Overview
Maximizing Medtech trial partnerships in Argentina for success necessitates leveraging local regulatory expertise, achieving cost-effectiveness, and accessing diverse patient populations. This approach is essential for enhancing the efficiency and effectiveness of clinical studies within the region.
Understanding and navigating the regulatory landscape, fostering collaborations with local institutions, and employing innovative trial designs are crucial elements that significantly contribute to these efforts. By addressing these key challenges, stakeholders can position themselves advantageously in the evolving Medtech landscape.
Introduction
In the rapidly evolving landscape of medical technology, Argentina stands out as a promising hub for clinical trials, presenting unique advantages that can significantly enhance the success of Medtech initiatives. The complex regulatory framework governed by the National Administration of Drugs, Foods and Medical Technology (ANMAT) necessitates a strategic approach to navigate this environment effectively.
By leveraging local expertise and fostering partnerships with healthcare providers, regulatory bodies, and research institutions, companies can streamline processes, reduce costs, and gain access to diverse patient populations.
Furthermore, innovative trial designs and effective patient engagement strategies are essential for optimizing outcomes and accelerating recruitment. As the Medtech sector continues to expand in Argentina, understanding and capitalizing on these opportunities will be crucial for companies aiming to make a lasting impact in the field.
Leverage Local Regulatory Expertise
The intricate landscape of Medtech trial partnerships in Argentina is significantly influenced by the country's regulatory framework, overseen by the National Administration of Drugs, Foods and Medical Technology (ANMAT), which imposes stringent requirements. However, leveraging regional regulatory expertise can greatly streamline this process.
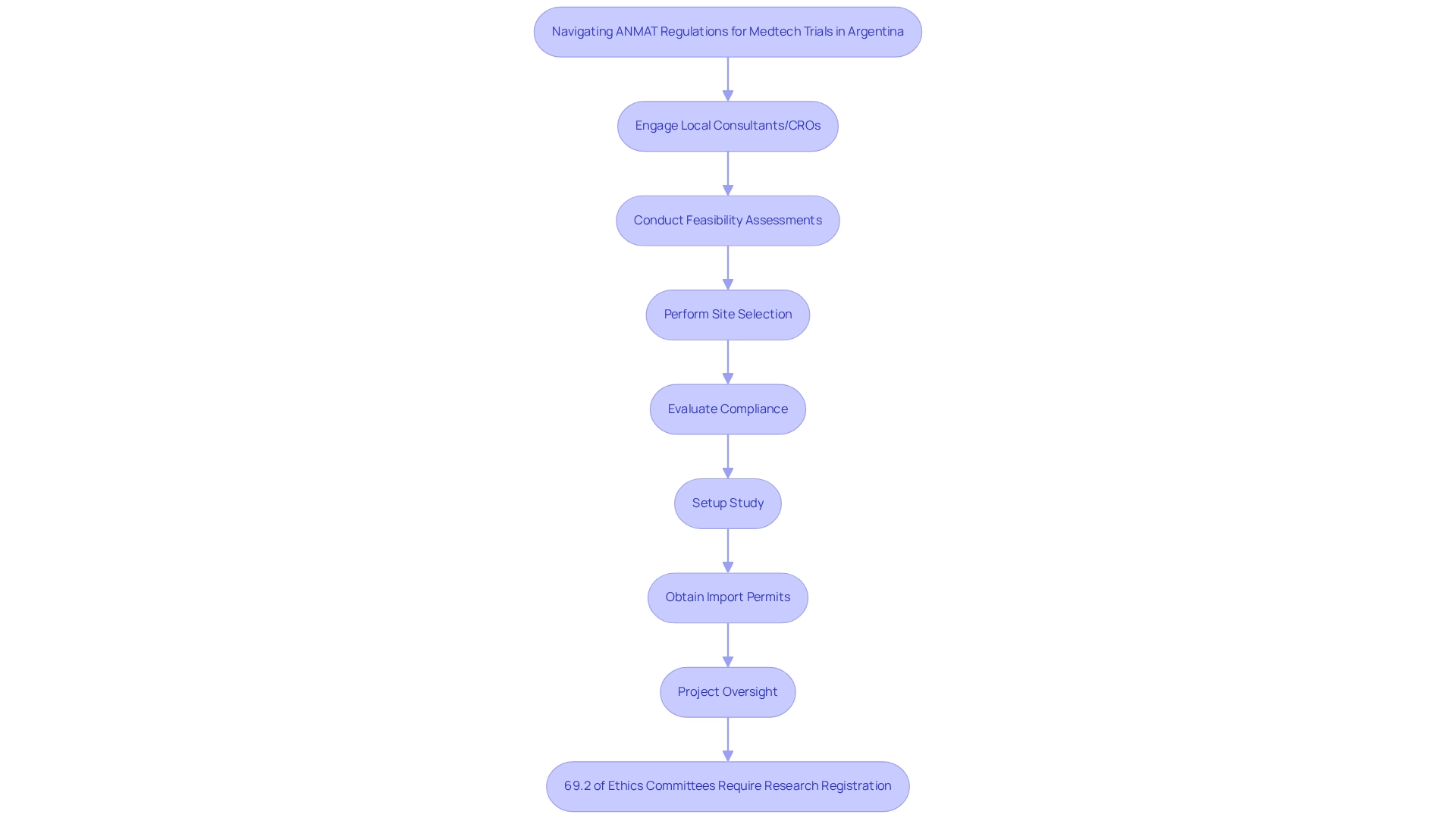
Engaging local consultants or contract research organizations (CROs) with a comprehensive understanding of ANMAT's protocols can markedly reduce approval timelines, allowing studies to commence swiftly. bioaccess® offers extensive management services for studies, encompassing:
- Feasibility assessments
- Site selection
- Compliance evaluations
- Setup
- Import permits
- Project oversight
- Reporting on both serious and non-serious adverse events
These are essential components for navigating these complexities. Notably, statistics reveal that 69.2% of ethics committees now require evidence of research registration for protocol approval, underscoring the necessity for thorough documentation. This requirement aligns with the anticipated growth of the Argentine research market, which stands to benefit from improved regulatory compliance and efficiency.
By mastering the intricacies of regional regulations, Medtech trial partnerships in Argentina can prepare comprehensive submissions that adhere to ANMAT's standards, thereby increasing the likelihood of successful outcomes. Case studies from the Dominican Republic exemplify how advancements in healthcare systems can enhance a country's attractiveness for clinical research, suggesting that similar strategies could yield benefits in Argentina.
