Introduction
Title: A Comprehensive Guide to 21 CFR 312: Navigating Clinical Trials with Compliance and Integrity
Clinical trials play a crucial role in advancing medical knowledge and improving patient outcomes. To ensure the safety, effectiveness, and integrity of these trials, the United States has established Title 21 of the Code of Federal Regulations (21 CFR 312). This cornerstone regulation sets forth the responsibilities and requirements for sponsors, investigators, and Institutional Review Boards (IRBs) involved in clinical trial conduct.
In this article, we will explore the key components of 21 CFR 312, including the IND application process, clinical trial protocol requirements, informed consent and subject protection, data collection and integrity, safety reporting requirements, IRB approval and oversight, as well as compliance and regulatory considerations. By delving into each of these areas, we aim to provide a comprehensive understanding of the regulations governing clinical trials in the United States.
Throughout this guide, we will emphasize the importance of compliance with 21 CFR 312 for the approval and marketing of investigational drugs. We will also highlight the role of various stakeholders, such as sponsors, investigators, and IRBs, in ensuring participant safety and the integrity of study data.
Join us as we navigate the complexities of 21 CFR 312 and explore best practices for successfully conducting clinical trials in a compliant and ethical manner. By following these regulations and guidelines, we can advance medical science, enhance patient care, and contribute to the development of innovative treatments that have the potential to transform lives.
What is 21 CFR 312?
Title 21 of the Code of Federal Regulations (21 CFR 312) serves as a cornerstone for clinical trial conduct in the United States, delineating the responsibilities and requirements for sponsors, investigators, and Institutional Review Boards (IRBs). Compliance with 21 CFR 312 is crucial for the approval and marketing of investigational drugs, ensuring the protection of participant well-being and the integrity of study data.
The FDA, as part of the U.S. Department of Health and Human Services, enforces these regulations to safeguard public health by ensuring the safety, effectiveness, and security of human drugs. One significant aspect of 21 CFR 312 is the orphan drug designation, which the FDA grants under section 526 of the act. This designation is pivotal for drugs intended to treat rare diseases or conditions, providing the sponsor with exclusive approval for seven years post-FDA approval, barring a clinically superior subsequent sponsor's application.
Moreover, the FDA's regulation extends to how prescription drug advertisements are presented to the public. The agency's recent final rule mandates that direct-to-consumer human prescription drug ads in television and radio formats clearly, conspicuously, and neutrally present the major side effects and contraindications.
To ease the registration and results reporting of experiments, the FDA makes use of the ClinicalTrials. Gov Protocol Registration and Results System (PRS). Sponsors and investigators must have a PRS account to register a study, emphasizing the importance of transparent reporting in medical research. This system plays a crucial part in preserving the integrity of medical experiments and ensuring adherence to FDA regulations.
With the main objective of progressing medical understanding and enhancing patient results, experiments are essential to the progress of new treatments. Individuals involved in medical experiments contribute to the transformation of healthcare and the potential to change lives, highlighting the value and impact of their participation.
Key Components of 21 CFR 312
Delving into 21 CFR 312, it's paramount to grasp its integral elements which encompass a plethora of critical topics. These subjects cover a wide range of explicit definitions that are essential for understanding the regulatory language, as well as the overall responsibilities that stakeholders must maintain during investigations. IND applications for the gateway for new drugs to enter clinical trials, while detailed clinical trial protocol requirements ensure the methodological rigor of these studies. Informed consent and subject protection are at the core of ethical considerations, ensuring participants' rights are safeguarded throughout the investigation process.
Data collection and integrity are the bedrock of reliable results, and safety reporting requirements ensure that any potential risks are communicated promptly and effectively. IRB approval and oversight provide an additional layer of ethical scrutiny and participant protection. Navigating compliance and regulatory considerations is a dynamic challenge that requires a thorough understanding of the evolving landscape of FDA regulations. Finally, adopting best practices for navigating 21 CFR 312 is essential for maintaining the highest standards of research integrity and regulatory compliance.
