Overview
The article primarily aims to serve as a comprehensive guide for mastering the Medtech trial approval process in Bolivia. It delineates essential steps, such as:
- Understanding regulatory requirements
- Preparing necessary documentation
- Submitting trial applications
- Implementing post-approval monitoring
This process is further streamlined and ensured for compliance with local regulations through the expertise of service providers like bioaccess.
Introduction
Navigating the complexities of Medtech trials in Bolivia necessitates a keen understanding of the regulatory landscape that governs clinical research. With the Ministry of Health and the Agencia Nacional de Medicamentos y Tecnología en Salud (AGEMED) at the forefront, researchers must familiarize themselves with essential laws and guidelines that dictate the approval process.
From the foundational Law No. 173 to the critical Good Clinical Practice (GCP) standards, each aspect plays a vital role in ensuring that trials not only meet regulatory requirements but also uphold ethical standards.
This article explores the necessary steps to successfully conduct Medtech trials in Bolivia, underscoring the importance of:
- Thorough documentation
- Strategic partnerships
- Ongoing compliance measures
These elements facilitate a smooth approval journey while safeguarding participant welfare.
Understand the Regulatory Landscape for Medtech Trials in Bolivia
To successfully navigate the Medtech approval process in Bolivia, understanding the regulatory environment is essential. The primary regulatory body overseeing clinical studies in Bolivia is the Ministry of Health, specifically through the Agencia Nacional de Medicamentos y Tecnología en Salud (AGEMED). Familiarize yourself with the following key regulations regarding the Medtech trial approval process in Bolivia.
- Law No.: This law governs the regulation of medical devices and establishes the framework for the Medtech trial approval process in Bolivia.
- Decree No. 292: This decree outlines the requirements for the registration and approval of medical devices, including the Medtech trial approval process in Bolivia and clinical study protocols.
- Good Clinical Practice (GCP) underscores the necessity of adhering to GCP guidelines, which are vital for the ethical and scientific quality of studies, particularly in relation to the Medtech trial approval process in Bolivia, where collaborating with a specialized service provider like bioaccess can greatly streamline your clinical study workflow.
