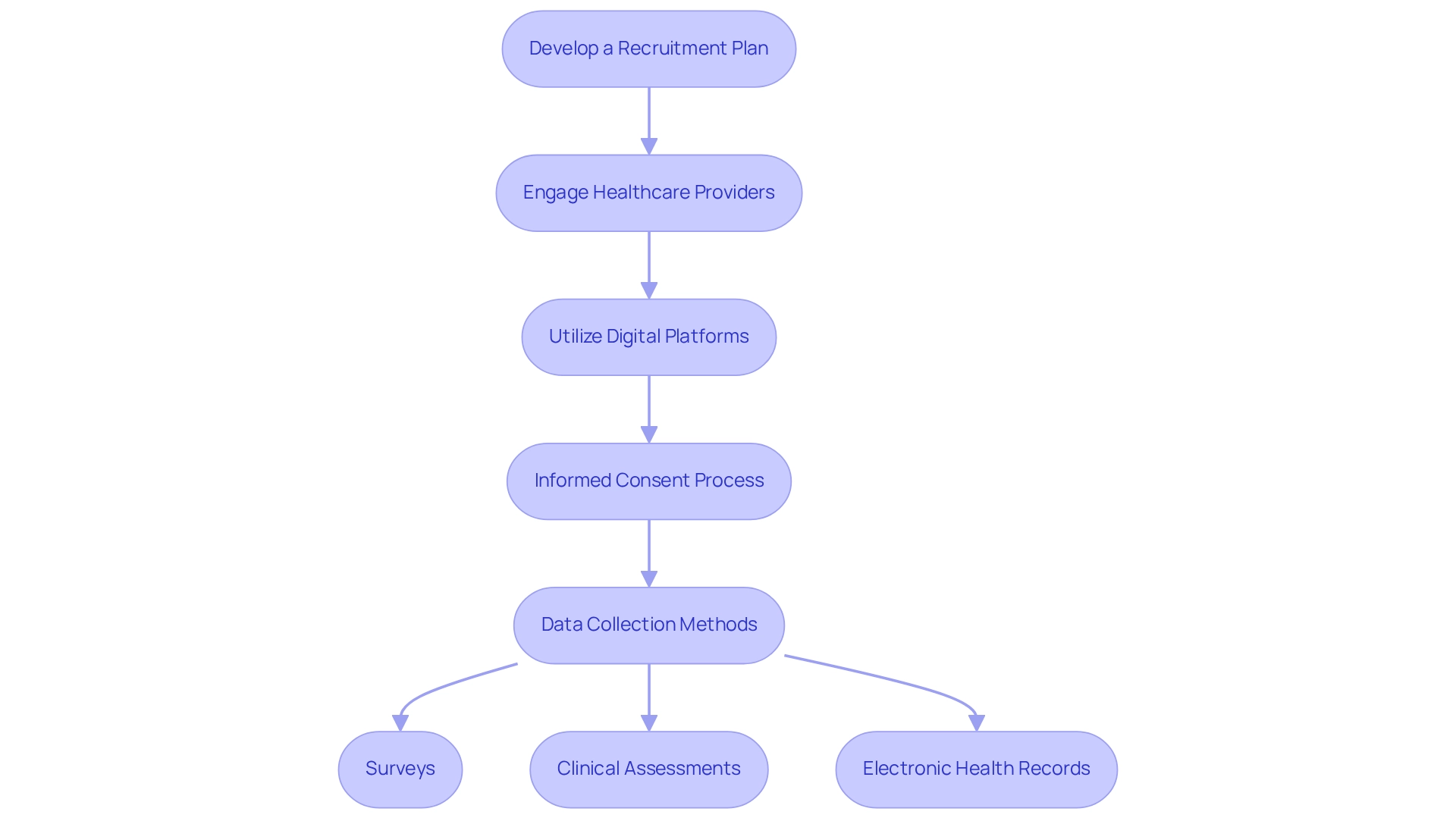
Implement Recruitment Strategies and Data Collection Methods
To effectively recruit participants and collect data in post-market studies, consider the following strategies:
-
Develop a Recruitment Plan: Identify potential sources for participants, including hospitals, clinics, and patient registries. Tailor your approach to the specific demographics of your target population to maximize engagement.
-
Engage Healthcare Providers: Collaborate with healthcare professionals who can refer qualified patients to your research. Offering clear information regarding the project's purpose and advantages is essential for encouraging their support and involvement. Engaging healthcare providers effectively can significantly impact recruitment success, as their endorsement often leads to increased participant trust and willingness to enroll.
-
Utilize Digital Platforms: Leverage social media and online patient communities to broaden your outreach. Develop captivating material that distinctly outlines the research and fosters involvement, leveraging the increasing trend of digital interaction in medical trials. Notably, the global clinical trial recruitment market is estimated to grow to $5.45 billion by 2030, underscoring the importance of effective recruitment strategies.
-
Informed Consent Process: Ensure that participants fully understand the study's purpose, procedures, and potential risks. Develop clear and concise consent forms that comply with ethical standards, enhancing trust and transparency.
-
Data Collection Methods: Select appropriate methods for data collection, such as:
- Surveys: Implement validated questionnaires to gather participant feedback effectively.
- Clinical Assessments: Conduct regular evaluations to monitor device performance and safety, ensuring thorough information capture.
- Electronic Health Records: Utilize existing medical records to supplement information collection, enhancing the richness of your dataset.