IND Application Process
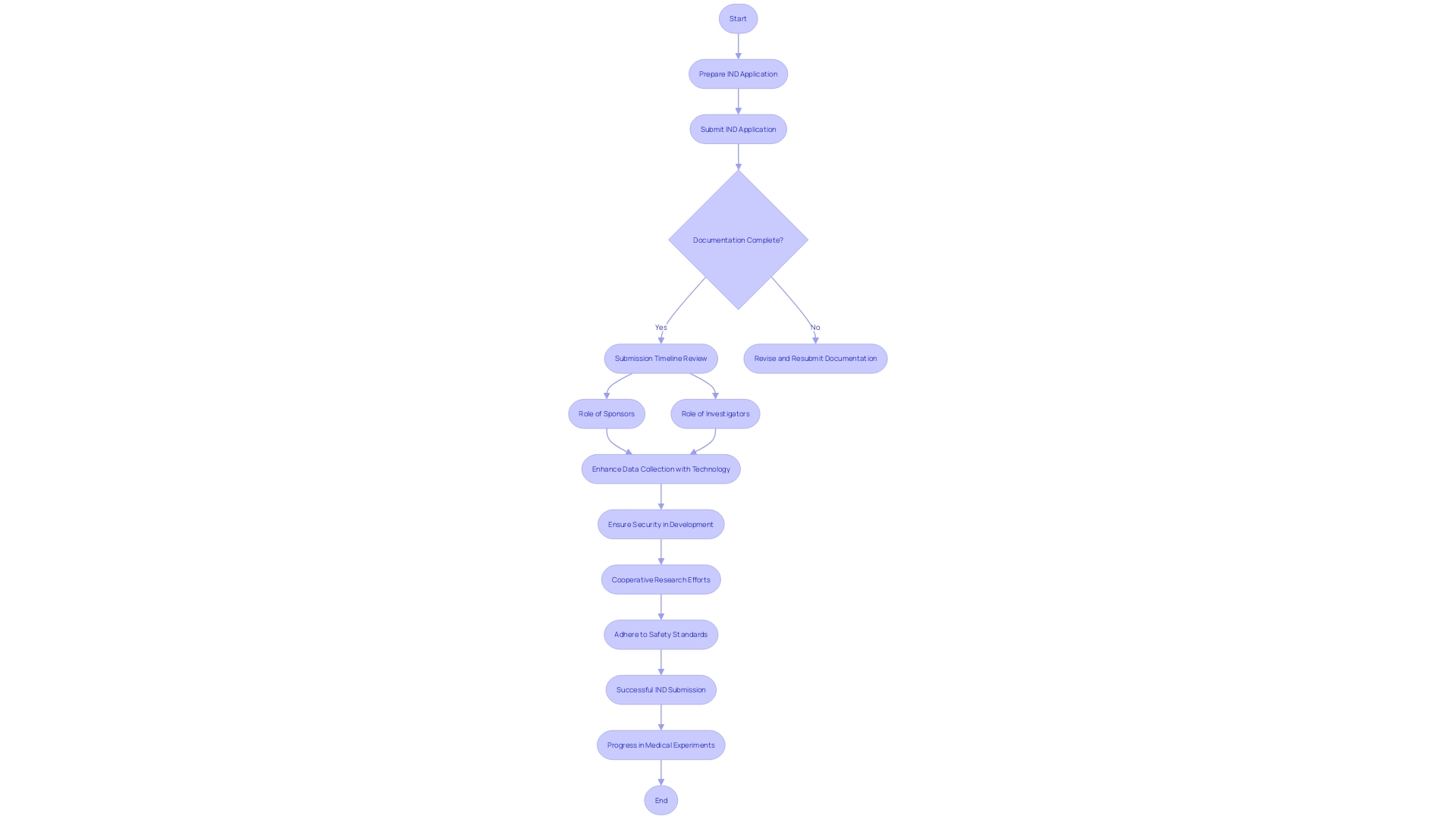
Starting the Investigational New Drug (IND) application process is a crucial step in the path of medical experiments. This crucial phase encompasses the meticulous preparation and submission of the IND application, which requires a clear understanding of the required documentation, adherence to submission timelines, and an awareness of the distinct roles and responsibilities that both sponsors and investigators hold. The IND application serves as the basis for ensuring that experiments for new drugs or treatments are conducted safely and within regulatory compliance. For example, a recent announcement from Intellia Therapeutics highlights the significance of successful IND submissions, as they get ready to progress their gene editing therapy, NTLA-2001, through medical experiments. The company's forward-looking statements are contingent upon regulatory approval through the IND process, demonstrating the critical nature of this step in drug development. Furthermore, involvement in medical experiments is crucial, not just for the progress of medical investigation but also for the potential health advantages to individuals with conditions such as diabetes. Involvement in experiments, which frequently covers several stages, directly contributes to the authentication of new treatments and technologies. As technology continues to transform the evaluation landscape, it facilitates more efficient and accurate data collection through digital tools and wearable devices, thereby enhancing the integrity of the IND application process. Furthermore, modern investigator training programs highlight the importance of security in medical product development, illuminating the intricacies and obligations that come with the IND process. These programs serve a wide range of stakeholders, including healthcare professionals and researchers, consolidating the collective effort necessary for successful conduct of medical experiments. The perspectives provided by these educational initiatives are echoed in the feelings of industry leaders, emphasizing the joint obligation of the research study community to maintain safety and effectiveness standards. Therefore, the IND application process not only signifies the beginning of medical tests but also demonstrates the cooperative endeavors that propel medical advancement.
