Introduction
Contract Research Organizations (CROs) play a vital role in the field of clinical research, encompassing a wide range of services in the pharmaceutical value chain. This article explores the challenges faced by CROs, the marketing strategies they employ, the role of content marketing in clinical trials, and showcases a case study of a company maximizing their clinical trial and consulting services.
It also delves into the key challenges and solutions in the industry, the marketing plans and execution strategies, and the outcomes achieved. Additionally, the article highlights the lessons learned and future directions for clinical trial companies to adapt and innovate in response to emerging healthcare trends.
Background and Context
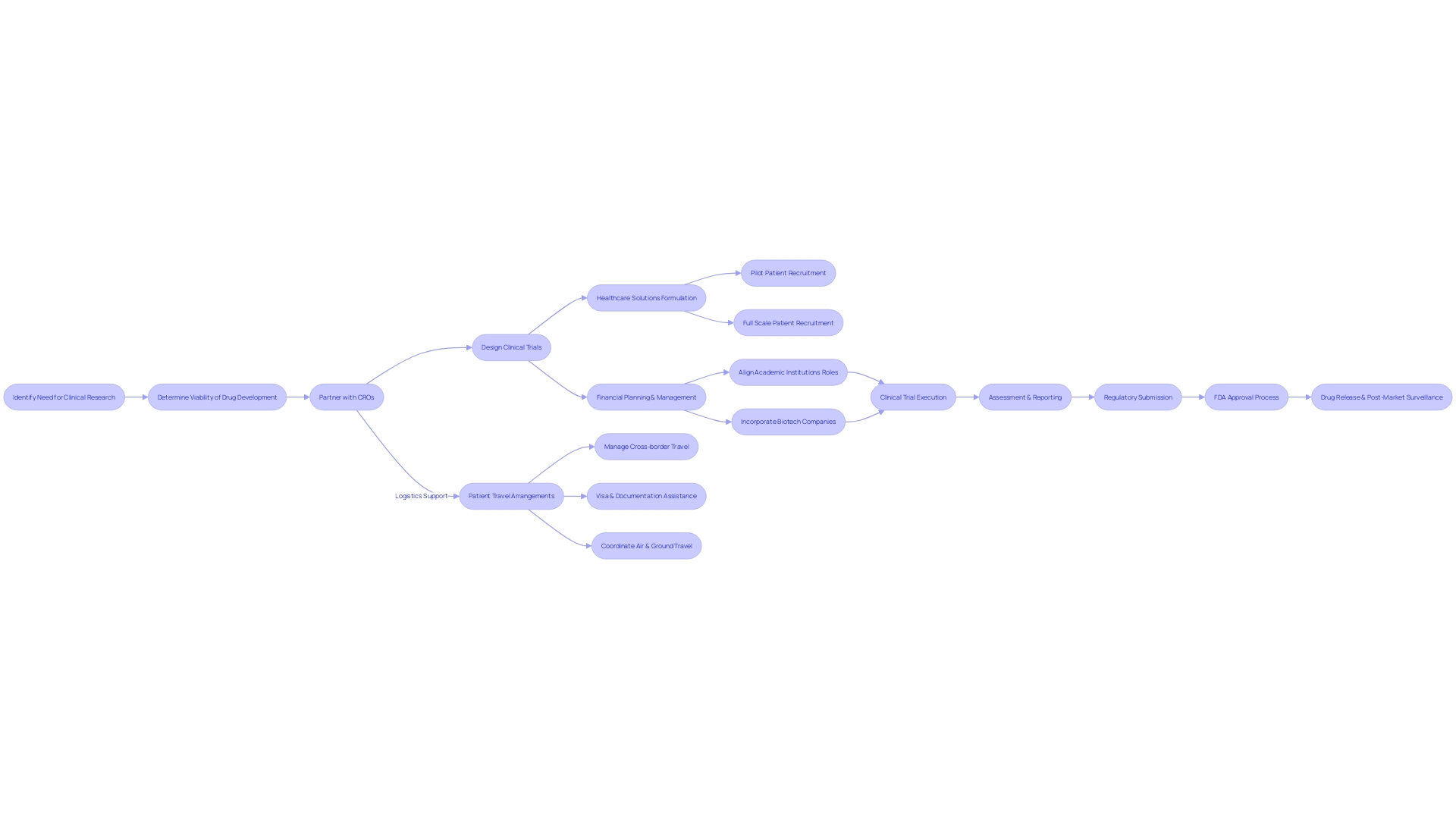
Contract Research Organizations (CROs) are at the forefront of clinical research, offering comprehensive services that span the full spectrum of the pharmaceutical value chain. One prime example is the CMIC Group in Japan, which has been an innovation leader in this space for over 30 years, providing end-to-end services from drug development to healthcare solutions.
