Introduction
The landscape of clinical research in Latin America is intricate, with regulatory environments varying significantly across countries. Navigating these complexities is crucial to ensure high-quality healthcare outcomes and the wellbeing of communities. Understanding the specific regulatory frameworks that govern first-in-human (FIH) and early-feasibility studies (EFS) is especially important given the heightened public expectations and the necessity for tailored care.
Networks like QuEST LAC are emerging to bolster research and evolve health systems, highlighting the need for expertise in this field. Recent developments in health policy and regulatory leadership underscore the dynamic nature of the regulatory scene. This article explores the key regulatory bodies and guidelines in Latin America, emphasizing the importance of staying abreast with regulatory updates.
Ethics and informed consent are also crucial considerations in the diverse and evolving landscape of Latin America, where linguistic and cultural heterogeneity must be taken into account. Navigating the approval process for FIH and EFS studies requires a thorough understanding of submission requirements and key considerations for successful approval. By adopting a strategic approach that aligns with regional best practices, researchers can navigate the complex regulatory terrain and contribute to advancements in healthcare and patient treatment options.
Understanding Regulatory Environments for Clinical Trials in Latin America
The landscape of clinical research in Latin America is intricate, with regulatory environments varying significantly across countries. Ensuring high-quality healthcare outcomes and the wellbeing of communities hinge on navigating these complexities adeptly. In Latin America, it is crucial to understand the specific regulatory frameworks that govern first-in-human (FIH) and early-feasibility studies (EFS), especially given the heightened public expectations and the necessity for systems that can deliver care tailored to diverse contexts.
Quality health systems are paramount, and the stakes are high: poor-quality healthcare is a major issue in low- and middle-income countries, with millions of deaths each year from treatable conditions. To address this, networks such as the Quality Evidence for The Transformation of Health Systems for Latin America and the Caribbean (Quest LAC) have emerged, aiming to bolster research and evolve health systems.
Chris, a seasoned biomedical engineer with over a decade of experience in clinical studies for advanced medical devices, exemplifies the type of professional dedication required in this field. Similarly, the ongoing changes in regulatory leadership, exemplified by the anticipated appointment of a new head for Colombia's INVIMA, highlight the dynamic nature of the regulatory scene.
Furthermore, recent developments in health policy have seen significant disruptions, such as Brazil's ANVISA grappling with a compromised controlled substances database, and Honduras's ARSA intensifying inspection and training actions. These shifts underscore the need for a deep comprehension of the regulatory climate that affects the healthcare industry and clinical research.
This context of fluctuating regulations and the push for improved healthcare systems is matched by a growing emphasis on open science practices. The potential for collaboration and innovation in the biomedical research ecosystem is vast, and submissions from individuals and groups across Latin America to contribute to this movement are highly encouraged.
Amidst these developments, the voice of experts like Kati, a health policy analyst, resonates with the call for attention to rare diseases in the region, advocating for equitable and timely care. This advocacy is mirrored by organizations seeking to expedite the drug development process, as highlighted by Alvea's initiative to bring new medicines to clinical trials swiftly.
The call to action is clear for those in the clinical research community: join forces to shape a landscape where clinical trials can flourish, fostering innovations that could transform healthcare and save lives. For industry professionals and veterans alike, the opportunity to contribute to this transformative change is immense, and the time to act is now.
Key Regulatory Bodies and Guidelines in Latin America
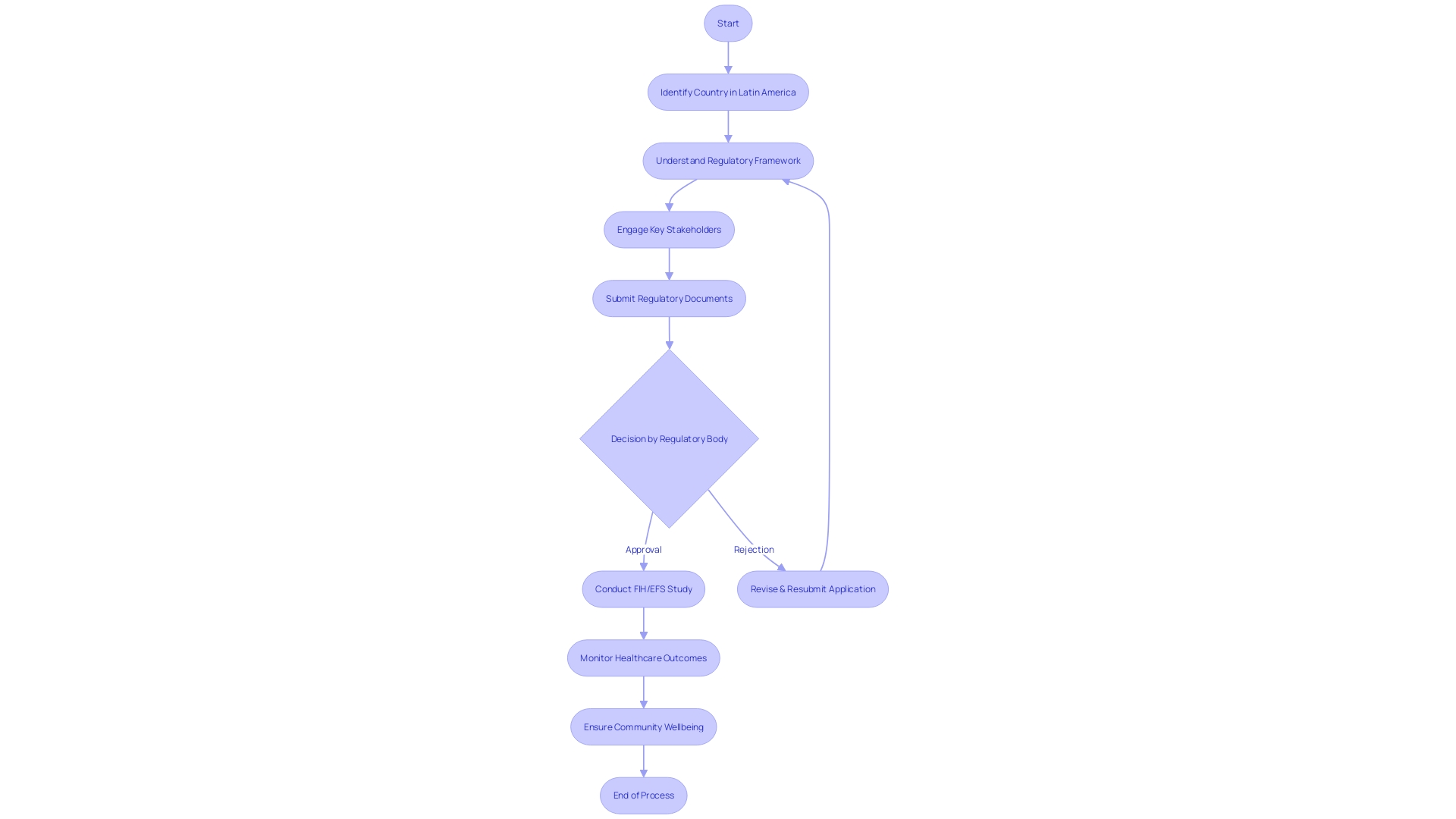