Clinical Trial Protocol Requirements
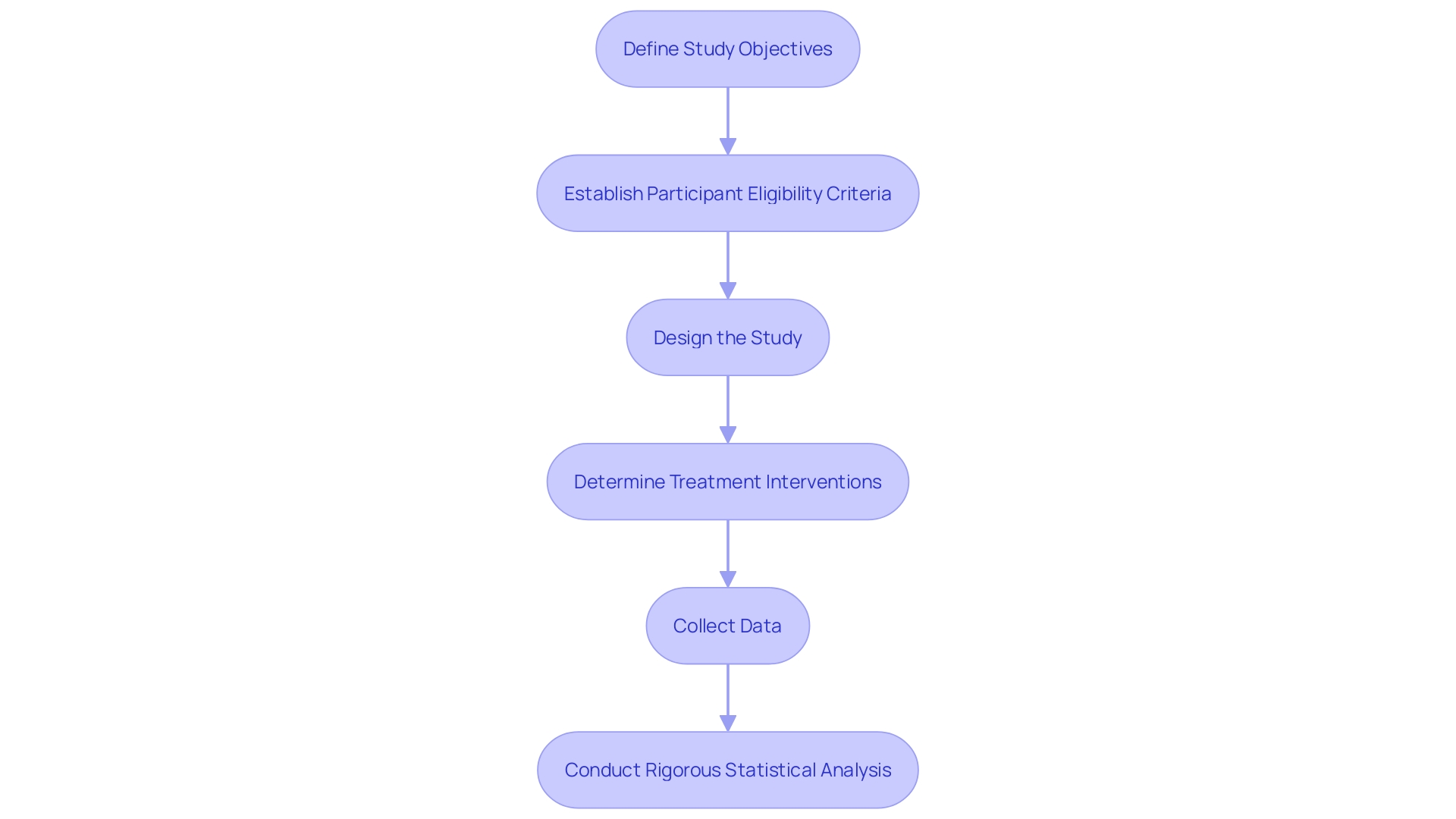
Creating a thorough experimentation plan is a foundation of every effective investigation, guaranteeing the credibility of the gathered information and the dependability of the results. A meticulously designed protocol should encompass clear study objectives, meticulously delineated participant eligibility criteria, a robust study design, well-defined treatment interventions, effective data collection methods, and a rigorous statistical analysis plan. Such protocols are crucial, especially in contexts where prediction rules (CPRs) guide decision-making at the point of care. By integrating CPRs into electronic health records, clinicians can apply evidence-based guidelines directly to patient care. For instance, CPRs have proven to accurately differentiate between viral and bacterial respiratory infections, supporting the judicious use of antibiotics and thus contributing to antibiotic stewardship efforts.
The imperative for stringent protocols is highlighted by the significant issue of antibiotic overprescription in outpatient settings. In the United States, approximately half of all outpatient antibiotic prescriptions for acute respiratory infections (Aris) are unwarranted, with a staggering 47 million unnecessary antibiotic prescriptions issued annually. This overprescription contributes to the alarming rise of antibiotic-resistant bacterial infections, which claim approximately 35,000 lives each year. The creation of sturdy research study protocols is thus crucial in facilitating research that can address these public health challenges and promote better stewardship practices.
Additionally, the FDA's responsibility in protecting public health emphasizes the significance of complying with regulatory standards in medical experiments. Recent guidance from the FDA emphasizes the need for clear and concise informed consent, which should facilitate participants' understanding of a study's purpose, potential risks and benefits, and overall structure. This guidance is a component of the FDA's wider strategy to oversee experiments during public health crises, where upholding methodological and statistical correctness is of utmost importance. The challenges faced during the COVID-19 pandemic, for instance, have underscored the need for flexible yet rigorous protocols that can adapt to rapidly changing circumstances while safeguarding participant safety and data integrity.