With over 20 years of experience in Medtech, bioaccess offers extensive clinical study management services, including feasibility assessments, site selection, compliance reviews, study setup, import permits, project management, and reporting. Their expertise encompasses various study types, such as Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies (PMCF). Leveraging bioaccess's expertise and customized strategy can ensure a more streamlined Medtech trial approval process in Bolivia for your application.

Prepare Essential Documentation and Compliance Requirements
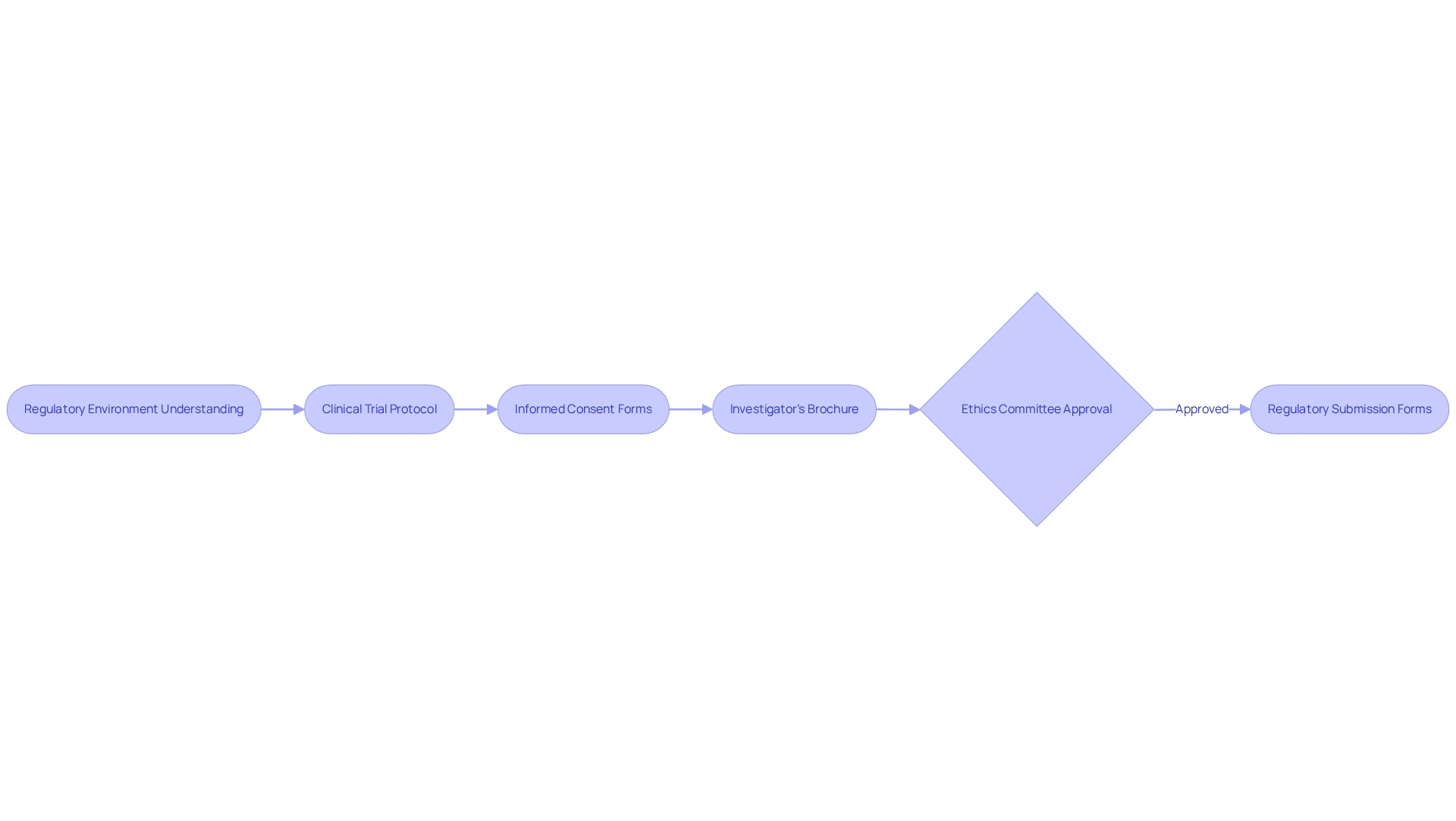
Once you grasp the regulatory environment, the next step is to prepare the essential documentation needed for the Medtech trial approval process in Bolivia. Key documents include:
- Clinical Trial Protocol: This document outlines the objectives, design, methodology, statistical considerations, and organization of the trial.
- Informed Consent Forms: Ensure that these documents are clear and adhere to ethical standards, allowing participants to comprehend the study's purpose and risks.
- Investigator's Brochure: This should provide comprehensive information about the investigational product, including preclinical and clinical data.
- Ethics Committee Approval: Obtain authorization from a recognized ethics committee to ensure that the study meets ethical standards.
- Regulatory Submission Forms: Complete all necessary forms required by AGEMED for trial approval.
Navigating the Latin American Medtech landscape requires recognizing the structural challenges and opportunities present within the region. Collaborating with local organizations, such as bioaccess®, can facilitate a smoother evaluation and enhance market access strategies. Furthermore, ensure that all documents align with local regulations and are translated into Spanish, considering cultural sensitivity in documentation to promote better understanding and adherence. This preparation will not only streamline the Medtech trial approval process in Bolivia but also demonstrate your commitment to compliance and transparency, thereby fostering trust with local stakeholders. Additionally, bioaccess® is committed to ensuring information security and addressing client concerns through established grievance and data protection procedures, reinforcing the significance of client trust in the clinical study process.
