Introduction
Chile's regulatory landscape for medical device clinical trials is both robust and intricate, playing a pivotal role in the global medical research ecosystem. Governed primarily by the Institute of Public Health (Instituto de Salud Pública, ISP), the framework ensures rigorous oversight and alignment with international standards, such as those established by the World Health Organization (WHO) and the International Conference on Harmonisation (ICH). This regulatory environment is designed to not only safeguard public health but also to facilitate the swift approval of low-risk trials, making Chile an increasingly attractive venue for clinical research.
Understanding this system is essential for organizations aiming to navigate the complexities of medical device trials in Chile, ensuring compliance with national and international regulations while advancing medical innovation and patient care.
Regulatory Framework for Medical Device Clinical Trials in Chile
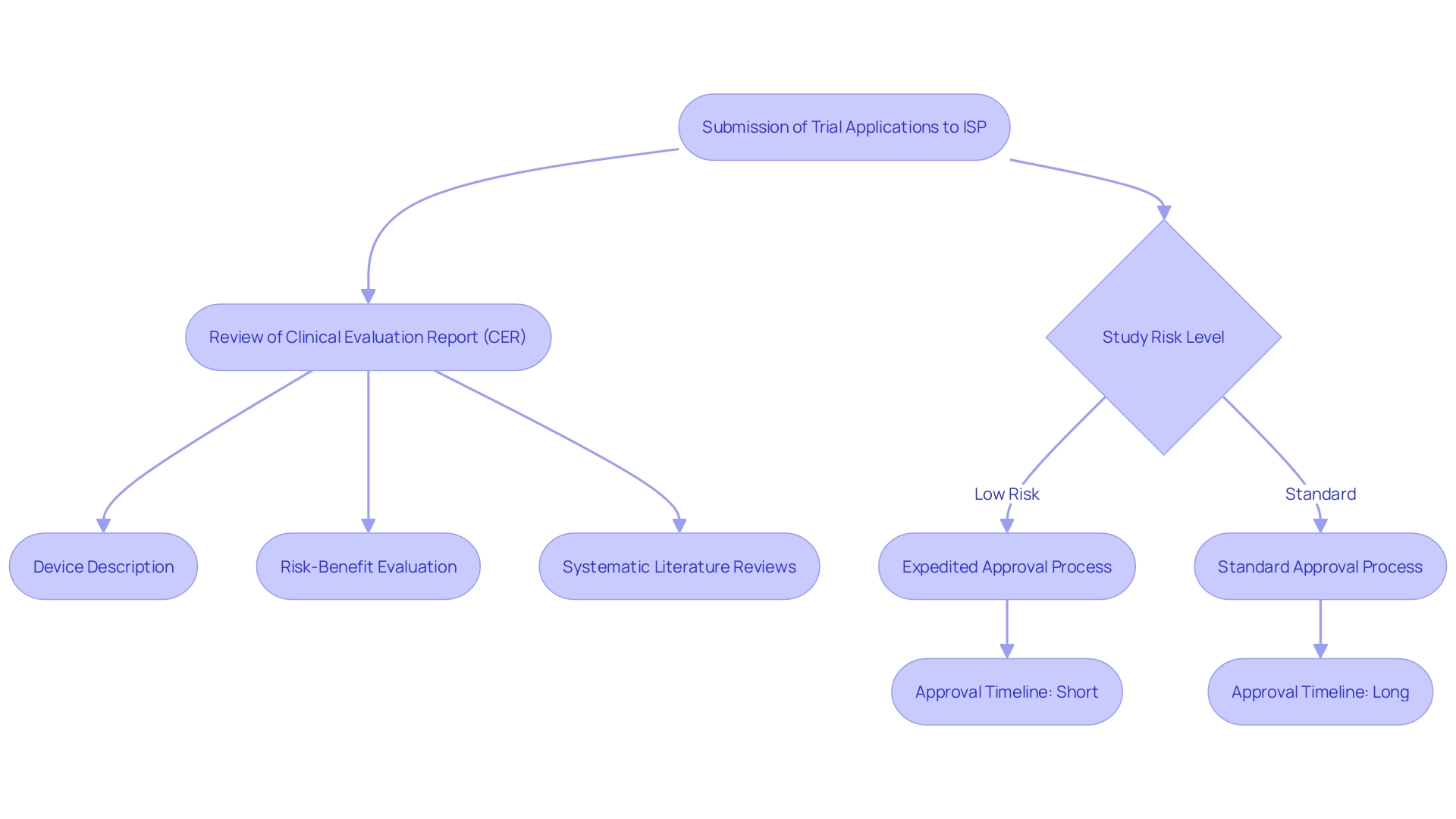
Chile has created a thorough set of rules overseeing studies related to medical instruments. The main organization supervising these trials is the Institute of Public Health (Instituto de Salud Publica, ISP), which is tasked with approving trial applications and ensuring adherence to national regulations. This framework aligns with international standards, such as those set by the World Health Organization (WHO) and the International Conference on Harmonization (ICH).