The advancement of protocols for testing also benefits from a worldwide outlook, integrating diverse investigations from continents like Europe, America, and Asia. However, it's noteworthy that perspectives from Africa and Australia are often missing, which could provide even richer insights into global health challenges. The studies and systematic reviews arising from these protocols address various themes, including interventions in psycho-oncology and the methodological validity of non-pharmacologic interventions. These contributions highlight common issues such as inadequate sample sizes, statistical power concerns, and the need to control for biases and confounding variables.
In summary, a well-constructed trial protocol is an indispensable tool for researchers, enabling them to design studies that are not only methodologically sound but also responsive to current public health needs. Whether the focus is on antibiotic stewardship, informed consent processes, or interventions across various medical fields, the protocol serves as a blueprint for achieving reliable and impactful study results.
Informed Consent and Subject Protection
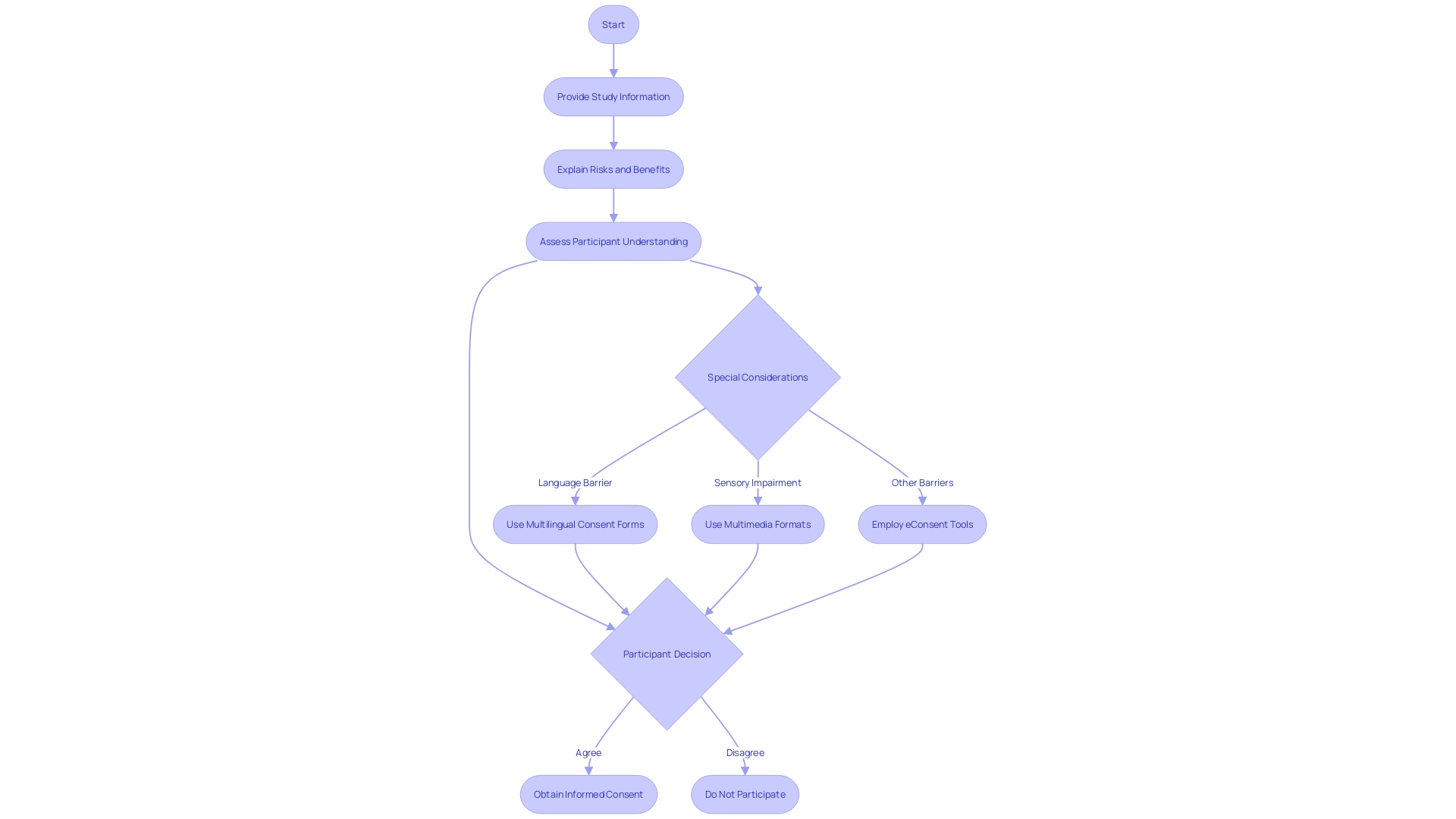
Ethical clinical investigation emphasizes the importance of informed consent, which is a process that ensures study participants are fully aware of the study's purpose, procedures, potential risks, and benefits, allowing them to make an informed decision about their participation. The informed consent document serves as a cornerstone in protecting the rights, safety, and well-being of subjects, and it should be presented clearly and concisely to facilitate understanding.