For instance, focusing on regional healthcare enhancements and regulatory alignment can bolster Medtech trial partnerships in Argentina, positioning the nation as a formidable competitor in the Medtech sector. Expert insights emphasize that adeptly navigating ANMAT regulations is crucial for the success of Medtech trial partnerships in Argentina, rendering regional regulatory knowledge an invaluable asset in this competitive arena.
As Tiffany Ryder, a healthcare advocate, aptly stated, 'Find your harshest critic, not your biggest fan. That’s where real product improvement happens.' Therefore, actively seeking critical feedback and engaging regional expertise can lead to more successful testing outcomes. To fully capitalize on these insights, Medtech companies should prioritize forging relationships with local regulatory experts to effectively navigate the complexities of ANMAT regulations.
Achieve Cost-Effective Clinical Trials
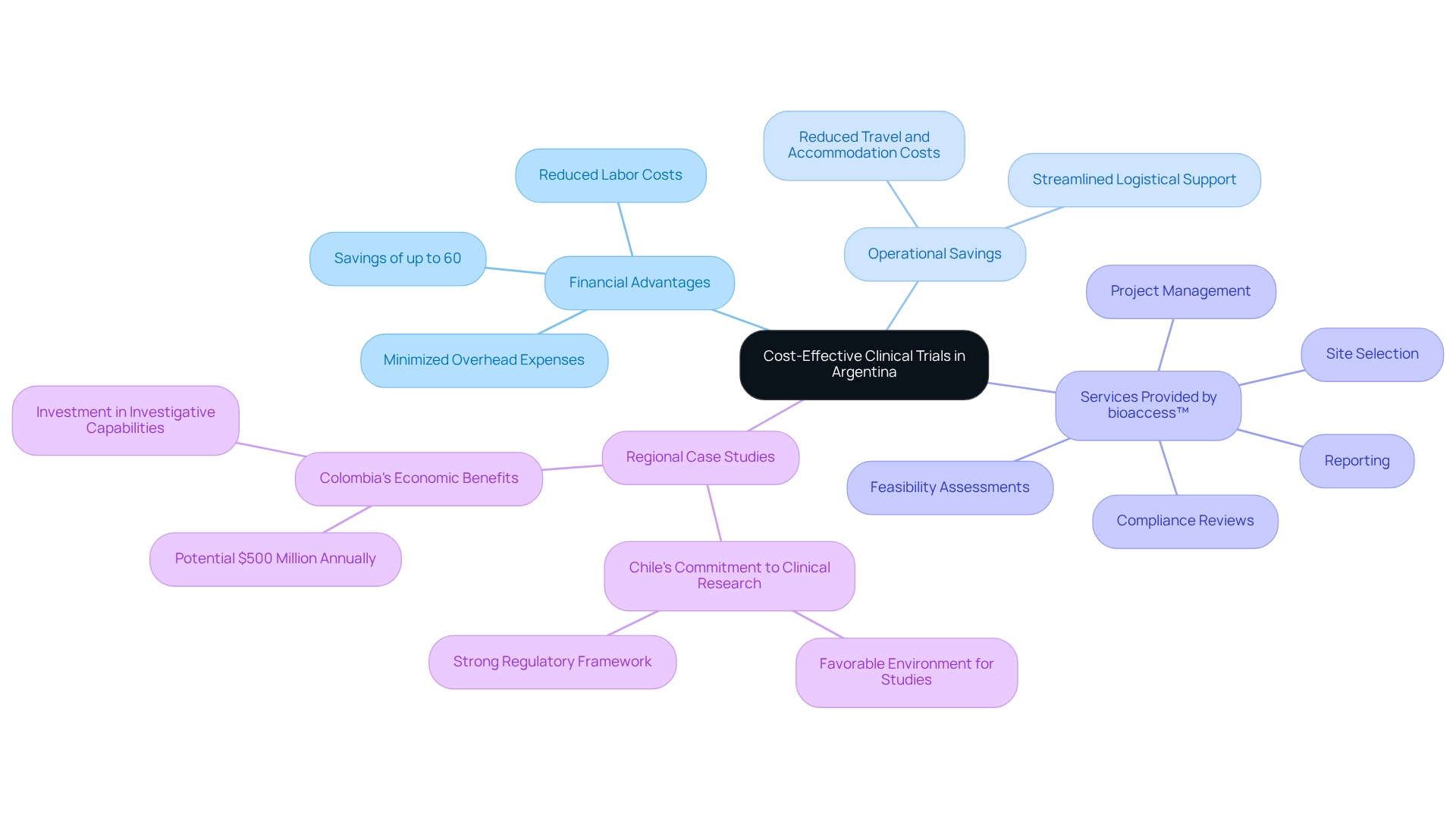
Argentina offers a highly cost-effective environment for Medtech trial partnerships, characterized by significantly reduced operational expenses, including labor and facility costs. Companies can achieve savings of up to 60% when conducting studies in Argentina compared to those in the U.S. or Europe. The U.S. Food and Drug Administration emphasizes the importance of considering all elements in planning, stating, 'Like every coin, there are two sides.'
To fully leverage these financial advantages, it is advisable for companies to establish Medtech trial partnerships in Argentina, which can streamline logistical support and minimize overhead expenses. Engaging local sites for patient recruitment not only enhances recruitment efficiency but also reduces travel and accommodation costs for study personnel, thereby maximizing overall cost-effectiveness in medical research.
bioaccess™ provides comprehensive research management services, including:
- Feasibility assessments
- Site selection
- Compliance reviews
- Setup
- Import permits
- Project management
- Reporting
These services significantly enhance the efficiency of trials in the region. The evolving healthcare system in the Dominican Republic supports the advancement of medical device research, further highlighting the region's potential.
Case studies, such as Chile's commitment to medical research, demonstrate successful instances of cost-efficient studies in Latin America, reinforcing the case for pursuing Medtech trial partnerships in Argentina. Additionally, Colombia's medical research initiatives could yield nearly $500 million in economic benefits annually, underscoring the broader economic landscape of medical studies in the area.
Access Diverse Patient Populations
Argentina's population presents a vibrant tapestry of ethnicities and health conditions, offering a unique opportunity for Medtech companies to engage with diverse patient populations. This variety is crucial for research studies aimed at evaluating the effectiveness of medical devices across different demographics.
