Introduction
Navigating the regulatory landscape of Latin America can be a daunting task for clinical researchers. With diverse requirements and procedures specific to each country, it is crucial to have a deep understanding of the regulatory bodies that oversee clinical research in the region. One such regulatory body is ANVISA in Brazil, which imposes stringent measures to ensure the safety and efficacy of products in the market.
However, the regulatory landscape is not static, and initiatives like the Request for Information are driving a shift towards transparency and accessibility in biomedical research. In this article, we will explore the challenges of navigating country-specific requirements, overcoming language and cultural barriers, addressing ethical considerations, and building strong partnerships with local experts. Through a case study of Inspira Technologies, we will also highlight a successful example of navigating the regulatory challenges in Latin America.
Join us as we delve into the intricate regulatory terrain of Latin America and uncover strategies for success.
Understanding the Regulatory Landscape
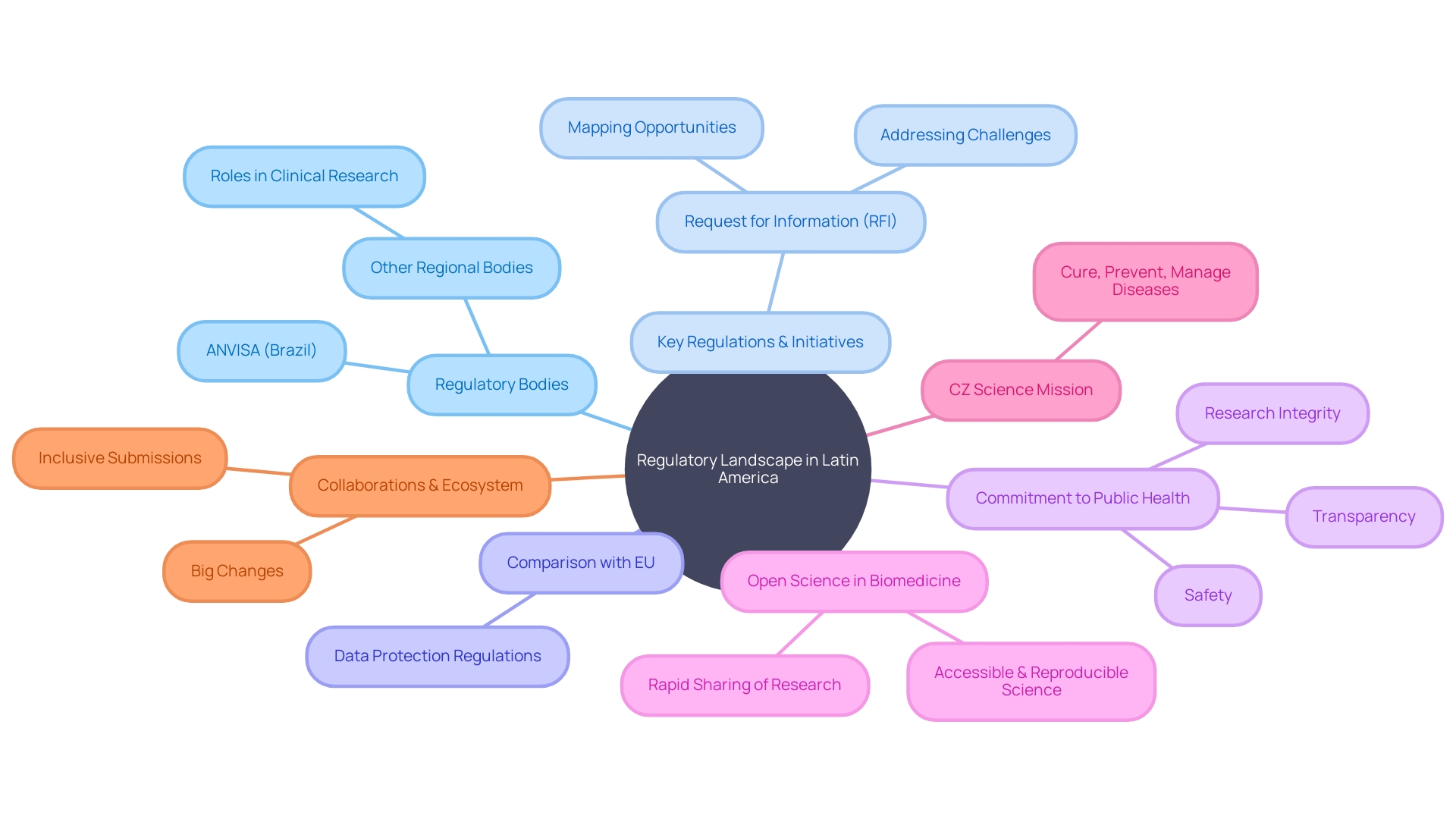
Navigating the diverse regulatory environments of Latin America requires a deep understanding of the requirements and procedures specific to each country for the initiation of first-in-human (FIH) and early-feasibility studies. A multitude of regulatory bodies across the region each plays a pivotal role in overseeing clinical research.