In accordance with the Declaration of Helsinki, which has served as a guiding ethical document for 60 years, ongoing revisions are being debated to tackle discrepancies and improve the ethical practice of scientific investigation. Furthermore, the draft guidance by regulatory bodies recommends presenting key information at the outset of the consent document. This includes the study's purpose, duration, procedures, possible risks and benefits, and compensation for research-related injuries.
The National Organization for Rare Disorders (NORD) endorses innovative approaches to informed consent, such as multimedia formats that cater to diverse participant needs. Electronic consent (eConsent) tools, which can include audio, video, and interactive elements, have proven effective in making the informed consent process more accessible and understandable. These tools are especially valuable in addressing language barriers, sensory impairments, and varying health literacy levels.
Additionally, there are significant advantages to participating in a study, including gaining insights into one's health. For instance, participants may receive critical health information that would otherwise remain undetected, as highlighted by the experience of Barbara, who discovered a life-threatening heart condition through her participation in a study found via an online matching portal.
These efforts are supported by recent initiatives to promote inclusive study design and offer career paths for scholars from diverse professional backgrounds. The constantly changing domain of clinical investigation persists in its efforts towards ethical integrity, participant comprehension, and inclusive engagement, guaranteeing that the pursuit of medical advancements upholds the dignity and autonomy of every individual involved.
Data Collection and Integrity
Gathering accurate and reliable data is crucial for the evaluation of investigational drugs, particularly in assessing their safety and efficacy. The complexity of this task is underscored by the need to consider a diverse range of participant characteristics, including demographic, social, economic, and health status-related factors. The representation of underserved groups in research is vital, as varying responses to interventions can be missed without inclusive participation, leading to biases and ultimately contributing to health inequity.
In the realm of data collection, traditional methods such as case report forms are being augmented by innovative electronic data capture systems and comprehensive data monitoring strategies. These advancements are essential in addressing data quality issues such as missing, ambiguous, or conflicting data, which are notably prevalent in patient-reported outcomes measures (Proms) completed outside of controlled settings. For instance, studies reported up to 44% of patients either missed or ambiguously marked items on paper-based questionnaires, highlighting the challenges of ensuring data integrity.
The reliability of research study information is additionally strengthened by following protocol-specified timelines, guaranteeing that recall periods are suitable and that data reflects an accurate health status. Real-world data, collected outside the controlled environment of clinical experiments, is also being recognized for its potential to broaden and complement formal study findings. This is exemplified by the EU's initiative to create catalogues for researchers to leverage real-world health data, enhancing the inclusivity and applicability of research outcomes.