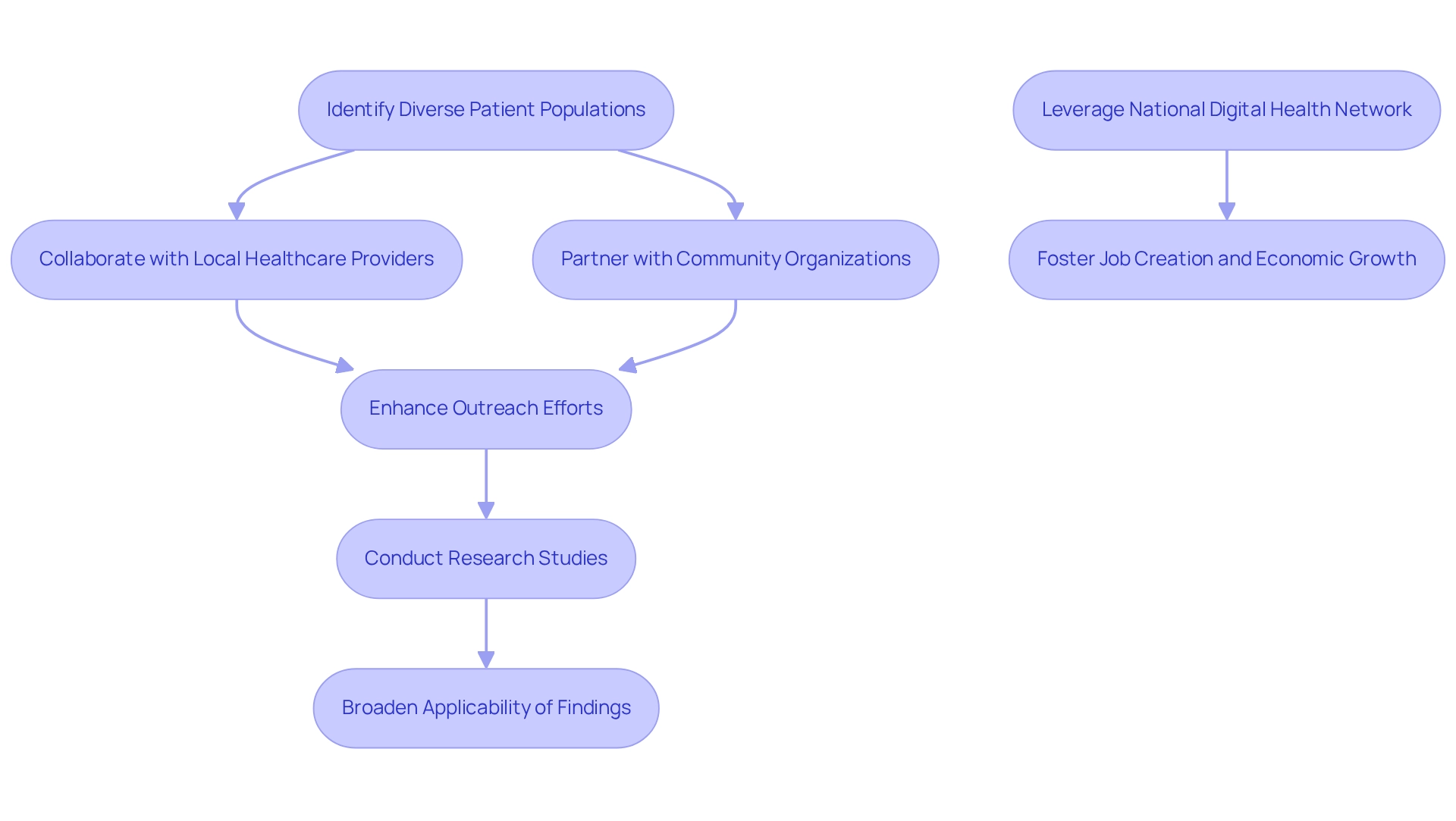
By collaborating with local healthcare providers and community organizations, companies can significantly enhance outreach efforts, ensuring that studies encompass participants from a wide array of backgrounds. Such collaboration not only enriches the data collected but also broadens the applicability of findings to a more extensive patient base.
Furthermore, the recognition of Argentina's national regulatory body, ANMAT, by the Pan American Health Organization enhances the nation's credibility within the global research regulatory approval process, positioning it as a desirable location for international studies.
As the Dominican Republic experiences an upward trend in attracting medical device trials, this regional development underscores Argentina's growing significance as a hub for Medtech innovation. Highlighting ethnic diversity in medical research transcends mere regulatory compliance; it serves as a strategic advantage that can yield more comprehensive and impactful outcomes in the Medtech sector.
To capitalize on these opportunities, research directors should actively seek collaborations with community organizations and leverage the national digital health network established by Argentina's Ministry of Health to improve patient experiences and healthcare delivery.
Additionally, by partnering with bioaccess®, a leader in comprehensive clinical trial management services—including feasibility studies, site selection, compliance reviews, project management, and reporting—companies can ensure their trials are not only efficient but also aligned with community needs, ultimately fostering job creation and economic growth in the region.