For instance, ANVISA in Brazil, akin to the FDA in the U.S., imposes stringent measures to safeguard public health, overseeing everything from food safety to pharmaceuticals. Under Law # 6360, health authorities must register products like medicines, cosmetics, dietary supplements, and medical devices. Such regulations ensure that products meet rigorous safety and efficacy standards before they are available on the market.
However, the regulatory landscape is not static. Initiatives like the Request for Information aim to identify and tackle challenges such as the adoption of open science practices in biomedical research. This inquiry into the state of open science in Latin America reflects a broader shift towards transparency and accessibility to accelerate scientific discovery and development.
Leading authorities are keen on improving the safeguards for critical data, especially in light of advancements in technology. A recent project compares biometric data protection regulations in Latin America with those in the European Union to pinpoint key areas where Latin America can bolster its data protection measures.
By considering best practices, observing real-time regulatory updates, and anticipating future shifts in policy, clinical researchers can better navigate the intricate regulatory terrain of Latin America's varied jurisdictions. As evidenced by the increase in high-level biosafety laboratories in the region and the continuous refinement of guidelines, there is a tangible commitment to enhancing public health safety and research integrity.
Navigating Country-Specific Requirements
Conducting successful first-in-human (FIH) and early-feasibility studies (EFS) for medical devices in Latin America presents a web of country-specific regulatory challenges. Recognizing that each nation within this diverse region has its tailored framework, clear strategies must be crafted for effective navigation. Key insights suggest starting with well-informed target market selection, which pivots on disease incidence and prevalence data.
Such granularity in choosing locations reflects the respective markets’ characteristics and lay the groundwork for focused Voice of Customer (VoC) research.
Choosing a handful of representative locations aids in uncovering notable similarities which, despite not encapsulating every nuance of each country, can be significantly influential for strategizing research efforts. Furthermore, establishing robust local partnerships emerged as crucial for seamless on-site operations and navigating through the intricate local regulations. Such alliances often form the bridge between foreign device manufacturers and the local ecosystem.
Recent news from Inspira Technologies epitomizes the importance of understanding and adapting to regional demands. Their expansion into eight Central American markets, contingent upon regulatory approval, and their recent usability study underscore the commitment to meeting and exceeding rigorous regulatory expectations. Alongside such milestones, industry reports have highlighted a rise in regulatory pressures globally, urging companies to remain agile and informed.
With the FDA categorizing devices based on risk and corresponding regulatory scrutiny, and a similar stratified landscape present across Latin America, manufacturers are urged to adapt their strategies accordingly. A comprehensive understanding of these classifications can not only streamline regulatory navigation but also potentially expedite market entry. And as the industry braces for a continuous surge in regulatory demands, leveraging such strategic insights to fine-tune preparedness and efficiency in documentation remains critical.
Overcoming Language and Cultural Barriers
Undertaking medical device studies in Latin America presents unique operational hurdles due to the linguistic and cultural diversity of the region. Beyond language proficiency, the key to navigating these challenges lies in a profound understanding of local contexts and establishing trustworthy collaborations within each target market. Because low- and middle-income countries (LMICs) within Latin America significantly differ in their healthcare needs and infrastructures, pinpointing the most relevant markets according to disease prevalence and other health data is critical.
Region-specific strategies and building connections with local partners facilitate smoother operations and avert the risks associated with "parachute" or "helicopter research"—terms denoting studies that fail to involve or benefit the local communities sufficiently. Furthermore, considering that clinical trial data from Latin America might not be cross-applicable to regulatory environments of countries like Japan or China, it is of paramount importance to harness local expertise in both trial execution and navigating regulatory landscapes. Insightful local partnerships, coupled with a strategic focus on representative locations, empower researchers to conduct studies that resonate with local cultural sensitivities and deliver meaningful outcomes for all involved.
Addressing Ethical Considerations
Medical device manufacturers undertaking first-in-human and early-feasibility studies in Latin America must account for ethical nuances specific to the region. These studies are grounded in the fundamental principle of protecting the rights and welfare of research participants. Healthcare professionals assessing medical devices, such as the INSPIRA ART100 blood circulation device in a usability study in Boston, must ensure the assessments consider the ethical significance of the technological impact on human subjects, amidst cultural and social contexts of the intended user base.