Furthermore, with the increasing importance of patient-focused pharmaceutical development, the incorporation of connected devices, wearables, and decentralized solutions is transforming data strategies. These approaches enable the capture of a broader spectrum of data, providing richer insights into patient behavior and trends. Such strategies require a careful approach to data management, one that is guided by advanced analytics to interpret the large amounts of data generated in medical experiments.
Ultimately, the objective is to maintain the integrity and quality of the data collected, thereby facilitating more informed decisions in drug development while ensuring patient well-being and the applicability of research findings to diverse populations.
Safety Reporting Requirements
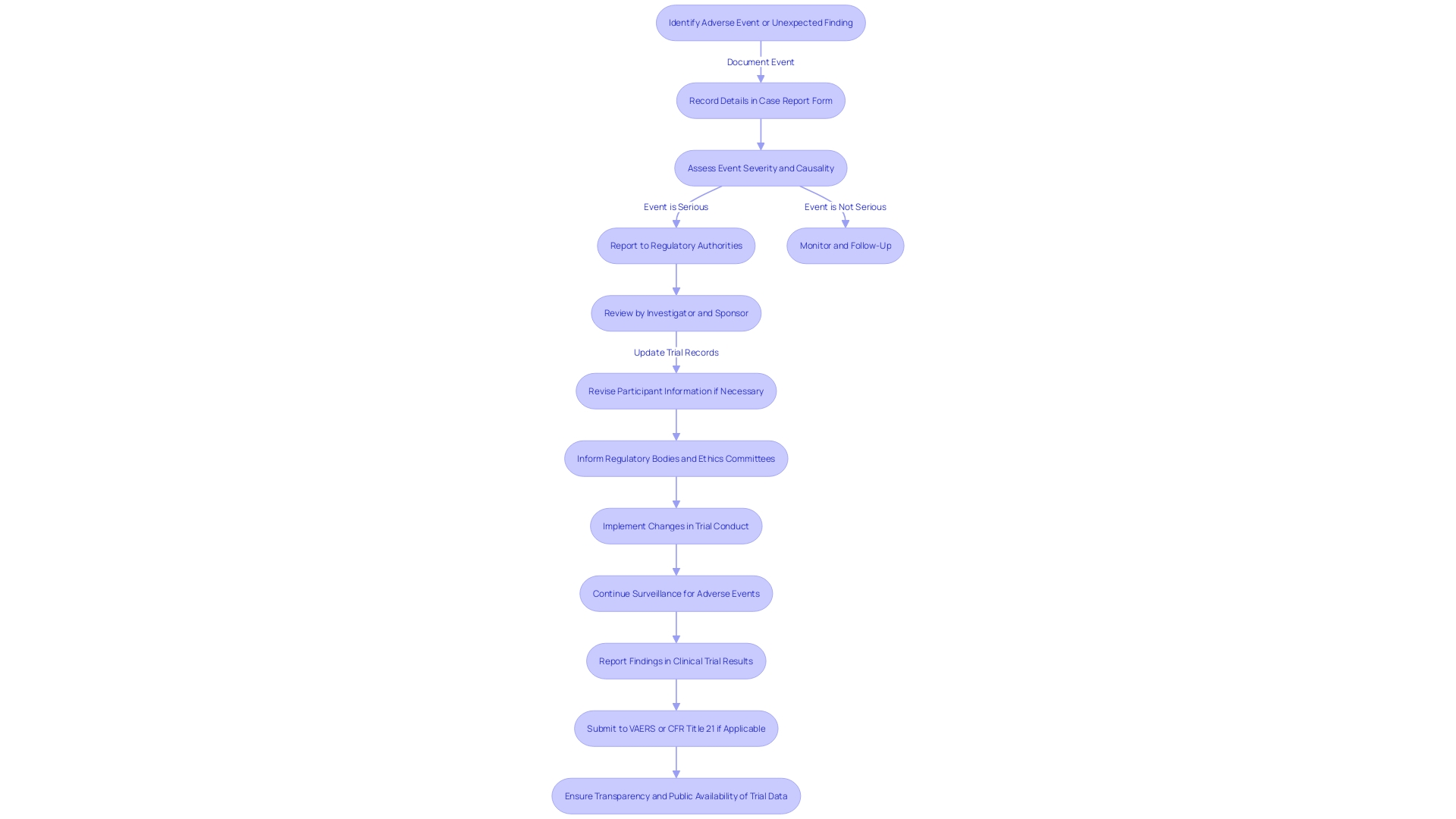
The Code of Federal Regulations (CFR) Title 21 is crucial in ensuring that clinical trials are conducted with the utmost concern for participant well-being. Specifically, 21 CFR 312 lays out stringent reporting requirements aimed at protecting participants by ensuring that adverse events and unexpected findings are promptly reported. Adverse events, serious adverse events, and unanticipated problems are carefully monitored by both investigators and sponsors, who have a shared responsibility in the surveillance and communication of potential concerns.
Under this regulation, any event associated with the manufacturing, testing, processing, packing, labeling, or storage of a licensed biological product must be reported if it represents a deviation from current good manufacturing practices or unexpected events that could impact product purity, potency, or adhere to good manufacturing practices. The expectation is that these reports are not only timely but also include all relevant information to enable a thorough investigation.
The importance of reporting is emphasized by the Vaccine Adverse Event Reporting System (VAERS), which has been instrumental in identifying vaccine concerns. Similarly, in clinical experiments, such watchfulness in reporting the well-being of participants ensures that interventions are secure for individuals, who are often fragile people starting an experiment as a final option for treatment, as observed in experiments for conditions like transthyretin-mediated amyloidosis.
As the regulations evolve, it is anticipated that by August/September 2024, new guidelines regarding data governance will be implemented, as per the draft document from the International Council for Harmonisation (ICH) E6 GCP (revision three), which emphasizes the protection of participants and the reliability of research outcomes.
Furthermore, the FDA’s recent final rule on direct-to-consumer prescription drug advertisements in television and radio formats exemplifies the commitment to clear and transparent communication regarding the profiles of medical interventions. This level of communication applies to reporting on the well-being of participants in medical studies, where a clear and precise account is crucial.
The combined endeavor of all parties involved in a medical examination, encompassing producers, healthcare experts, and investigators, is vital in adding to the protection of medicinal goods and interventions. The reporting process is inclusive and accessible, with translations available to bridge language barriers and facilitate wider engagement in the reporting process. In the end, these careful safety reporting standards in trials serve to protect public health and maintain the integrity of medical studies.