Accelerate Patient Recruitment
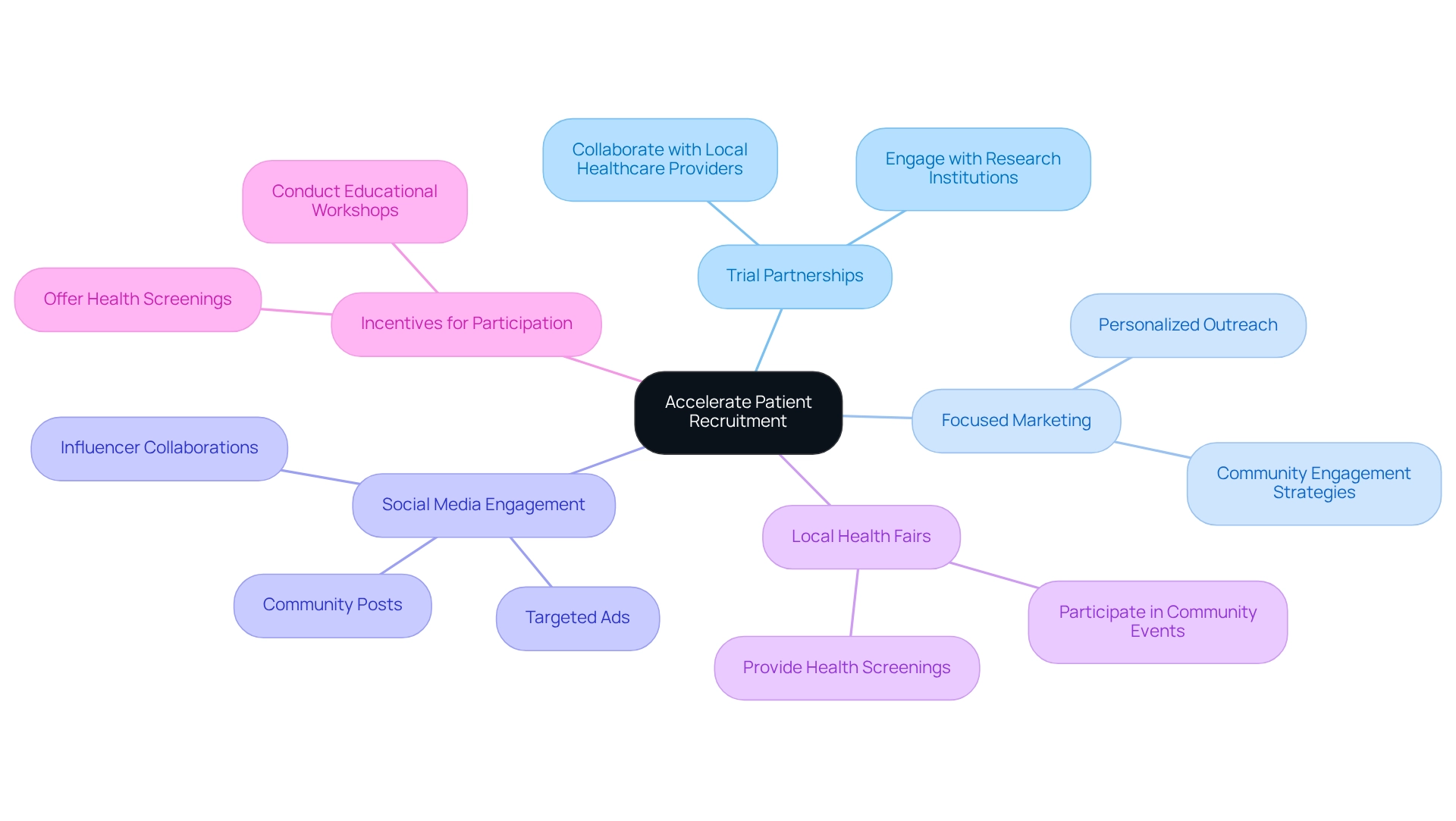
To efficiently boost patient recruitment in Argentina, Medtech firms must engage in trial partnerships and employ focused marketing strategies that resonate with regional communities. The 2025 State of Marketing & Trends Report reveals that over 70% of marketers believe personalized outreach significantly enhances engagement, underscoring the necessity for tailored approaches.
Leveraging social media platforms is crucial, as they serve as powerful tools for outreach and engagement. As Pete Cashmore, founder of mashable.com, aptly states, "We’re living at a time when attention is the new currency." This highlights the importance of engaging with multiple channels to capture the interest of potential participants.
Furthermore, participating in local health fairs and forming partnerships with healthcare providers can significantly enhance visibility and attract potential participants. Providing incentives, such as health screenings or educational workshops, can further encourage individuals to participate in research studies.
A case study on athenahealth illustrates this point; their introduction of a new Patient Intake feature streamlined the recruitment process, saving time and enhancing healthcare efficiency. Establishing a comprehensive database of potential participants will further streamline recruitment, ensuring that eligible candidates are readily available when needed.
By implementing these strategies and utilizing the expertise of bioaccess® in overseeing extensive research services—including Early-Feasibility, First-In-Human, Pilot, Pivotal, and Post-Market Follow-Up Studies—Medtech trial partnerships in Argentina can enhance their recruitment efforts, promote successful studies, and contribute to community economic growth and healthcare advancement.
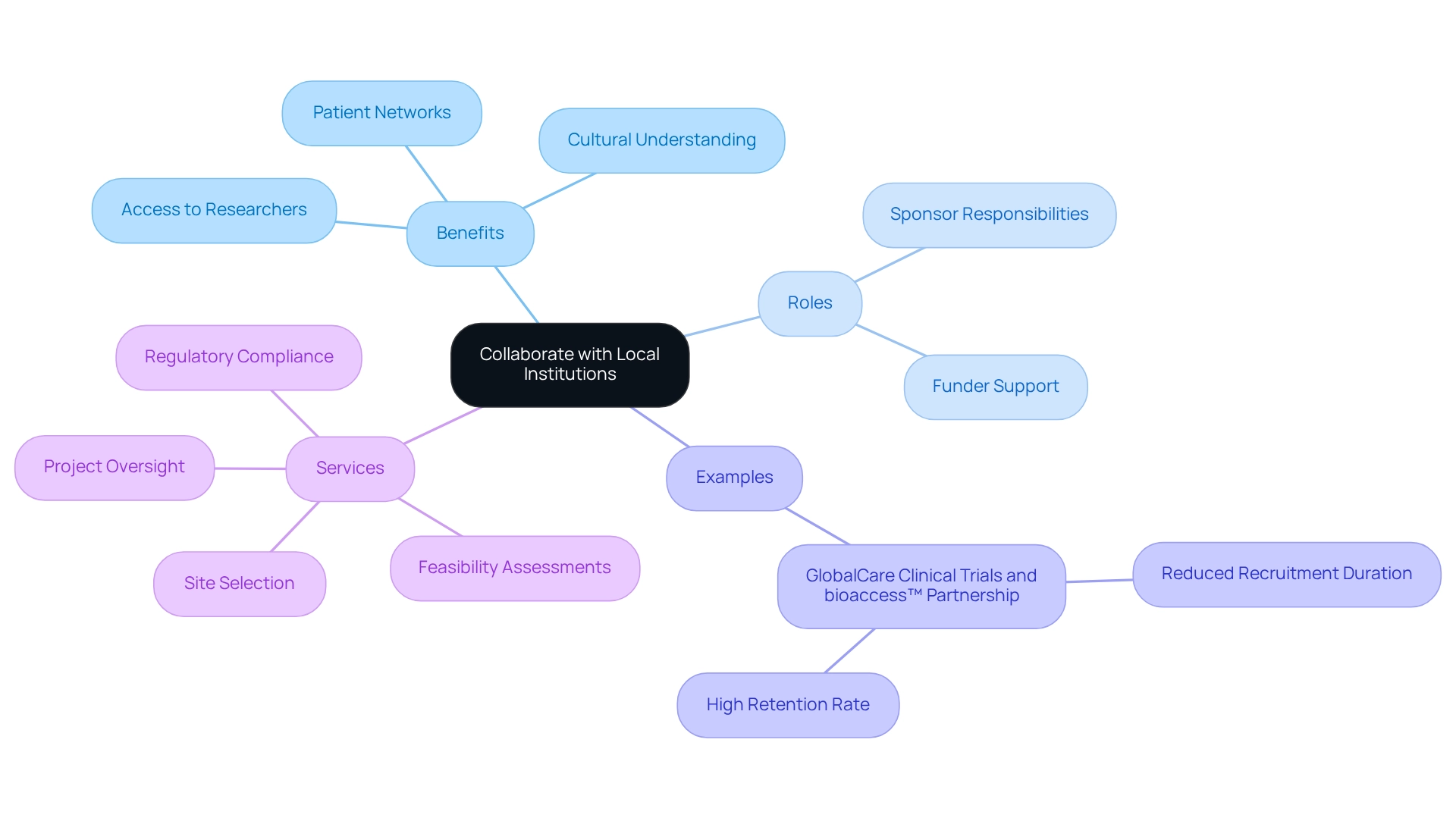
Collaborate with Local Institutions
The success of clinical studies in Argentina is fundamentally anchored in Medtech trial partnerships with regional hospitals, universities, and research organizations. These partnerships grant access to experienced researchers, specialized facilities, and established patient networks, which are essential for conducting studies efficiently and ethically. By leveraging the expertise of regional institutions, Medtech trial partnerships can enhance the credibility of their trials, making them more attractive to potential participants and stakeholders. Additionally, collaboration with local entities cultivates a deeper understanding of regional regulatory requirements and cultural nuances, ultimately leading to improved patient recruitment and retention.