Furthermore, Latin America is characterized by its diverse political histories and varying levels of technology infrastructure, which present unique challenges in maintaining ethical consistency across studies. Strategic guidance on ethical considerations is drawn from analyzing regional legislation compared with EU regulations, recognizing the variability and aiming to harmonize practices with international standards. Concrete case vignettes, alongside a set of guiding questions, help underscore relevant ethical dilemmas.
Moreover, Latin America’s pivotal role in global health—exemplified by Brazil's leadership in the G20 presidency year—heightens the importance of scalable and sustainable ethical frameworks to ensure equitable advancement in medical research.
Building Strong Partnerships with Local Experts
Forging alliances with domestic specialists and relevant entities is a pivotal strategy for effectively navigating the complex and dynamic regulatory landscapes of Latin America. Local expertise, from regulatory consultants to clinical research organizations and ethics committees, is invaluable for understanding unique regional challenges and developing appropriate methodologies for First-in-Human (FIH) and early-feasibility studies (EFS). Such collaboration is not just beneficial but a necessity for ensuring that research adheres to the diverse and evolving data protection and privacy laws, as exemplified by the proactive approaches of Argentina, Uruguay, Mexico, Peru, and Colombia towards monitoring and regulating the use of personal data.
Experiences shared by experts across 11 Latin American countries during a regional workshop highlight the importance of collaborative work in AI research which could serve as a model for cooperation in other scientific areas. Scientists are increasingly recognizing the need for collective efforts to address global issues. Analogously, data protection authorities in various Latin American nations are mapping out strategies to inform future regulatory work in the context of advancing technology and digitalization.
Approaching regulatory challenges in Latin America demands more than ad hoc solutions; it calls for establishing foundational partnerships that are informed by on-the-ground realities and the expertise of those who navigate them daily. This region-specific insight informs alliances and paves the way for harmonizing public policies and joint research efforts—key elements noted by the growing debate on open versus FAIR data management and the embrace of open science principles within the context of Latin American research data policy.
Case Study: Successful Navigation of Regulatory Challenges
A notable example of navigating the rigorous regulatory environment in Latin America can be observed through the journey of Inspira Technologies. As they expanded their innovative medical product offerings into eight countries in Central America, with a focus on Mexico, the journey was a testament to strategic planning and execution. Their experience underscores the intrinsic challenges of entering new markets with variable compliance demands.
Despite potential hurdles, their INSPIRA ART100 blood circulation medical device punctuated this success story: from the completion of a meticulous Usability Study in Boston, Massachusetts, which harnessed the expertise of healthcare professionals to vet the device's human factors, to anticipated recurring revenues from disposable kits projected at up to $28 million over five years post-regulatory approval.
The industry's preparedness to meet increasing regulatory requirements in the medical device realm is crucial. This is emphasized by the Food and Drug Administration's (FDA) classification system in the US, which delineates devices from low to high risk and necessitates varying levels of scrutiny. This classification exemplifies the imperative of a medical device manufacturer to navigate the regulatory maze efficiently.
Especially so, considering that high-risk class three devices, like pacemakers, represent 10% of the FDA-regulated devices and undergo prolonged approval processes. Central to this success is an understanding of the regulatory landscape shifts, as well as strategies and priorities delineated by industry professionals in managing these evolving demands.
Inspira Technologies' case exemplifies the significance of a multi-faceted approach that considers market incentives, intellectual property, and ethical considerations in a dynamic regulatory environment. Their narrative offers a concrete example of industry resilience and adaptability, reflecting the broader themes of regulatory navigation strategies for first-in-human and early-feasibility studies within Latin America's distinct setting. It's an informative read for those seeking to align their regulatory compliance strategies with the demands of this rapidly advancing sector.
Conclusion
In conclusion, navigating the regulatory landscape of Latin America requires a deep understanding of country-specific requirements and procedures. ANVISA in Brazil and other regulatory bodies play a crucial role in ensuring safety and efficacy standards. The region is witnessing a shift towards transparency and accessibility in biomedical research, driven by initiatives like the Request for Information.
To succeed in this complex environment, clinical researchers must consider strategies such as well-informed target market selection and establishing strong local partnerships. Overcoming language and cultural barriers is crucial, as is addressing ethical considerations that are specific to the region. Building collaborations with local experts and regulatory consultants is essential for navigating the diverse regulatory landscapes.
Inspira Technologies provides a successful case study of navigating these challenges. Their expansion into Central America and usability study exemplify the importance of understanding compliance demands and adapting to the regulatory environment. Such accomplishments highlight the need for industry preparedness to meet increasing regulatory requirements.
In summary, by understanding country-specific requirements, overcoming language and cultural barriers, addressing ethical considerations, and building strong local partnerships, clinical researchers can successfully navigate the regulatory terrain of Latin America.