This regulatory framework includes several critical components. For example, the Clinical Evaluation Report (CER) includes a comprehensive device description, an evaluation plan, systematic literature reviews, data from studies, and a state-of-the-art report. The CER also encompasses a risk-benefit evaluation, summarizing adverse incidents, device malfunctions, and possible safety issues, along with a meta-summary of overall medical evidence supporting the device's safety and performance.
Furthermore, Chile's oversight framework seeks to accelerate the approval procedure for low-risk studies, with initial submissions for Phase III and IV studies handled within 14 days, considerably quicker than the standard 30 days. This efficiency is vital considering the rising quantity of research studies and the escalating intricacy of device regulations worldwide.
Comprehending Chile's legal structure is crucial for entities aiming to carry out research studies in the area. The nation's adherence to global benchmarks and its effective authorization procedures render it a desirable site for healthcare device evaluations, aiding in the worldwide progress of scientific inquiry and patient treatment.
Key Regulatory Bodies and Their Roles
Numerous important regulatory organizations have crucial functions in the supervision of medical device studies in Chile. The Instituto de Salud Publica (ISP) acts as the main authority in charge of assessing and authorizing medical studies. Furthermore, the Ministry of Health (Ministerio de Salud) plays a crucial role in developing health policies and regulations, ensuring that tests comply with national health standards. The National Ethics Committee provides crucial ethical oversight, ensuring that all clinical trials comply with established ethical guidelines.
The categorization of healthcare instruments into four risk levels (class I-IV) based on potential dangers requires that compliance criteria differ accordingly, with higher-risk devices facing more thorough assessment procedures. The collaboration between these bodies not only streamlines the approval process but also ensures compliance with comprehensive regulations. This cooperative approach is essential for maintaining the quality, safety, efficacy, and performance of medical devices before they reach the market, ultimately safeguarding public health.
In the broader context, global governance frameworks and standards play a pivotal role in shaping national guidelines. For instance, the integration of expedited approval processes, enhanced stakeholder coordination, and joint payer/HTA guidance on study design and data needs can significantly improve the system's efficiency. By connecting pricing strategies to data uncertainty and improving public comprehension of the oversight processes, the governing bodies can promote a more transparent and effective clinical trial environment.
Ultimately, ensuring that healthcare equipment meets strict oversight criteria before market entry is crucial. This not only involves rigorous scientific and ethical scrutiny but also necessitates unprecedented levels of coordination among various regulatory entities. Such measures are fundamental to advancing healthcare research and improving patient outcomes in Chile.
Ethical Considerations and Approval Process
Ethical factors have an essential influence on the execution of medical studies for devices in Chile. The approval process mandates the submission of an ethical review application to an Institutional Review Board (IRB) or Ethics Committee. This thorough evaluation examines the risk-benefit ratio, informed consent procedures, and the protection of vulnerable populations, ensuring that the studies adhere to the highest ethical standards.
In Chile, the importance of ethical oversight is underscored by the necessity for compliance with stringent guidelines established for the protection of human participants. Researchers are assigned the responsibility of ensuring that all ethical guidelines are strictly adhered to in order to obtain the necessary approvals, which are essential for the initiation of any medical study. This process includes a detailed review of consent practices, which are pivotal for safeguarding participant rights and ensuring transparency.
The ethical considerations extend beyond basic compliance. They encompass broader social implications, particularly in ensuring equitable access to the benefits of medical research. Enhancing ethical standards in clinical studies can help tackle global divides, as shown by the difference in participation rates between high-income countries (HICs) and low- and middle-income countries (LMICs). For instance, in 2022, there were significantly more trials in HICs compared to LMICs, despite the latter's higher disease burdens.
Moreover, the ethical review process in Chile aligns with global standards that advocate for patient-centered research. This approach is essential for generating robust evidence that informs healthcare and public health practices. 'Adjusting ethical supervision to be more attuned to the particular risks and purposes of research studies is an urgent necessity, as highlighted by specialists who advocate for 'right-sizing' participant consent methods to strengthen the ethical structure regulating medical research.'.