The sponsor bears the responsibility for initiating a research project and ensuring compliance with regulations, while the funder provides financial support, highlighting the operational dynamics inherent in these partnerships. Successful cases illustrate how Medtech trial partnerships have not only streamlined operations but also enriched the overall research environment. A notable example is GlobalCare Clinical Trials' collaboration with bioaccess™, which enhanced ambulatory services in Colombia, resulting in a remarkable reduction of over 50% in recruitment duration and a retention rate exceeding 95%. This partnership exemplifies how effective collaborations can boost operational efficiency while ensuring adherence to regulatory standards and Good Clinical Practice.
bioaccess™ offers an extensive array of services, including feasibility assessments, site selection, regulatory compliance, and project oversight, all of which are vital for advancing medical device evaluations. As sociologist William H. Whyte aptly stated, "The great enemy of communication, we find, is the illusion of it." This emphasizes the critical role of effective communication in nurturing successful collaborations. By strategically positioning themselves through local partnerships, Medtech trial partnerships in Argentina can significantly amplify their medical initiatives.
Implement Innovative Trial Designs
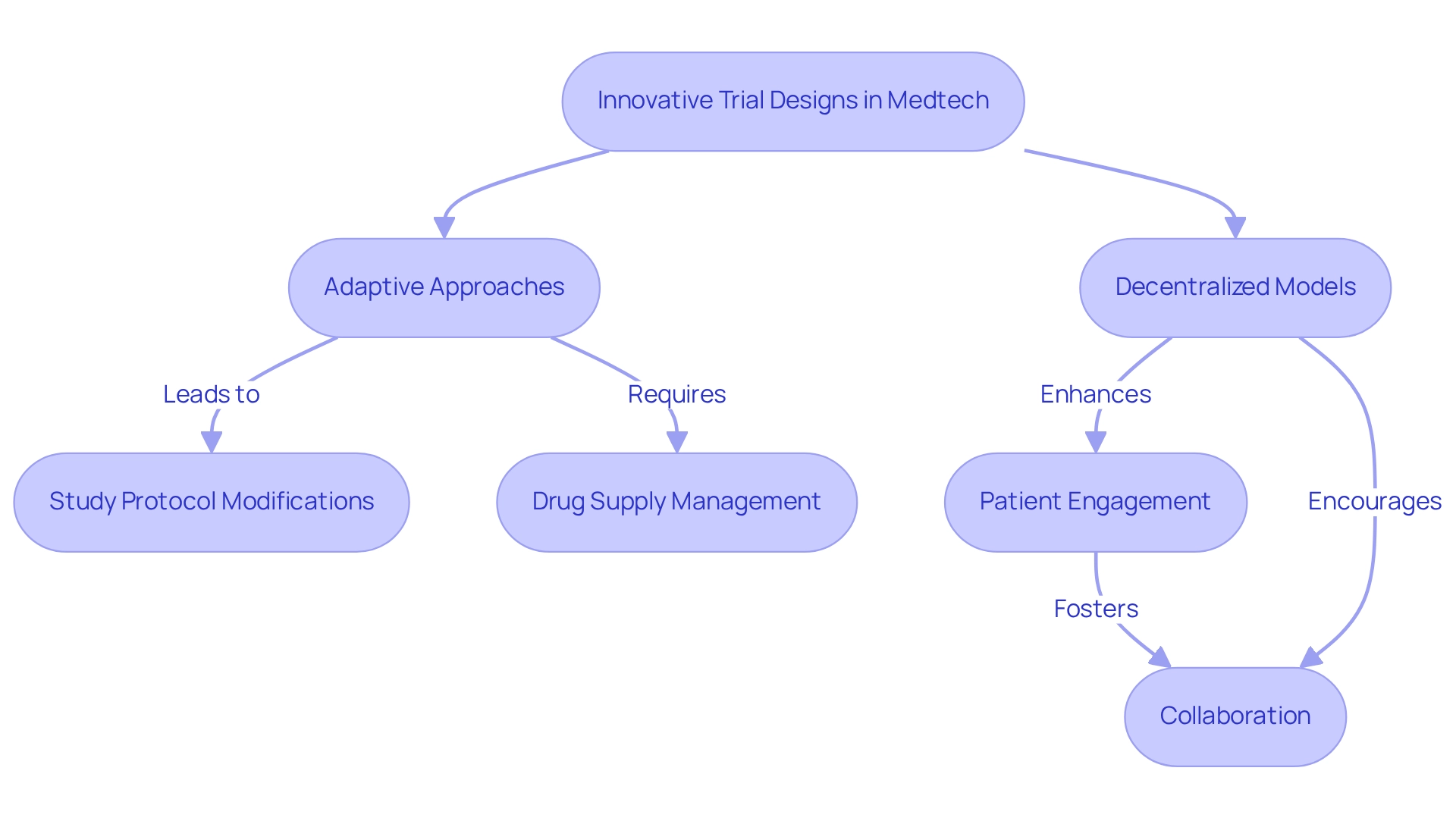
Implementing innovative experimental designs, including adaptive approaches and decentralized models, can significantly enhance the efficiency of Medtech trial partnerships in Argentina. With over 20 years of expertise in Medtech, bioaccess® employs adaptive studies that permit modifications to the study protocol based on interim results, facilitating quicker decision-making and improved resource allocation. For example, the TG02 and temozolomide study utilized a Bayesian Optimal Interval (BOIN) design to determine optimal dosing for high-grade gliomas, exemplifying the effectiveness of adaptive methodologies in real-world applications.