Such CROss address complex challenges, like aiding a Pennsylvania patient suffering from a rare disease to participate in a trial in Turkey, overcoming daunting logistical and linguistic barriers. The intricacies involved in navigating the pharmaceutical R&D financial ecosystem is documented in a white paper for the Dutch government, illustrating the roles played by various entities, including academic institutions in drug targeting and biotech companies in drug discovery.
The paper underscores the financial and strategic decisions crucial in the early stages of clinical development. As articulated by experts, the challenges in the field are manifold, especially given the vast number of rare diseases, each requiring tailored research approaches. Funding limitations have historically impeded natural history studies, although recent changes show promise. It's clear that CROss are not just facilitators but pivotal players in orchestrating the complexities of clinical trials, ensuring that each 'link in the chain' is optimized for success.
Marketing Strategies for Clinical Trials
Clinical trial companies face a unique challenge when it comes to marketing their services. Unlike consumer goods, medical therapies require addressing the complexities of patient recruitment and trial accessibility.
For example, a patient in rural Pennsylvania diagnosed with an ultra-rare condition may be presented with the opportunity to join a clinical trial in Turkey, illustrating the hurdles of attracting participants to international studies. While eager to join potentially lifesaving research, this patient and their family are confronted with daunting logistical considerations - obtaining travel visas, navigating language barriers in documents, and arranging travel.
To effectively navigate such challenges, clinical trial companies need to implement a nuanced marketing strategy that goes beyond standard practices, such as targeted online advertising or social media campaigns. These strategies can help raise awareness, but they must be accompanied by thoughtful support, such as partnerships with healthcare professionals and patient advocacy groups that can help patients navigate the complexities of participating in a clinical trial.
The difference between consumer and medical marketing is vast and intricate, necessitating an approach that accounts for the risk-averse nature of decision-makers and participants. As emphasized by industry experts, it is crucial for clinical trial companies to influence decision-making well in advance. Planning is essential, as noted by transaction advisory professionals who have identified that the majority of pivotal decisions are made years before a clinical study concludes. To optimize each link in the trial chain, companies must invest time and energy into ensuring that future choices are 'bulletproof.' In designing and executing their marketing strategies, clinical trial firms must consider the entire patient journey and anticipate the needs and concerns that arise throughout the trial process, thereby increasing accessibility for potential participants and enhancing the overall reach and efficacy of clinical research.