IRB Approval and Oversight
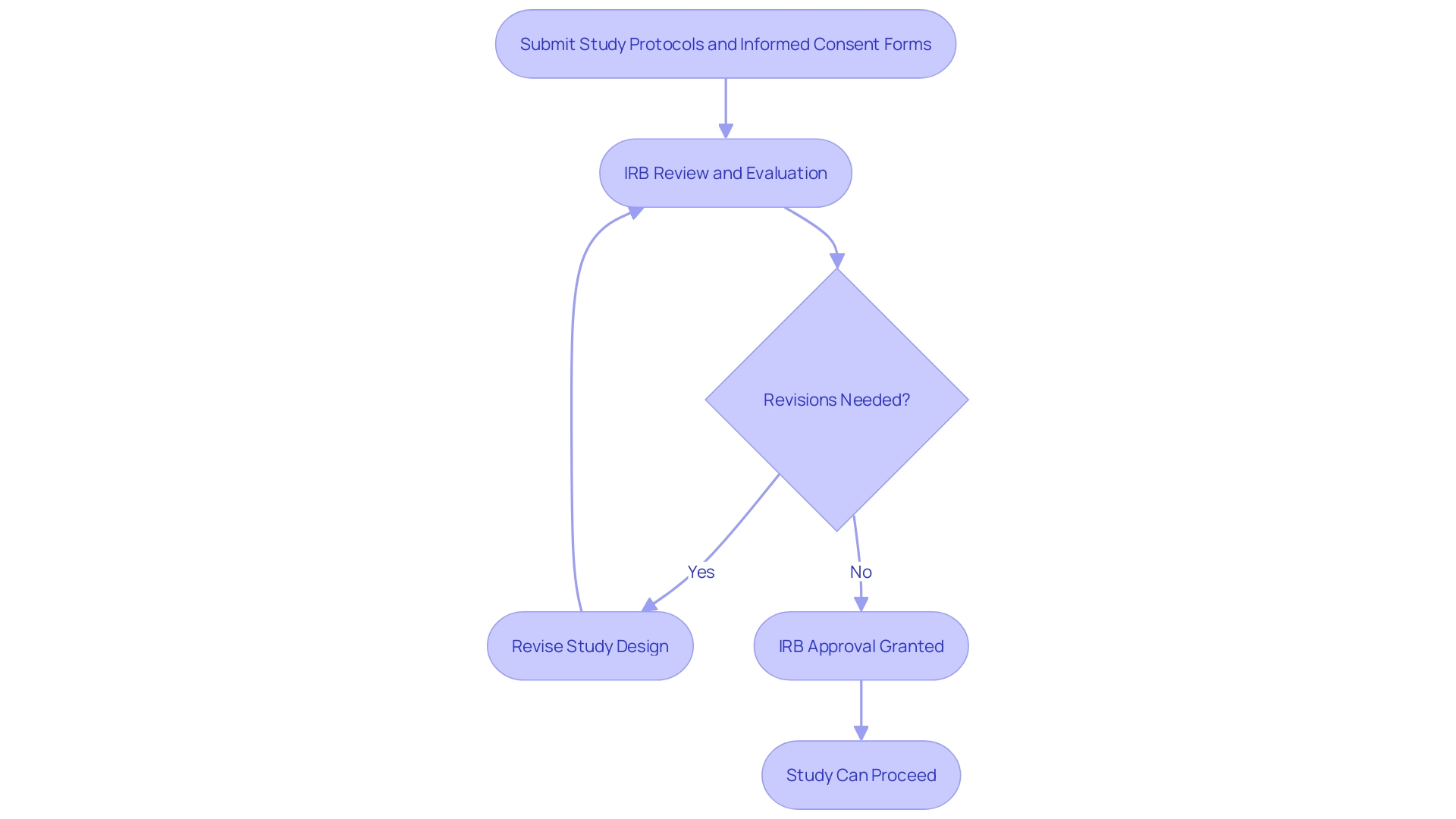
Institutional Review Boards (IRBs) are vital in the field of clinical studies, responsible for safeguarding human subjects participating in studies. Researchers seeking IRB approval must present comprehensive study protocols, informed consent forms, and other pertinent documents for review. This process ensures that the study is conducted ethically, in alignment with the foundational principles set forth in the Declaration of Helsinki, which is regarded as the cornerstone document of medical research ethics. The IRB evaluates the submitted materials, assessing the potential risks and benefits, and has the authority to request revisions to the study design. This review process, while thorough, can extend over several weeks or months, influenced by the frequency of the IRB meetings.
In addition to the initial approval, IRBs are responsible for continuous oversight of the study project. Their ongoing monitoring activities are crucial for maintaining ethical standards throughout the study's duration. This vigilance is a response to historic lapses in ethical investigation practices, such as those highlighted by the Tuskegee Syphilis Study, which underscored the need for stringent oversight.
The significance of effective and properly conducted medical investigation cannot be emphasized enough, as it directly affects the FDA's ability to make informed decisions regarding the safety and effectiveness of medical products. Acknowledging this, the FDA has been proactive in improving investigative methodologies and enhancing the dependability of data, which in turn promotes medical advancements and safeguards participant rights. To further streamline research practices, the FDA is also working towards the harmonization of its regulations with the U.S. Department of Health and Human Services' Common Rule, thereby optimizing the efficiency of medical research while upholding the highest standards of human subject protection.
Compliance and Regulatory Considerations
Understanding the intricacies of 21 CFR 312 is an essential part of managing trials. This code section outlines the essential requirements for investigational new drug applications, encompassing stringent recordkeeping practices, meticulous reporting requirements, and the readiness for FDA inspections. Following these regulations is not just a legal duty but also a foundation in safeguarding participant safety and ensuring the accuracy and reliability of data.
Considering the diverse aspects of clinical studies, starting from initial phase one investigations with healthy volunteers to subsequent-stage examinations involving affected patients, the importance of adherence is especially significant. The significance of this compliance was emphasized by the FDA Modernization Act of 1997, which paved the way for more transparent experiment data through the creation of ClinicalTrials.gov. This openness is crucial, as even experiments with unfavorable outcomes contribute valuable understanding to the medical community, aiding in the formation of future research directions.
