Overview
The article addresses effective clinical trial strategies within Peru's Medtech sector, underscoring the critical need to:
- Navigate regulatory frameworks
- Select optimal clinical sites
- Engage local stakeholders
- Incorporate cultural insights
- Implement targeted recruitment strategies
It articulates the necessity of compliance with regulations, highlights the significance of experienced sites for achieving higher success rates, and elucidates the advantages of community engagement and culturally tailored approaches to enhance participant recruitment and retention.
Introduction
In the dynamic landscape of clinical research, navigating the regulatory framework in Peru is crucial for researchers aiming to conduct successful trials. Governed by the National Institute of Health, the approval and oversight of clinical studies in Peru involve a complex interplay of laws, ethics, and compliance measures.
Understanding the following is essential for ensuring patient safety and maintaining public trust:
- The Clinical Trials Law
- The role of ethics committees
- The submission process
With recent updates aimed at streamlining these processes, researchers must stay informed to leverage opportunities for efficient trial initiation.
This article delves into key strategies for optimizing clinical trial management in Peru, including:
- Selecting the right sites
- Engaging local stakeholders
- Incorporating cultural insights
Ultimately, these strategies enhance the likelihood of successful outcomes in this vibrant market.
Navigate Peru's Regulatory Framework for Clinical Trials
Peru's research study environment is primarily governed by the National Institute of Health (INS), which holds the responsibility for the authorization and oversight of research investigations. Researchers must navigate several key regulations to ensure compliance:
- Research Studies Law: This law establishes the framework for conducting research studies, emphasizing ethical considerations and safety protocols for participants. Ratified through Supreme Decree No 021-2017-SA, it lays the groundwork for regulatory compliance in the country. The implications of this regulation encompass stringent requirements for ethical oversight and patient safety, which are essential for maintaining public trust in clinical research.
- Ethics Committees: All clinical studies require review and approval from an accredited ethics committee. This step is crucial for safeguarding the rights and well-being of participants, ensuring that ethical standards are upheld throughout the research process.
- Submission Process: Researchers must submit comprehensive documentation, including detailed protocols, informed consent forms, and safety reporting plans, to the INS for approval prior to commencing any study. This process is designed to ensure that all aspects of the examination are thoroughly vetted for compliance with regulatory standards.
Recent updates to the regulatory framework in 2025 have introduced new guidelines aimed at streamlining the approval procedure, potentially reducing timelines for initiation. Staying informed about these changes is vital for researchers looking to implement successful Medtech clinical trial strategies in Peru. For instance, bioaccess® has effectively utilized its expertise in navigating these regulations, particularly concerning the submission process and collaboration with ethics committees. Furthermore, bioaccess® offers extensive services, including feasibility assessments, site selection, and project management, which support effective Medtech clinical trial strategies in Peru, enhancing the likelihood of successful approvals and expediting the path to commercialization for innovative Medtech solutions. Additionally, bioaccess®'s commitment to data protection ensures that client concerns are addressed with compliance and transparency, bolstering confidence in the research process. It is also important to note that investigational products may be used under post-study access if they have benefited the research subject, as determined by the principal investigator, adding another layer of consideration for researchers within the regulatory landscape.
Choose Optimal Clinical Sites for Your Trials in Peru
Selecting clinical trial sites in Peru necessitates a meticulous evaluation of several pivotal factors to guarantee successful outcomes.
-
Site Experience: It is imperative to prioritize sites with a robust history of conducting clinical trials, particularly within the Medtech sector. Statistics reveal that sites with extensive experience boast a higher success rate in effectively navigating challenges and delivering reliable results. For instance, recent data indicates that experienced sites in Peru have achieved a 30% higher success rate in trial completion compared to their less experienced counterparts.
-
Participant Population: A thorough analysis of the local community's demographics is essential to ensure access to the target participant group for your study. Understanding the demographic landscape is crucial for recruitment and retention. By 2025, the demographics show an increasing number of individuals with conditions pertinent to Medtech clinical trial strategies in Peru, rendering it an attractive site for clinical research.
-
Infrastructure: Assessing the site's facilities is vital, ensuring they are equipped with necessary medical equipment and staffed by qualified personnel. A well-equipped location can significantly enhance testing efficiency and patient safety.
-
Regulatory Compliance: It is critical to verify that the site adheres to local regulations and maintains a solid track record of successful audits by regulatory bodies. This compliance is essential for preserving the integrity of the experiment and facilitating smoother approval processes.
As Jason Hannon, President and CEO of Mainstay Medical, asserts, "We have real-world data showing that when you turn this therapy loose, physicians will be able to get the same results." This statement underscores the necessity of selecting sites capable of delivering reliable outcomes.