The Role of Content Marketing in Clinical Trials
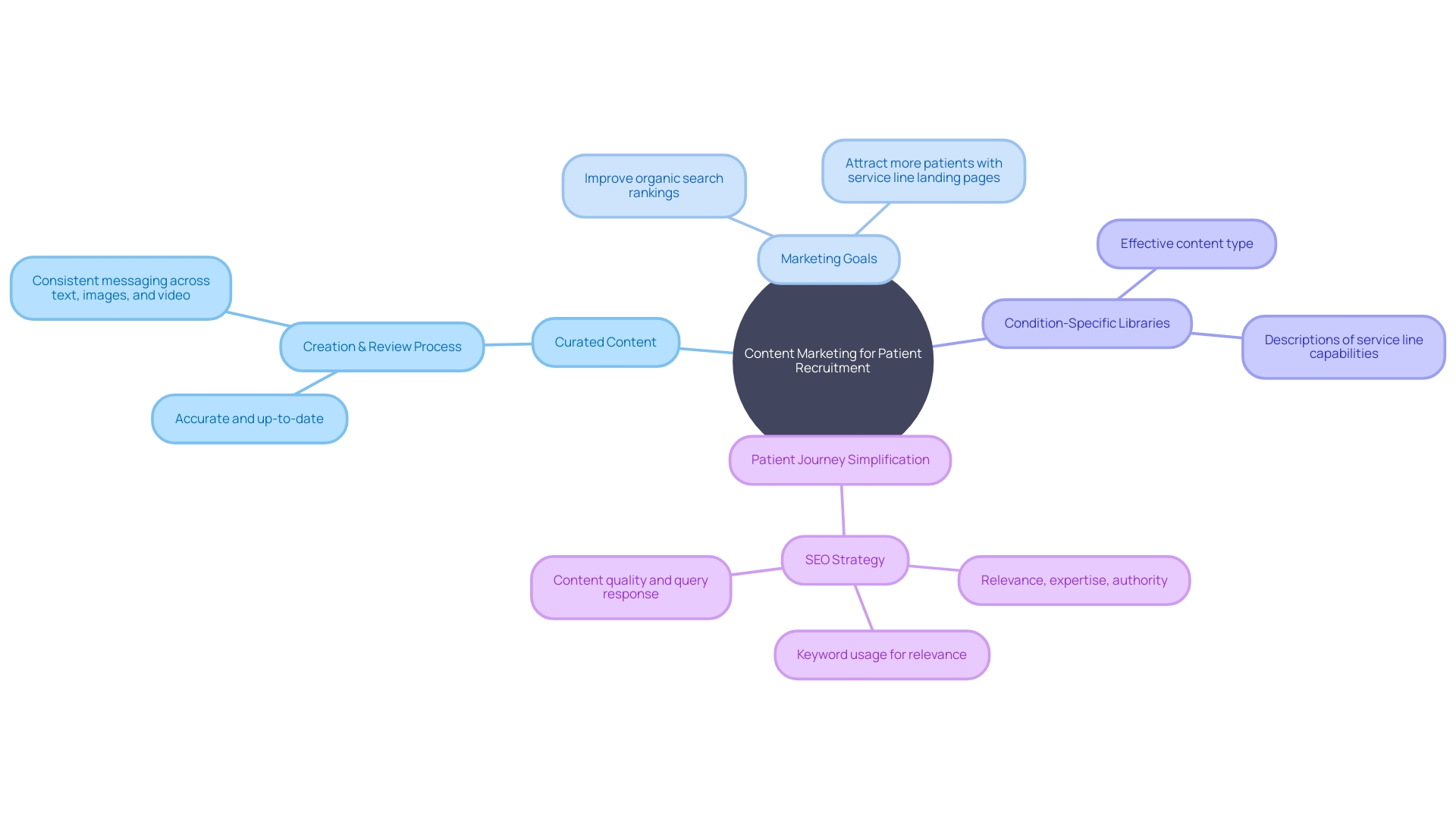
In confronting the challenges of patient recruitment for clinical trials, clinical trial companies must harness the informative and persuasive power of content marketing. For instance, consider a patient with an ultra-rare disease in rural Pennsylvania who needs to navigate the complexities of international clinical trial participation.
Addressing their concerns through curated content - such as detailed guides on securing visas, comprehending foreign paperwork, and coordinating travel - can be an instrumental part of a content strategy aimed at patient engagement and support. Central to this strategy is establishing clear marketing goals, whether improving organic search rankings for broader outreach or crafting service line landing pages that resonate with potential participants.