By implementing these strategies, you can significantly enhance participant recruitment and ensure that your data collection methods yield reliable and valid results in the post-market study design for Argentina. Simplifying eligibility criteria, as demonstrated in the case analysis 'Simplifying Eligibility Criteria,' can expand the pool of qualified candidates, tackling health disparities and enhancing treatment results. Additionally, the recent partnership between bioaccess® and Caribbean Health Group seeks to establish Barranquilla as a premier location for clinical trials in Latin America, backed by Colombia's Minister of Health, which emphasizes the potential for local economic development and healthcare enhancement through effective clinical research.
Addressing health disparities through diverse recruitment is essential for effective treatments, making these strategies not only practical but also ethically imperative.
Monitor Progress and Ensure Compliance Throughout the Study
To effectively monitor your study and ensure compliance, it is essential to implement the following strategies:
- Establish Monitoring Protocols: Clearly define the frequency of progress evaluations and the specific metrics to track, such as recruitment rates and quality indicators.
- Conduct Regular Meetings: Schedule consistent meetings with your research team to evaluate progress, address challenges, and share updates, fostering open communication.
- Implement Quality Control Measures: Develop robust procedures for information verification and validation, ensuring the accuracy and reliability of collected information.
- Compliance Audits: Conduct regular audits to assess conformity with regulatory requirements and research protocols. This includes thorough reviews of informed consent processes and data collection methodologies, which are crucial for maintaining compliance with INVIMA regulations as a Level 4 health authority.
- Report Adverse Events: Create a systematic approach for reporting and addressing any adverse events or issues that arise during the study. Ensure all incidents are meticulously documented and reported to ANMAT as mandated.
As Michael J. Martens from the Division of Biostatistics at the Medical College of Wisconsin mentions, "The authors express gratitude to Dr. Marian Ewell for her groundbreaking contributions to oversight in the BMT CTN." This highlights the importance of robust safety monitoring in clinical trials. Furthermore, recent research has examined less frequent testing where stopping criteria are evaluated after every fourth patient, emphasizing the necessity for flexible monitoring protocols.
By adopting a proactive approach to monitoring and compliance, you can significantly enhance the quality and credibility of the post-market study design for Argentina, ultimately leading to better patient outcomes. Moreover, insights from the case examination titled 'Future Research Directions in Safety Monitoring' suggest that employing time-to-event methods for toxicity detection could enhance the ability to identify safety signals, underscoring the significance of thorough monitoring strategies. Leveraging bioaccess®'s expertise in clinical trial management can further support these efforts, ensuring a successful study outcome.

Conclusion
Navigating the complexities of post-market studies in Argentina necessitates a comprehensive understanding of the regulatory landscape established by ANMAT. By adhering to crucial regulations, such as those articulated in ANMAT Resolution 6677/10, and ensuring continuous post-market surveillance, companies can uphold the integrity of their research while prioritizing participant safety. Engaging with ethics committees and remaining adaptable to evolving regulations is vital for compliance and successful market entry.
Defining clear objectives and meticulously designing study protocols are essential steps in laying a solid foundation for post-market studies. By identifying key outcomes, formulating hypotheses, and employing robust data collection methods, organizations can significantly enhance the scientific rigor of their research. Leveraging expert insights, particularly from seasoned partners like bioaccess®, can streamline this process and guarantee adherence to regulatory standards.
Effective recruitment strategies and comprehensive data collection methods are pivotal to the success of post-market studies. Collaborating with healthcare providers and utilizing digital platforms can substantially improve participant engagement. Furthermore, ensuring an informed consent process fosters trust and transparency, ultimately leading to more reliable results.
Monitoring progress and ensuring compliance throughout the study is paramount for maintaining the quality and credibility of research efforts. Establishing robust monitoring protocols, conducting regular audits, and systematically addressing adverse events contribute to improved patient outcomes and regulatory adherence.
In summary, a proactive and informed approach to post-market studies not only enhances compliance but also fortifies market entry strategies. By prioritizing ethical practices and participant safety, organizations can adeptly navigate the intricacies of the regulatory framework in Argentina, achieving sustained success in their research endeavors.
Frequently Asked Questions
What is the role of ANMAT in post-market studies in Argentina?
ANMAT is the primary authority overseeing trials in Argentina, and understanding its regulatory framework is essential for designing post-market studies.
What does ANMAT Resolution 6677/10 entail?
ANMAT Resolution 6677/10 outlines guidelines for good practices in trials, emphasizing ethical considerations and the importance of participant safety, which are crucial for maintaining the integrity of the study.
What are the post-market surveillance requirements for medical devices in Argentina?
Companies must meet stringent obligations for ongoing monitoring of medical devices after approval, including timely reporting of adverse events and conducting regular safety assessments to ensure compliance with safety standards.
Why is ethics committee approval necessary before starting research?
Securing authorization from a recognized ethics committee is required to ensure that the research aligns with ethical standards and protects participant rights throughout the research process.
How does the regulatory framework evolve in Argentina regarding quality management systems?
ANMAT requires companies to adapt their quality management systems within 120 days of new regulations taking effect to ensure compliance and maintain the credibility of the research.
What is the taxation range on imported medical devices under Mercosur?
The taxation on imported medical devices falls under the Mercosur Common External Tariff, ranging from 0% to 16%, which can significantly influence the cost structure for medical device companies.
How can bioaccess® assist companies in navigating post-market study design and regulations?
bioaccess® specializes in clinical trial management services and can help clients navigate regulatory requirements, post-market study design, and financial implications, facilitating a smoother market entry strategy in Argentina.