Utilizing resources such as the Peruvian Clinical Trials Registry (REPEC) can assist in identifying suitable sites and streamlining the selection process. Engaging with local experts and stakeholders early in the planning phase can further enhance site selection. A case study involving Mainstay Medical illustrates that by collaborating with medical directors from various payers and developing studies based on their feedback, the team significantly improved study results. This proactive engagement resulted in a higher likelihood of successful reimbursement and market acceptance.
By concentrating on these critical factors and leveraging the expertise of bioaccess®, a leader in Medtech research in Latin America, recognized for its collaboration with Caribbean Health Group to enhance Medtech clinical trial strategies in Peru, you can optimize your clinical trial site selection process.
Engage Local Stakeholders for Enhanced Recruitment
To enhance patient recruitment in Peru, implementing the following strategies for engaging local stakeholders is essential:
-
Collaboration with Healthcare Providers: Establish partnerships with local hospitals and clinics to identify potential participants and secure their support. This partnership not only enhances access to a broader group of individuals but also fosters trust within the community. As Arthur Conan Doyle noted, "It is a capital mistake to theorize before one has data," underscoring the importance of data-driven approaches in recruitment efforts.
-
Community Outreach: Arrange informational sessions and workshops focused on educating the community regarding the initiative's objectives and advantages. Addressing concerns directly can alleviate apprehensions and foster a supportive environment for participation. The case study on "Community-Centered Approaches to Clinical Trial Diversity" illustrates how engaging with communities can lead to better health outcomes and increased participation.
-
Involvement of Patient Advocacy Groups: Engage with organizations that represent patient interests to broaden outreach efforts and enhance credibility. These groups can assist in connecting researchers and potential participants, ensuring that the study resonates with the community.
-
Utilize Local Media: Leverage local newspapers, radio, and social media platforms to disseminate information about the proceedings and its significance. Effective communication through these channels can raise awareness and encourage participation. Significantly, Clinical Leader has underscored the significance of media attention in research studies throughout Latin America and Colombia, stressing the role of local media in promoting community involvement. Furthermore, 16% of clinical studies are classified as 'Other,' emphasizing the variety of study types and the necessity for customized recruitment strategies.
-
Incorporate Technology: Implement blockchain technology for secure and transparent data sharing among stakeholders of the study. This not only improves data integrity but also fosters trust, which is crucial for patient recruitment. bioaccess® is committed to ensuring information security and client trust, addressing any concerns through established grievance and data protection procedures.
The Medtech clinical trial strategies in Peru can greatly enhance recruitment and retention rates, ensuring that studies are conducted efficiently and effectively. By nurturing strong connections with healthcare providers and the community, clinical studies can achieve improved results and aid in advancing medical knowledge.
Incorporate Cultural Insights into Trial Design
Integrating cultural insights into Medtech clinical trial strategies in Peru is essential for enhancing participation and ensuring positive outcomes. The key strategies include:
-
Understanding Local Beliefs: Conducting thorough research on the cultural beliefs and practices of the target population is imperative. This understanding ensures that the trial design is respectful and relevant, fostering trust among potential participants. As Alam W. Watts pointed out, we often consider the languages and visuals presented by our society, highlighting the importance of understanding these influences on individuals' perceptions. Steve Garchow's experience underscores that a deep understanding of local healthcare practices and patient needs is vital for effective engagement in Medtech clinical trial strategies in Peru.
-
Tailoring Communication: Utilizing culturally appropriate language and communication methods when interacting with participants is crucial. This approach not only enhances comprehension but also fosters connection, making individuals feel valued and acknowledged. Garchow's insights on the necessity for culturally aware marketing strategies further emphasize the importance of this approach in Medtech clinical trial strategies in Peru.
-
Flexible Protocols: Developing study protocols that allow for adaptability in participation is essential. Adjusting to local customs and practices can significantly enhance comfort and willingness to engage in the study. Garchow's emphasis on the need for local groups to modify global strategies to meet specific country requirements reinforces this approach.
-
Feedback Channels: Establishing avenues for participants to provide input on the process is vital. Actively listening to their feedback ensures that their voices are heard and considered, resulting in a more inclusive and responsive testing environment. The importance of local engagement, as highlighted by Garchow, strengthens the value of incorporating participant feedback into study design.
The significance of community involvement is further illustrated by case studies, such as those demonstrating partnerships in African studies, which confirm that effective collaboration with local leaders and health workers is essential for successful study outcomes. By integrating these cultural factors and addressing barriers to participation, researchers can cultivate a more inclusive environment that enhances Medtech clinical trial strategies in Peru, promotes engagement, and ultimately improves the quality and efficacy of research studies.