In summary, ethical considerations in Chile's evaluations for healthcare devices are comprehensive, ensuring that assessments are conducted with the utmost integrity and respect for human rights. These measures not only safeguard participants but also aid in the overall progress of healthcare knowledge and fair healthcare results.
Challenges and Solutions in Conducting Medical Device Clinical Trials in Chile
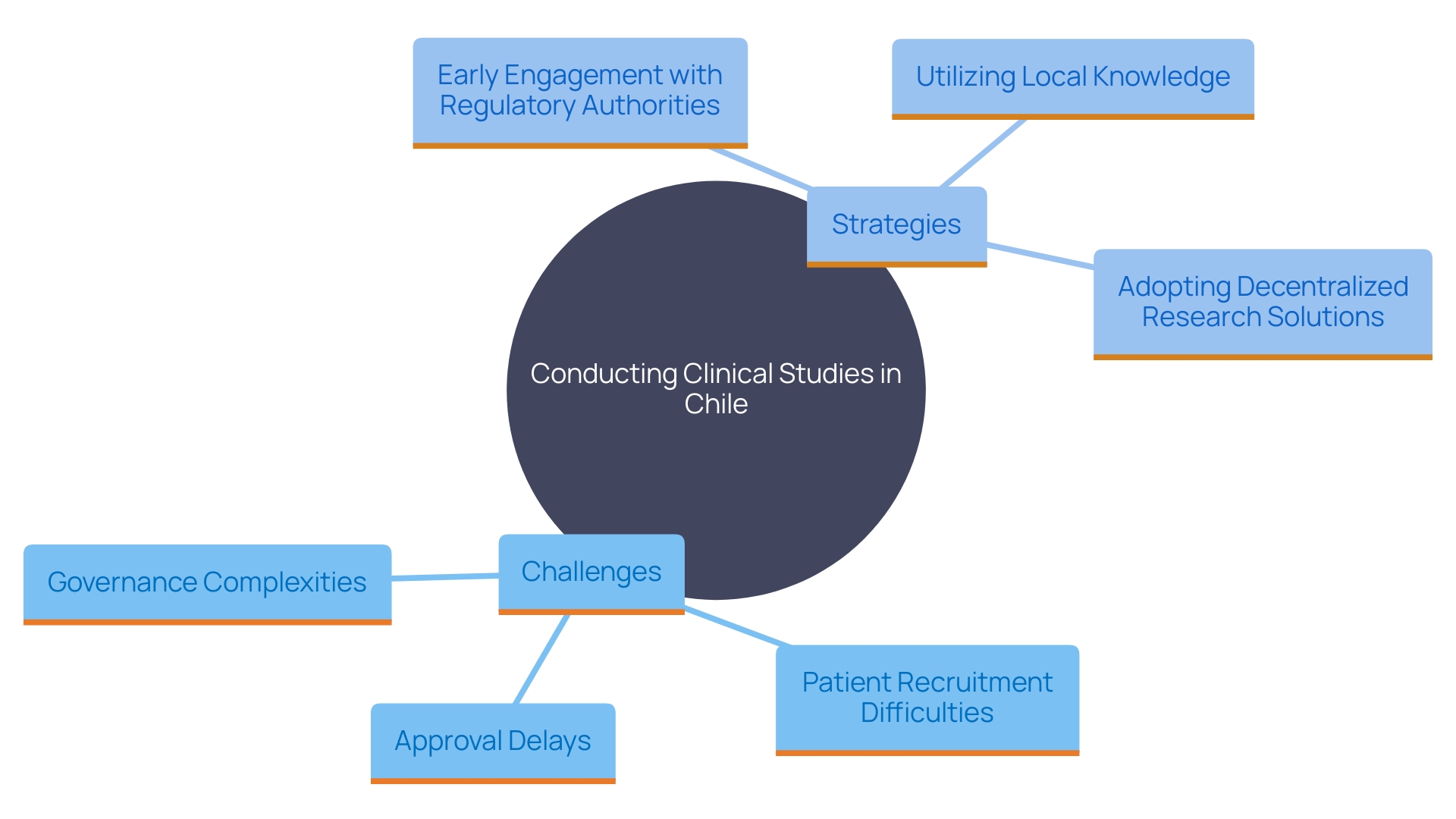
Carrying out clinical studies for medical equipment in Chile includes various challenges, like approval delays, differing interpretations of guidelines, and difficulties in patient recruitment. To effectively address these challenges, sponsors must engage early with regulatory authorities and ethics committees, ensuring open lines of communication. Utilizing local knowledge and building connections with healthcare providers can greatly improve patient recruitment and retention, resulting in successful study outcomes.