Nevertheless, adaptive studies introduce complexities, particularly in drug supply management during multi-arm investigations, where potential imbalances between centers and fluctuations in drug demand may occur when arms are discontinued. A tailored central system for randomization, provided by bioaccess®, is essential to minimize errors and ensure timely communication of drug supply requirements to pharmacies.
Decentralized studies leverage telemedicine and remote monitoring to enhance patient engagement and retention, effectively alleviating the travel burden for participants. Insights from bioaccess® experts underscore the significance of these strategies in improving patient participation rates. By adopting these innovative approaches, Medtech firms, particularly those partnering with bioaccess®, can optimize their processes, mitigate logistical challenges, and potentially accelerate their market entry through Medtech trial partnerships in Argentina.
Bioaccess® offers comprehensive management services for studies, including Early-Feasibility Studies, First-In-Human Studies, and Post-Market Follow-Up Studies, ensuring that evaluations are conducted efficiently and effectively within the Latin American context. This ultimately contributes to better patient outcomes and expedited access to transformative medical devices. As emphasized by bioaccess® team members, collaboration is vital in these pioneering study designs.
Streamline Logistics and Supply Chain
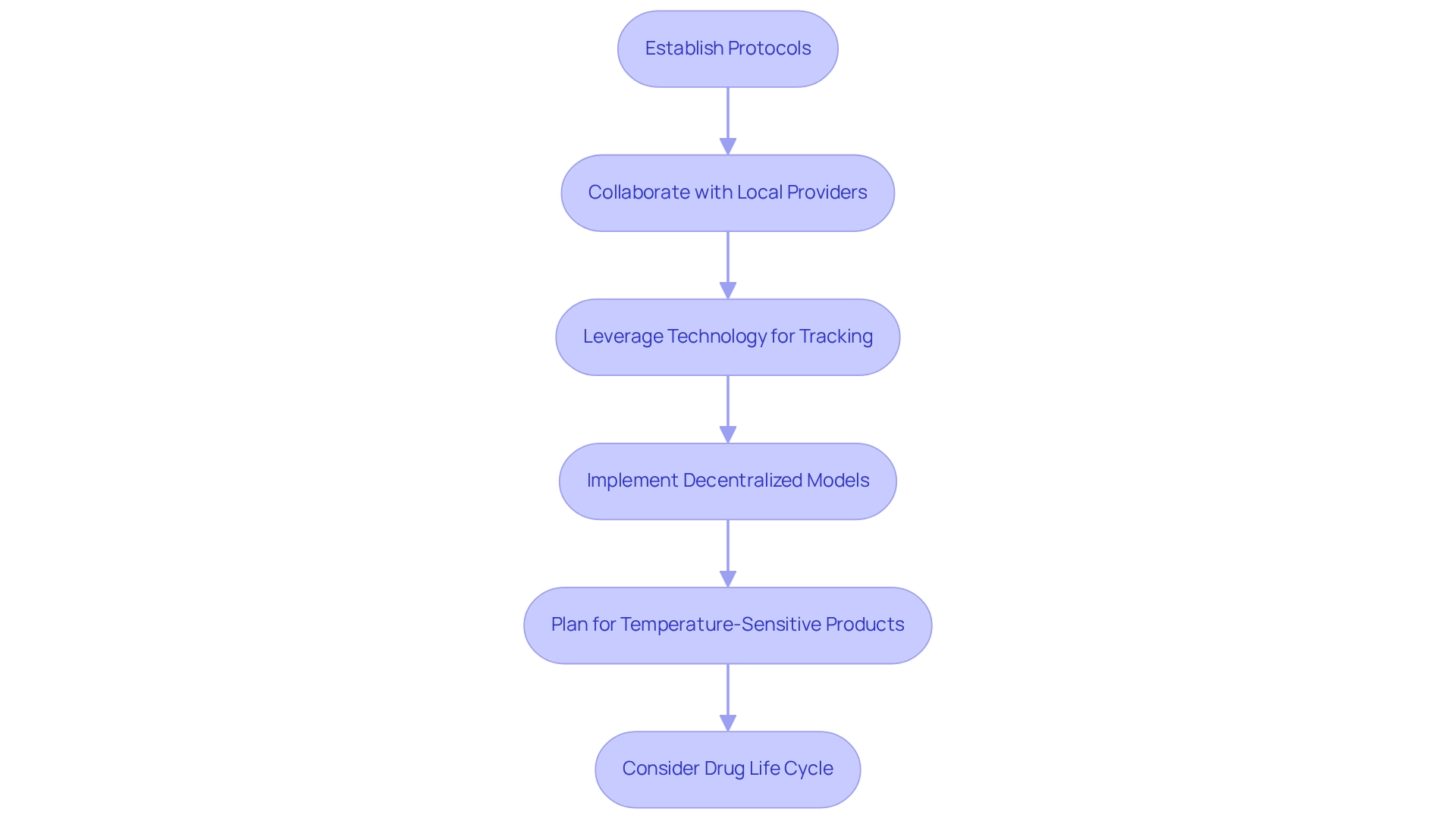
To efficiently optimize logistics and supply chain management for research studies in Argentina, Medtech firms must establish robust protocols for the transportation and storage of study materials. Collaborating with local logistics providers who possess a deep understanding of the regulatory landscape is crucial for ensuring compliance and operational efficiency. Leveraging technology for real-time tracking of shipments and inventory management significantly enhances visibility, thereby reducing the risk of delays. In fact, technology investments represent 40% of the overall cost of ownership in medical studies, underscoring the importance of incorporating advanced solutions.
Moreover, implementing decentralized study models can expand participant access beyond conventional locations, addressing logistical issues inherent in research. Effective strategies for transporting materials include meticulous planning for temperature-sensitive products and employing automated systems to optimize processes. Insights from supply chain experts indicate that considering the complete drug life cycle during research planning can prevent future complications and extra costs. As Ian Davison, an RTSM Subject Matter Expert, observes, "Since the protocol directives influence the supply chain in numerous ways, taking into account the complete drug life cycle during the research planning process aids in avoiding future issues or additional costs."