By building condition-specific libraries and detailing service line capabilities, clinical trial companies can create a repository of valuable information that also aids in simplifying the patient journey. Ken Getz, an industry expert, highlights the shift in clinical trial design 'from solely focusing on great science to now also prioritizing great execution,' which necessitates leveraging content to demystify trial participation. This not only improves operational efficiency but also addresses a potential participant's concerns more directly and humanely, thereby increasing the likelihood of their involvement. Such a compassionate and structured approach to content marketing holds the potential to bolster trust and credibility, encouraging a higher rate of informed and willing participation in critical medical research.
Case Study: Maximizing Clinical Trial and Consulting Services
X Pharmaceuticals, a stalwart in clinical trial and consulting services, embarked on enhancing the impact of medical research through a strategic revamp of their operational framework. The company homed in on refining their internal processes to increase efficiency, broadened the scope of their research facilities network, and harnessed cutting-edge technology to streamline clinical operations.
An illustrative case points to the seamless journey of a patient from rural Pennsylvania, grappling with an ultra-rare disease sans FDA-approved treatments, who was presented with the chance to join a crucial clinical trial overseas. This instance underscored the intricate logistics involved in cross-border healthcare services, highlighting the essential support system required by patients to navigate the complexities of international trials, including visas, language barriers, and travel arrangements.
In dialogue with experts from the industry, it emerged that many companies could have significantly benefited from strategic planning earlier in the clinical trial phases. Historical data from around 1,200 points suggest that careful, proactive decision-making could augment chances of success in about 80% of cases. X Pharmaceuticals embraced this philosophy, dedicating more time and resources to ensure each strategic decision during trials was robust, considering it like reinforcing each link in a chain for optimal longevity and strength. Their approach underscored the importance of foresight in clinical trial planning, underscoring the value of a methodically thought-through chain of decisions that form a reliable backbone for company operations.
Key Challenges and Solutions
X Pharmaceuticals, recognizing the need to enhance their clinical trial services, addressed prominent challenges head-on. A critical obstacle was identifying and securing suitable research locations.
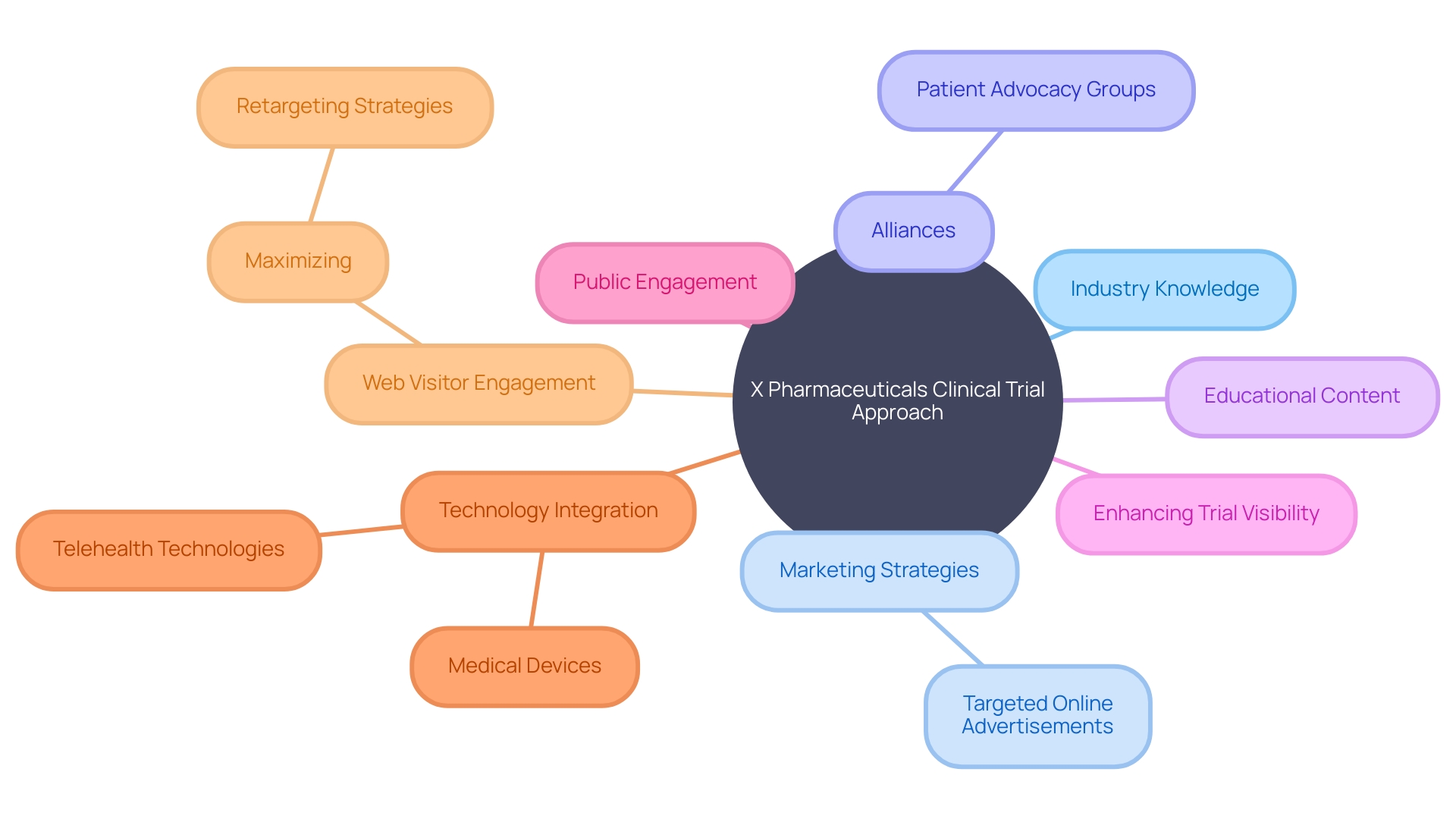