Submit Your Trial Application and Navigate the Review Process
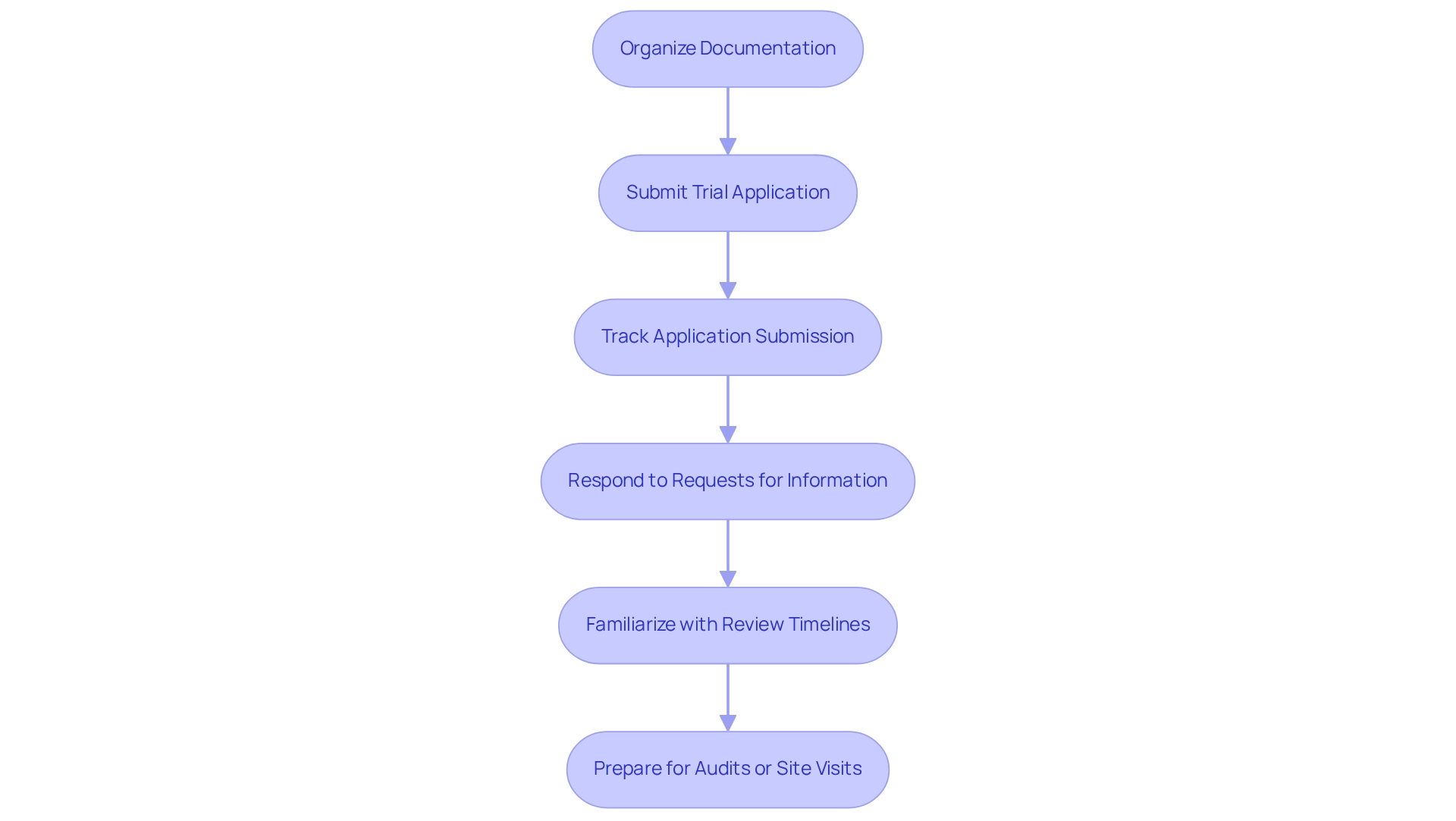
After organizing your documentation, the subsequent step in the Medtech trial approval process in Bolivia is to submit your trial application to the relevant authority. Begin by compiling your application: ensure that all necessary documents are included and arranged according to the organization's guidelines, which may encompass feasibility studies and compliance reviews to meet local requirements. Utilize the online submission portal for efficiency in the Medtech trial approval process in Bolivia, and confirm your submission to track your application while proactively engaging with the organization during the evaluation process. Respond promptly to any requests for additional information or clarification, leveraging your expertise in regulatory affairs to facilitate discussions. Familiarize yourself with the expected review timelines of the Medtech trial approval process in Bolivia and follow up if you do not receive feedback within the stipulated period. Understanding these timelines is crucial for managing expectations and planning accordingly.