Additionally, with bioaccess®'s 20+ years of expertise in managing Early-Feasibility, First-In-Human, Pilot, Pivotal, and Post-Market Follow-Up Research, companies can leverage Medtech trial partnerships in Argentina that offer a comprehensive approach to clinical project management. This includes feasibility assessments, site selection, compliance reviews, and project oversight. The case study titled "Planning for Complexity in Clinical Trials" highlights the unique challenges faced, particularly with biopharmaceuticals that have short shelf lives or require strict temperature control. By optimizing these logistical elements and utilizing bioaccess®'s specialized services, companies can maintain trial timelines and minimize disruptions, ultimately advancing the development of innovative medical devices in the region while contributing to local economic growth and healthcare improvement.
Generate Real-World Evidence
Producing real-world evidence (RWE) is essential for Medtech firms seeking to confirm their devices' efficacy beyond regulated research settings. By incorporating observational research and patient registries into their research frameworks, these companies can gather essential data on device performance in routine healthcare environments. Collaborating with local healthcare providers not only facilitates access to comprehensive patient data but also strengthens the credibility of the evidence gathered.
In Argentina, bioaccess® has proven essential in carrying out observational research that confirms medical devices, particularly in the context of Medtech trial partnerships, highlighting their influence on research outcomes. With over 20 years of experience in Medtech, their expertise in managing Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies (PMCF) enhances the overall understanding of device efficacy and safety. Recent studies have shown that observational designs can enhance randomized controlled experiments (RCTs) by addressing situations where RCTs may be unfeasible. This dual approach not only enhances the comprehension of device effectiveness and safety but also offers important insights that RCTs cannot, especially in intricate medical situations, as emphasized in the case analysis 'The Contribution of Observational Research to Nephrology.'
Moreover, RWE plays a pivotal role in regulatory submissions, offering insights that can significantly influence product development and marketing strategies. Ingrid Hopper, NHMRC Early Career Fellow and Head of Drug and Device Registries, highlights the significance of incorporating observational research into trials, stating that they can offer crucial data that supports the validation process. As the Medtech landscape evolves, the incorporation of real-world evidence generation into medical research is becoming progressively essential, ensuring that innovations fulfill the genuine needs of patients and healthcare providers alike. By utilizing observational research, bioaccess® enables Medtech trial partnerships in Argentina to not only confirm their devices but also contribute to a more comprehensive understanding of their effects on patient care and local healthcare enhancement. To maximize the benefits of medical research, consider partnering with bioaccess® to enhance your study's credibility and effectiveness.

Enhance Patient Engagement Strategies
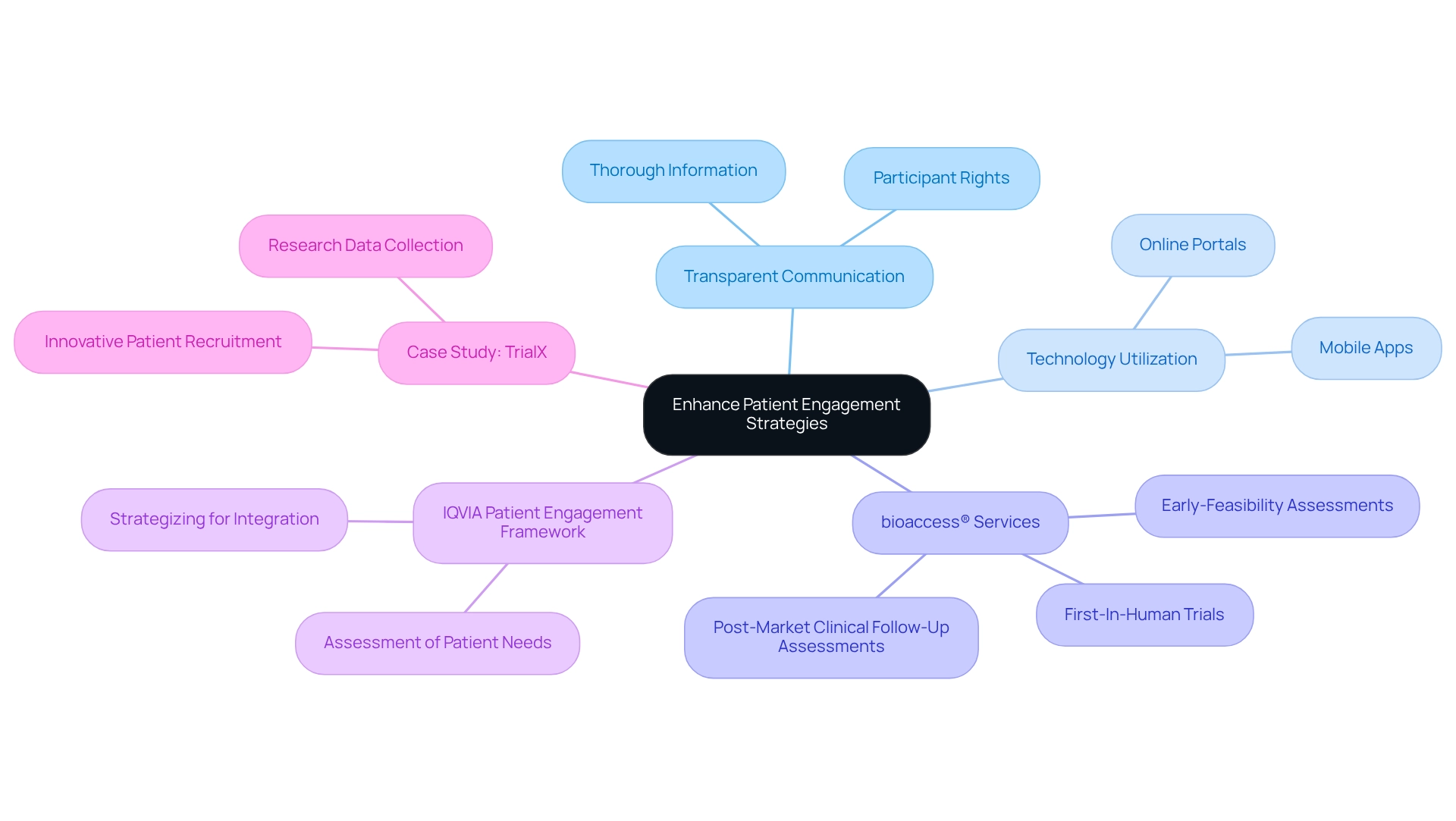
Improving patient involvement approaches is essential for the success of Medtech trial partnerships in Argentina. Organizations must emphasize transparent communication, ensuring that participants receive thorough information regarding the process and their rights. Leveraging technology, such as mobile apps and online portals, facilitates continuous communication and support, making it easier for participants to stay informed and engaged.
bioaccess®, with over 20 years of expertise in Medtech, provides expedited medical device research services, including:
- Early-Feasibility Assessments
- First-In-Human Trials
- Post-Market Clinical Follow-Up Assessments
These services significantly enhance patient involvement through organized communication and support systems. Our team possesses specialized expertise and adaptability in managing clinical studies, ensuring that each research project is customized to address the distinct requirements of the local market.