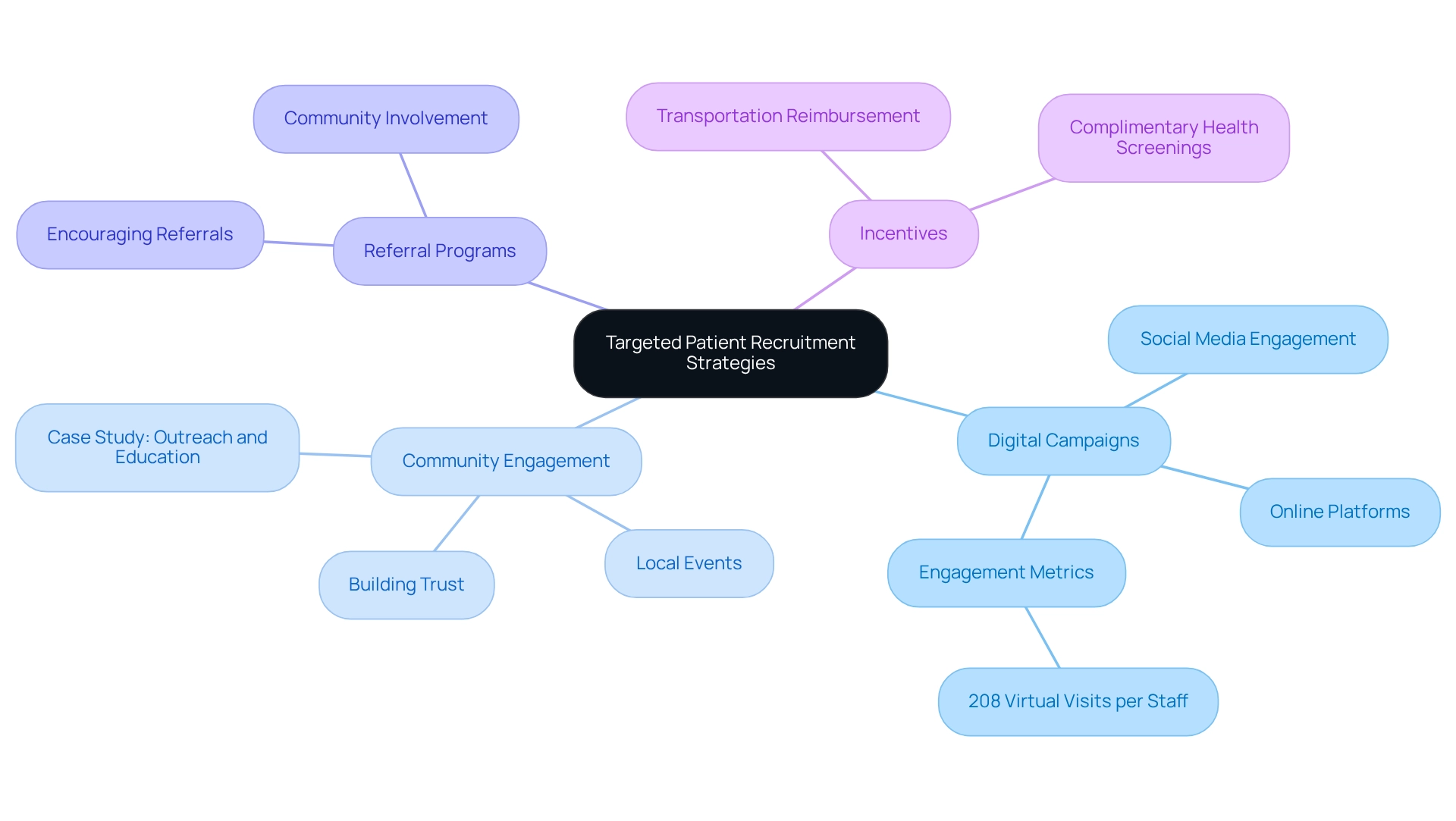
Implement Targeted Patient Recruitment Strategies
To effectively implement targeted patient recruitment strategies in Peru, consider the following approaches:
-
Digital Campaigns: Leverage social media and online platforms to engage potential participants, particularly younger demographics who are more active online. Effective digital campaigns can significantly improve visibility and interest in medtech clinical trial strategies in Peru. Notably, health centers have an average of 208 virtual visits per medical care staff, indicating a strong existing engagement that can be tapped into.