However, maintaining compliance with the FDA regulations is not without challenges. A comprehensive survey conducted by Mr. Keyes and the Taskforce revealed a diverse landscape of registration and reporting practices among academic institutions. After analyzing more than 47,000 records of medical experiments, the survey unveiled the necessity for enhanced support systems within organizations to fulfill regulatory expectations.
In light of these challenges, it is fundamental to develop robust strategies to mitigate potential risks associated with non-compliance. These strategies include staying abreast of regulatory updates, as evidenced by the 'Final Rule' of 2017, which sought to address low compliance rates by clarifying existing laws.
It is also crucial to understand the nuances of FDA terms, such as 'Orphan-drug designation', which plays a pivotal role in drug development and market exclusivity. The implications of such designations extend to various facets of drug development, including the development of treatments for subsets of non-rare diseases, or 'orphan subsets,' which may require special consideration due to unique drug properties like toxicity or mechanism of action.
Essentially, ensuring adherence to the regulations outlined in 21 CFR 312 is not solely about adhering to guidelines—it involves upholding the utmost standards of safety and efficacy in medical studies. As the FDA continues to safeguard public health through rigorous regulation of medical products, professionals involved in research must prioritize regulatory compliance as an integral part of their mission to advance medical science and enhance patient care.
Best Practices for Navigating 21 CFR 312
Mastering 21 CFR 312 is crucial for the management of experiments, ensuring adherence to the FDA's stringent regulations. This segment delves into robust methodologies for navigating study management intricacies, optimizing stakeholder engagement, and incorporating the latest regulatory amendments. To streamline study management, it's important to consider the dynamic nature of data element requirements on platforms like ClinicalTrials.gov, which have evolved significantly over the past 25 years. Understanding these changes is vital for accurate trial reporting.
For instance, recent modernization efforts at ClinicalTrials. Gov have focused on the clarity and utility of data presentation, both for submitted information and for highlighting omissions by study sponsors. Likewise, the FDA provides guidance on situations where data from medical trials is crucial for 510(k) submissions, offering examples where technological disparities or new hazards require extra medical proof.
Case studies, such as Cardinal Health's collaboration with a client on an investigational drug application, demonstrate the importance of an all-encompassing regulatory strategy to ensure prompt and comprehensive IND submissions. News reports mirror the feeling, with the FDA emphasizing the role of well-thought-out scientific investigation in product security and effectiveness determinations. Steps to harmonize human subject protection regulations with the HHS Common Rule are examples of efforts to balance research advancement with participant safety.
Understanding orphan drug designations and the exclusivity implications is another critical area. The FDA grants such designations to encourage development for rare diseases, ensuring exclusive approval to sponsors under specific conditions. Furthermore, the eCFR's structured display of regulations facilitates easier assimilation of these complex frameworks.
Integrating digital advancements like electronic consent (eConsent) can greatly improve patient understanding and involvement in medical studies. The use of multimedia, interactive glossaries, and simplified digital formats is instrumental in demystifying complex medical terminology and procedures for participants.
Professionals in the domain, like biomedical engineers with vast expertise in handling clinical studies, emphasize the significance of clinical experiments in medical progress. The various stages of clinical experimentation, starting from the early phases concentrating on well-being to subsequent stages evaluating effectiveness, are crucial in establishing novel treatments. Training courses for healthcare professionals and biomedical researchers are essential for developing a practical understanding of these safety concerns.
In conclusion, effective clinical trial management under 21 CFR 312 requires a multifaceted approach, combining an understanding of evolving data requirements, regulatory guidance, and modern technological tools to ensure compliance and advance medical product development.
Conclusion
In conclusion, complying with 21 CFR 312 is vital for conducting clinical trials in a compliant and ethical manner. This regulation sets forth the responsibilities and requirements for sponsors, investigators, and IRBs involved in clinical trial conduct. Compliance with 21 CFR 312 is not only a legal obligation but also crucial for participant safety and data integrity.
Key components of 21 CFR 312 include the IND application process, clinical trial protocol requirements, informed consent and subject protection, data collection and integrity, safety reporting requirements, IRB approval and oversight, as well as compliance and regulatory considerations. Understanding and adhering to these components is essential for maintaining clinical research integrity and regulatory compliance.
By following these regulations and guidelines, we can advance medical science, enhance patient care, and contribute to the development of innovative treatments. Compliance with 21 CFR 312 is about upholding the highest standards of safety and effectiveness in clinical trials.
In summary, navigating 21 CFR 312 requires a comprehensive understanding of its key components and a commitment to compliance and regulatory considerations. By upholding these standards, we can ensure participant safety, maintain data integrity, and contribute to the advancement of medical knowledge and patient outcomes. Effective clinical trial management under 21 CFR 312 requires a multifaceted approach, combining understanding, compliance, and the use of modern tools to ensure regulatory compliance and promote medical product development.