Be prepared for potential audits or site visits from AGEMED as part of the evaluation process. Ensure that all testing locations comply with regulatory standards, as this is essential for a successful study within the Medtech trial approval process in Bolivia. Navigating this process effectively can expedite your approval. Partnering with bioaccess® offers comprehensive clinical study management services, including site selection, project management, and reporting, ensuring a smoother journey through the regulatory landscape.
Implement Post-Approval Monitoring and Compliance Strategies
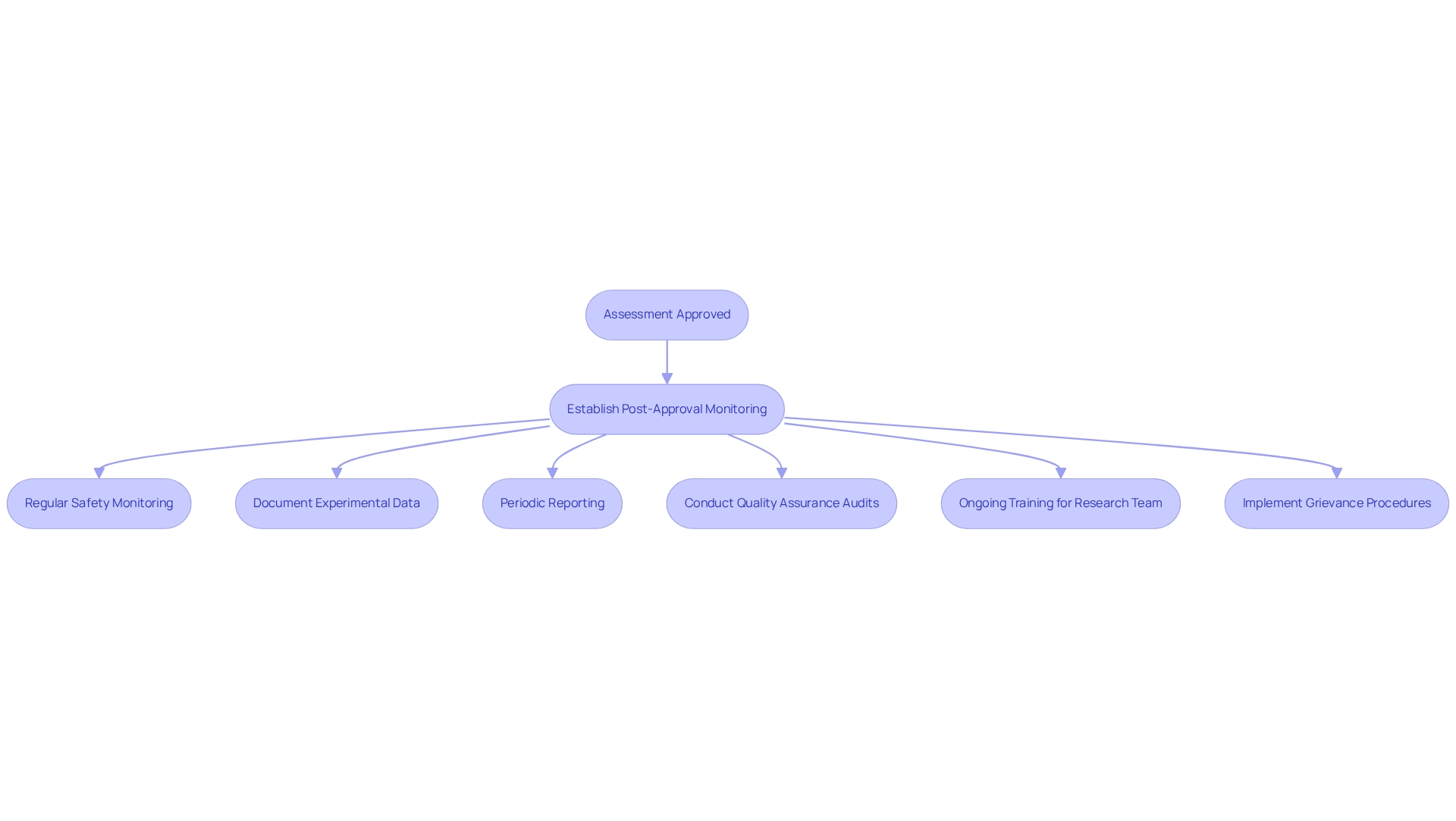
Once your assessment is approved and in progress, establishing robust post-approval monitoring and adherence strategies is essential. Regular safety monitoring is crucial; continuously monitor participant safety and report any adverse events to the designated authority as required. Additionally, ensure that all experimental data is accurately documented and preserved in accordance with GCP guidelines. Periodic reporting is also vital; submit regular progress reports to AGEMED, detailing study status, participant enrollment, and any issues encountered. bioaccess® offers comprehensive reporting services, including study status updates and inventory management, to streamline this process.