Their strategic solution included forming alliances with esteemed research institutions and healthcare facilities, effectively broadening their operational network. Further complicating their mission was navigating the intricate web of regulatory requirements.
To maintain unerring compliance, X Pharmaceuticals enlisted the expertise of seasoned regulatory professionals and adopted cutting-edge software systems. These tools and talent were instrumental in ensuring adherence to regulations throughout the clinical trial lifecycle, from preliminary steps to post-study analysis. Moreover, the company took note of the evolving landscape where patients, like the one from rural Pennsylvania considering a clinical trial for an ultra-rare disease in Turkey, face daunting logistical hurdles. X Pharmaceuticals' commitment to regulatory excellence and expansive collaboration underscores their dedication to overcoming operational challenges and enhancing patient accessibility to clinical trials.
Marketing Plan and Execution
Pioneering advancements in clinical trial execution and management, X Pharmaceuticals has embraced a multifaceted approach to underscore their consulting and trial services. Coupling their deep industry knowledge with innovative marketing strategies, they have leveraged targeted online advertisements designed to resonate with potential participants, while also nurturing alliances with patient advocacy groups to enhance trial visibility and public engagement. Drawing upon the successful model of industry leader CMIC Group, X Pharmaceuticals offers an array of services spanning the entire spectrum of medical research, echoing CMIC's commitment to providing customized solutions to meet the unique challenges of drug development and patient care.
Reflecting the increasing importance of digital platforms in healthcare delivery, X Pharmaceuticals has crafted educational content that underscores the transformative impact of clinical trials. This includes highlighting the significance of medical devices, as defined by the WHO, which assist in diagnosis, treatment, and improve patient quality of life. Through a synergistic mix of media, they encapsulate the value of clinical trials in advancing therapeutic breakthroughs and fostering a deeper understanding of the role such trials play in public health.
In a world where rare diseases and complex healthcare journeys are a reality, X Pharmaceuticals recognizes the profound influence of telehealth technologies in overcoming barriers to trial participation. Addressing scenarios where patients face cross-border travel for trial involvement, the company acknowledges the logistical and emotional challenges faced by families, aiming to simplify access to life-saving trials. This empathic approach, paired with an eye towards the intricate dance of maximizing web visitor engagement through retargeting strategies, signals X Pharmaceuticals' commitment to not only driving medical research forward but also ensuring its touchpoints with participants are meaningful and effective.