-
Community Engagement: Organize local events to raise awareness about the trial and its benefits, fostering personal connections with potential contributors. This strategy not only builds trust but also encourages community involvement, which is crucial for successful recruitment. The case study titled "Outreach and Education for Effective Collaboration" emphasizes the importance of outreach and educational resources in building trust and improving engagement in research activities.
-
Referral Programs: Encourage existing members to refer friends and family, leveraging personal networks to enhance recruitment efforts. This approach can create a sense of community and shared purpose among participants.
-
Incentives: Provide incentives for participation, such as transportation reimbursement or complimentary health screenings, to encourage enrollment. These incentives can significantly increase participation rates by addressing common barriers to entry.
Moreover, the partnership between bioaccess™ and Caribbean Health Group to establish Barranquilla as a premier location for research studies highlights the importance of Medtech clinical trial strategies in Peru and the significance of strategic alliances in improving recruitment initiatives. By providing extensive research study management services—including feasibility assessments, site selection, compliance evaluations, study setup, import permits, project management, and reporting—researchers can ensure studies are sufficiently powered to produce significant results. Community health centers play an essential role in this process, as emphasized in the case study "The Role of Community Health Centers in Advancing Health Equity," which demonstrates how their established connections within various populations can enhance research and improve health equity. As Dr. Eric Topol noted, "The crowd will see you now, the patient will see you now... Everyone but the doctor," emphasizing the importance of patient visibility in the clinical trial process.
Conclusion
Navigating the regulatory framework for clinical trials in Peru is essential for researchers aiming for successful outcomes. Understanding the Clinical Trials Law, the role of ethics committees, and the submission process is fundamental to ensuring compliance and maintaining public trust. With recent updates designed to streamline these processes, staying informed is critical for effective trial initiation.
Selecting optimal clinical sites is equally important, requiring careful consideration of site experience, patient demographics, infrastructure, and regulatory compliance. Engaging local stakeholders, including healthcare providers and community organizations, can significantly enhance patient recruitment efforts, fostering trust and participation.
Incorporating cultural insights into trial design further enriches the research process, ensuring that trials are respectful and relevant to local populations. Implementing targeted recruitment strategies, such as digital campaigns and community engagement, can also drive participation.
Ultimately, the success of clinical trials in Peru hinges on a holistic approach that combines regulatory knowledge, strategic site selection, stakeholder engagement, cultural sensitivity, and innovative recruitment strategies. By leveraging these elements, researchers can enhance the likelihood of achieving successful trial outcomes, contributing to the advancement of medical knowledge and public health in this vibrant market.
Frequently Asked Questions
What governs the research study environment in Peru?
The research study environment in Peru is primarily governed by the National Institute of Health (INS), which is responsible for the authorization and oversight of research investigations.
What is the Research Studies Law in Peru?
The Research Studies Law establishes the framework for conducting research studies, emphasizing ethical considerations and safety protocols for participants. It was ratified through Supreme Decree No 021-2017-SA and includes stringent requirements for ethical oversight and patient safety.
What role do Ethics Committees play in clinical studies in Peru?
All clinical studies in Peru require review and approval from an accredited ethics committee to safeguard the rights and well-being of participants and to ensure that ethical standards are upheld throughout the research process.
What is the submission process for researchers in Peru?
Researchers must submit comprehensive documentation, including detailed protocols, informed consent forms, and safety reporting plans, to the INS for approval before commencing any study, ensuring compliance with regulatory standards.
What recent updates have been made to the regulatory framework in Peru?
In 2025, new guidelines were introduced to streamline the approval procedure, potentially reducing timelines for initiation of research studies.
How can bioaccess® assist researchers in Peru?
Bioaccess® provides expertise in navigating regulations, particularly concerning the submission process and collaboration with ethics committees. They offer services such as feasibility assessments, site selection, and project management to support successful Medtech clinical trial strategies in Peru.
What is the importance of participant population analysis in clinical trials?
Analyzing the local community's demographics is essential for ensuring access to the target participant group for the study, which is crucial for recruitment and retention.
What factors should be evaluated when selecting clinical trial sites in Peru?
Key factors include site experience, participant population demographics, infrastructure, and regulatory compliance to ensure successful outcomes.
Why is site experience important in clinical trials?
Sites with a robust history of conducting clinical trials, especially in the Medtech sector, have a higher success rate in navigating challenges and delivering reliable results.
How can the Peruvian Clinical Trials Registry (REPEC) assist in site selection?
The REPEC can help identify suitable clinical trial sites and streamline the selection process, enhancing the likelihood of successful outcomes.
What is the significance of engaging local experts in the planning phase of clinical trials?
Engaging local experts and stakeholders early in the planning phase can improve study results and increase the likelihood of successful reimbursement and market acceptance.