The adoption of decentralized research solutions, hastened by the COVID-19 pandemic, provides a feasible approach to address recruitment challenges. DCTs enable studies to be carried out from a distance, utilizing electronic patient-reported outcomes (ePRO) and electronic clinical outcome assessments (eCOA), as well as digital monitoring solutions and in-home clinical services. This method not only enhances participant retention but also lowers the overall expense of the study. As per a study conducted by Zelta, 89% of sponsors are presently employing decentralized technology to assist with at least one of their experiments.
Establishing experiments in smaller nations instead of well-known research centers can offer benefits like increased recruitment rates and more involved investigator research personnel. However, these countries may lack suitable research support personnel, necessitating additional training on experimental practices and guidelines. Furthermore, governance complexities and logistical challenges, such as import laws and regional political instability, can affect product availability.
Involving various groups in research studies is essential for guaranteeing thorough and fair scientific investigation. 'In spite of the logistical difficulties, heightened oversight assistance and interest in local research locations can boost the possibilities for carrying out medical studies in areas with historically low involvement rates, such as Africa.'. As highlighted by Révérien Uwacu, supply management advisor for research studies, tackling these obstacles is crucial for scientific, ethical, and health-related reasons.
In summary, early involvement with regulatory authorities, utilizing local knowledge, and adopting decentralized research solutions are key strategies for navigating the challenges of conducting studies for medical devices in Chile. By implementing these approaches, sponsors can enhance patient recruitment and retention, ultimately leading to successful study outcomes.
Best Practices for Navigating Clinical Research Regulations in Chile
To effectively navigate the research regulations in Chile, it is essential to adopt best practices such as maintaining clear documentation throughout the study process, ensuring compliance with local laws, and conducting thorough training for research personnel. Establishing a comprehensive project management plan that includes timelines, responsibilities, and checkpoints can also facilitate smoother operations. Consistently informing stakeholders about policy changes and keeping a proactive stance on compliance will further improve the likelihood of successful trial execution.
In addition to these best practices, it is crucial to engage the community and stakeholders in the research process. Sharing research results with the community before the publication of the manuscript ensures transparency and mutual understanding. Utilizing tools like the PhoeniX Toolkit and REDCap can streamline data management and enhance study efficiency. The PhoeniX Toolkit provides validated measures of phenotypic traits and environmental exposures, while REDCap offers an easy-to-use platform for clinical study management and data capture. Furthermore, platforms like ResearchMatch can help connect researchers with potential study participants, ensuring a diverse and representative sample.
A grasp of the compliance documentation across various fields, as outlined by both national and global health authorities, is essential. This includes exploring roles and methodologies associated with regulatory documentation and evaluating different models and strategies for fostering collaboration within the Regulatory Affairs field. By leveraging these resources and maintaining a proactive approach to compliance, clinical trials in Chile can be conducted more efficiently and ethically, ultimately leading to improved patient outcomes and advancements in medical knowledge.
Conclusion
The regulatory framework for medical device clinical trials in Chile is robust and designed to prioritize public health while facilitating medical research. The Instituto de Salud Pública (ISP) plays a critical role in overseeing compliance with international standards set by organizations like the World Health Organization (WHO) and the International Conference on Harmonisation (ICH). This alignment enhances the safety and efficacy of clinical trials, making Chile an attractive venue for research.
Key regulatory bodies, including the Ministry of Health and the National Ethics Committee, ensure the ethical and scientific integrity of trials. The classification of medical devices into various risk levels requires tailored regulatory approaches, particularly for higher-risk devices, thus safeguarding public health and improving patient outcomes.
Ethical considerations are paramount, with Institutional Review Boards (IRBs) conducting thorough reviews to protect participants’ rights and ensure informed consent. This focus on ethical standards and diversity in trial populations underscores a commitment to equitable healthcare.
While challenges such as regulatory delays and recruitment issues exist, strategies like decentralized clinical trials and early engagement with regulatory bodies can effectively mitigate these obstacles. Adopting best practices, including comprehensive documentation and community engagement, further streamlines compliance with Chile's regulatory framework.
In summary, understanding and adhering to Chile's regulatory landscape for medical device clinical trials is essential for advancing medical research and enhancing patient care. This commitment to ethical and regulatory standards will continue to solidify Chile's role in the global medical research arena.