Results and Outcomes
X Pharmaceuticals has reaped the rewards of targeted strategies to enhance their clinical trial operations and advisory services. Through the expansion of their research network into a broader geographical spread, they have unlocked the potential to conduct studies within varying environments and populations.
This adaptation has also sparked a revolution in process efficiency, leveraging cutting-edge technology to fast-track trial milestones. Notably, this evolution has materialized in reduced trial durations, a boon for speeding up the delivery of medical advancements.
The ability to finalize trials more swiftly has not only broadened their reach for participant involvement but has also bolstered X Pharmaceuticals' reputation within the realm of top-tier clinical trial entities. According to industry experts, the foresight to refine decision-making processes well in advance of study readouts is critical. Indeed, an analysis of over 1,200 data points reveals that a staggering 80% of decisions could be better informed given more thorough planning and attention. By refining the very decisions that comprise the sequential links in their operational chain, X Pharmaceuticals has demonstrated an exemplary commitment to optimizing trial outcomes for the benefit of all stakeholders.
Lessons Learned and Future Directions
The intricate nature of clinical trial logistics is exemplified by a scenario of a patient from rural Pennsylvania, who suffers from an ultra-rare disease with no established treatment under the Food and Drug Administration (FDA). They face the prospect of participating in a clinical trial situated in Turkey, but this requires navigating the complexities of international travel.
The patient and their family must grapple with the procurement of travel visas, deciphering foreign language documents, and coordination of transportation—challenges that can be overwhelming and could deter participation in the trial. This case underlines the necessity for clinical trial companies to expand their research network and streamline internal processes to improve access to vital studies.
Raman et al. mention in their article, "Responses are lightly edited for clarity.
BSp: What drove Treehill to consider offering this service in the first place? AP: What we've noticed over the past 20 years of being a transaction advisory is that a lot of the companies that we were meeting..." continues the dialogue, highlighting the importance of reflection and better preparation in research planning.
This insight underscores that decisions made years in advance of the study's outcome could benefit substantially from a more thorough and time-intensive review process. The analogy of optimizing each link in a chain is pertinent, suggesting that efficiency in clinical trial operations must be sought consistently and proactively. In response to emerging healthcare trends and the increase in electronic health records-sourced trials, clinical trial companies must adapt and innovate. Integrating advanced technology and collaborating with research institutions can significantly elevate the effectiveness of operations. By doing so, clinical trial organizations can ensure smoother operational transitions that could lead to better-prepared trials and, ultimately, a more significant impact on medical research advancements.
Conclusion
In conclusion, Contract Research Organizations (CROs) are vital players in clinical research, optimizing each link in the chain for successful trials. They employ unique marketing strategies, including partnerships with healthcare professionals and patient advocacy groups, to go beyond standard practices. Content marketing plays a crucial role in engaging and supporting patients throughout their journey.
X Pharmaceuticals' case study exemplifies the strategic revamp of clinical trial services, emphasizing proactive decision-making and thorough planning. They have addressed challenges such as securing research locations, navigating regulatory requirements, and improving participant accessibility. Their marketing plan includes targeted online ads, alliances with patient advocacy groups, and educational content.
Results show a broader research network, increased efficiency, reduced trial durations, and an enhanced reputation. The importance of refining decision-making and optimizing outcomes through thorough planning is highlighted. Lessons learned include expanding research networks, streamlining processes, and better preparation in research planning.
CROs must adapt to emerging healthcare trends, integrating technology and collaborating with research institutions. In summary, CROs are pivotal in clinical research, overcoming challenges and striving for optimal trial outcomes. By implementing effective marketing strategies, enhancing operational efficiency, and embracing innovation, CROs contribute to advancements in medical research and improve patient access to clinical trials.