Quality assurance audits should be conducted regularly to ensure compliance with regulatory requirements and identify areas for improvement. bioaccess® can assist in this area by offering project management and monitoring services tailored to your study's needs. Engage in thorough feasibility studies and select suitable research locations and chief investigators to ensure the project's success; bioaccess® specializes in these areas, providing expert guidance to optimize your trial setup.
Ongoing training for your research team on adherence and regulatory updates is essential to ensure everyone is informed and aligned. bioaccess® emphasizes the significance of review processes and feedback on study documents to help your team remain current with country requirements. Moreover, ensure that your team is aware of the grievance and data protection procedures in place, as these are essential for maintaining client trust and addressing any concerns that may arise during the trial.
By implementing these strategies, you can maintain compliance and ensure the integrity of your clinical trial throughout its duration, while also benefiting from bioaccess®'s commitment to data protection and client trust.
Conclusion
Successfully conducting Medtech trials in Bolivia relies on a thorough understanding of the regulatory landscape and a meticulous approach to documentation and compliance. Key regulations, such as Law No. 173 and Good Clinical Practice (GCP) guidelines, establish the foundation for ethical and scientifically sound research. By collaborating with experienced organizations like bioaccess, researchers can streamline the approval process and significantly improve their chances of success.
Preparation is crucial; essential documents, including clinical trial protocols and informed consent forms, must be carefully crafted and aligned with local regulations. Engaging with the regulatory authority, AGEMED, throughout the review process fosters transparency and trust—critical elements in navigating the complexities of clinical trials.
Once trials commence, implementing robust post-approval monitoring and compliance strategies is essential to ensure participant safety and data integrity. Regular reporting, quality assurance audits, and ongoing training for the research team are vital components of maintaining compliance. By prioritizing these elements, researchers not only meet regulatory standards but also contribute to the advancement of Medtech in Bolivia, ultimately benefiting participants and the broader healthcare community.
Frequently Asked Questions
What is the primary regulatory body for Medtech approval in Bolivia?
The primary regulatory body overseeing clinical studies in Bolivia is the Ministry of Health, specifically through the Agencia Nacional de Medicamentos y Tecnología en Salud (AGEMED).
What key regulations govern the Medtech trial approval process in Bolivia?
The key regulations include Law No., which governs the regulation of medical devices, and Decree No. 292, which outlines the requirements for the registration and approval of medical devices, including clinical study protocols.
Why is Good Clinical Practice (GCP) important for Medtech trials in Bolivia?
Good Clinical Practice (GCP) is vital for ensuring the ethical and scientific quality of studies, particularly in relation to the Medtech trial approval process in Bolivia.
How can bioaccess assist with the Medtech trial approval process in Bolivia?
Bioaccess can streamline the clinical study workflow by offering extensive clinical study management services, including feasibility assessments, site selection, compliance reviews, study setup, import permits, project management, and reporting.
What types of studies does bioaccess have experience with?
Bioaccess has experience with various study types, including Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies (PMCF).
How does leveraging bioaccess's expertise benefit the Medtech trial approval process in Bolivia?
Leveraging bioaccess's expertise and customized strategy can ensure a more streamlined Medtech trial approval process for your application in Bolivia.