The IQVIA Patient Engagement Framework assists in evaluating and strategizing for the integration of patient needs and preferences, further underscoring the significance of clear communication and technology application in studies. Moreover, involving patients in the trial design process fosters a sense of ownership and commitment, which can significantly boost retention rates.
A notable example is TrialX, which has effectively enhanced medical research through innovative patient recruitment strategies and technology. By concentrating on these strategies, Medtech firms can not only enhance the participant experience but also improve the quality of the data gathered, ultimately resulting in more successful trial outcomes.
As Michael Crichton wisely remarked, "Health is akin to money; we never fully grasp its worth until it is gone," emphasizing the essential necessity for patient involvement in research studies.
Establish Long-Term Partnerships
Forming long-lasting alliances with regional stakeholders—such as healthcare providers, regulatory agencies, and research institutions—is essential for attaining ongoing success in Medtech trial partnerships in Argentina. For instance, the partnership between bioaccess™ and Caribbean Health Group aims to establish Barranquilla as a prominent location for clinical studies in Latin America. Such collaborations enhance the efficiency and effectiveness of clinical research initiatives.
Backed by Colombia's Minister of Health, this collaboration seeks to create a robust ecosystem that simplifies research processes and fosters trust within the local healthcare community. This trust is vital for the success of upcoming evaluations, as it encourages increased involvement and engagement from all parties concerned.
Moreover, statistics suggest that organizations with established collaborations experience a substantial rise in success rates, with some research demonstrating up to a 30% enhancement in results. This underscores the critical value of collaboration in the Medtech sector.
Recent discussions emphasize the need for participants in research studies to cultivate a culture where data sharing is the expected norm, committing to responsible approaches that maximize advantages while minimizing risks.
bioaccess® offers extensive services, including feasibility studies, investigator selection, regulatory compliance, and project management, which are crucial for advancing medical device evaluations. Their dedication to data protection and transparent grievance procedures ensures that client concerns are addressed, thereby enhancing trust.
Furthermore, addressing challenges in sharing trial data is imperative; small firms often face resource limitations, yet collaborations can provide the necessary support to overcome these obstacles. As the landscape of clinical research evolves, the ability to forge and maintain Medtech trial partnerships in Argentina will be a key differentiator for companies striving to thrive in this dynamic environment.
Conclusion
Argentina's emergence as a leading hub for clinical trials in the Medtech sector is underscored by its unique advantages, including regulatory expertise, cost-effectiveness, and access to diverse patient populations. By leveraging local knowledge and forming strategic partnerships with healthcare providers and research institutions, Medtech companies can navigate the complexities of the National Administration of Drugs, Foods and Medical Technology (ANMAT) regulations, ultimately leading to more efficient trial processes and successful outcomes.
The substantial cost savings that can be realized in Argentina compared to other regions, alongside the ability to engage with a varied demographic, positions the country as an attractive option for clinical research. Innovative strategies such as adaptive trial designs and enhanced patient engagement techniques further bolster the potential for accelerated recruitment and improved participant retention.
Moreover, the importance of collaboration cannot be overstated. Establishing long-term partnerships with local stakeholders not only fosters trust but also enhances the overall credibility and effectiveness of clinical trials. By integrating real-world evidence into the research framework and prioritizing clear communication strategies, Medtech companies can ensure that their innovations meet the actual needs of patients and healthcare providers.
In conclusion, Argentina offers a fertile ground for Medtech initiatives, where understanding and capitalizing on local opportunities can lead to significant advancements in the field. Embracing these strategies will be essential for companies seeking to thrive in this evolving landscape, ultimately contributing to improved healthcare outcomes and economic growth in the region.
Frequently Asked Questions
What is the role of ANMAT in Medtech trial partnerships in Argentina?
The National Administration of Drugs, Foods and Medical Technology (ANMAT) oversees the regulatory framework for Medtech trial partnerships in Argentina, imposing stringent requirements that must be met for approval.
How can local consultants or CROs assist in Medtech trials?
Engaging local consultants or contract research organizations (CROs) with expertise in ANMAT's protocols can significantly streamline the approval process and reduce timelines for starting studies.
What services does bioaccess® provide for Medtech studies?
bioaccess® offers a range of management services for studies, including feasibility assessments, site selection, compliance evaluations, setup, import permits, project oversight, and reporting on adverse events.
What recent trend has been observed regarding ethics committees in Argentina?
Statistics show that 69.2% of ethics committees now require evidence of research registration for protocol approval, highlighting the need for thorough documentation.
How does Argentina's regulatory environment impact the research market?
Improved regulatory compliance and efficiency are expected to drive growth in the Argentine research market, making it more attractive for Medtech trial partnerships.
What insights can be drawn from case studies in the Dominican Republic?
Case studies suggest that advancements in healthcare systems can enhance a country's attractiveness for clinical research, indicating that similar strategies could benefit Argentina.
What are the financial advantages of conducting Medtech trials in Argentina?
Argentina offers a cost-effective environment for Medtech trial partnerships, with operational expenses, including labor and facility costs, being significantly lower—up to 60% savings compared to the U.S. or Europe.
How can local patient recruitment enhance Medtech trials?
Engaging local sites for patient recruitment improves efficiency and reduces travel and accommodation costs for study personnel, maximizing overall cost-effectiveness.
What is the significance of Argentina's ethnic diversity for Medtech research?
Argentina's diverse population provides a unique opportunity for evaluating medical devices across different demographics, which enriches data and broadens the applicability of research findings.
How does collaboration with local healthcare providers benefit Medtech companies?
Collaborating with local healthcare providers and community organizations enhances outreach efforts, ensuring studies include participants from various backgrounds, leading to more comprehensive outcomes.
What role does the Pan American Health Organization play in Argentina's research credibility?
The recognition of ANMAT by the Pan American Health Organization enhances Argentina's credibility in the global research regulatory approval process, making it a desirable location for international studies.
How can Medtech companies leverage the national digital health network in Argentina?
Research directors should seek collaborations with community organizations and leverage the national digital health network established by Argentina's Ministry of Health to improve patient experiences and healthcare delivery.