For clinical trial leaders, understanding the nuances of regulatory environments in Latin America is paramount. Each country within the region has its own specific set of governing bodies and guidelines that must be followed to conduct first-in-human and early-feasibility studies. Navigating these regulations effectively is key to running successful trials.
Prominent regulatory agencies, such as ANVISA in Brazil and INVIMA in Colombia, are central to the approval and oversight of clinical trials. These bodies are responsible for ensuring that trials are conducted ethically and safely, adhering to stringent standards that protect participants and produce reliable data. For example, ANVISA has implemented digital measures to enhance access to medication labels and other crucial information.
Recent developments in the region have highlighted the importance of staying abreast with regulatory updates. Changes in leadership, such as the anticipated appointment of a new head of INVIMA, can influence regulatory processes. Additionally, the increased vigilance from agencies like Honduras's ARSA, which reported a significant rise in inspection actions and training activities, underscores the evolving nature of regulatory oversight in Latin America.
Understanding these regulatory frameworks not only facilitates compliance but also aligns with broader health priorities in the region. For instance, addressing mental health challenges is critical, as Latin America exhibits some of the highest rates of depression and anxiety globally. This is compounded by economic factors such as productivity loss and financial strain from untreated conditions, which can impede the successful execution of clinical trials.
Thus, aligning clinical research with these health imperatives can enhance the impact of trials on patient outcomes and public health.
In sum, thorough knowledge of the regulatory landscape and the ability to adapt to its changes are indispensable for conducting efficient and compliant clinical trials in Latin America. This ensures not only the integrity of the research but also its potential to contribute to the betterment of health across the region.
Ethics and Informed Consent in Latin American Clinical Trials
Upholding ethical principles and ensuring informed consent are indispensable in clinical research. In the diverse and evolving landscape of Latin America, these issues become even more crucial due to the linguistic and cultural heterogeneity of the population. The region's vibrancy is mirrored in its public health challenges, highlighting the importance of inclusive and representative research.
As the region strengthens its global health leadership, with Brazil poised to lead the G20 in 2024, the emphasis on ethical conduct in clinical trials becomes paramount. The Declaration of Helsinki serves as a guidepost for medical research ethics, emphasizing that informed consent is not merely a formality but a fundamental right that must be protected from coercion, threat, or undue influence.
The linguistic diversity, particularly the prevalence of Spanish speakers who may not be fluent in English, underscores the need for clear communication and culturally appropriate consent processes. It's imperative that participants receive complete and accurate information about the nature and potential impact of the research to make well-informed decisions. This is not only a matter of ethical research conduct but also of respecting individual autonomy and the diverse cultural fabric of Latin American societies.
Furthermore, the definition of vulnerable populations such as minors, pregnant women, neonates, prisoners, and those who are mentally impaired requires special attention to consent and protection requirements in Latin America. The region's efforts in confronting public health crises, such as the COVID-19 pandemic, have highlighted the value of local capacities and health cooperation, which must be leveraged to safeguard the rights of research participants.
In light of these considerations, clinical trials must be designed with an acute awareness of ethical standards, ensuring that informed consent is obtained transparently and respectfully. This approach not only honors the rights of participants but also contributes to the integrity and credibility of the research, fostering trust and advancing health equity in Latin America and beyond.
Navigating the Approval Process for First-in-Human and Early-Feasibility Studies
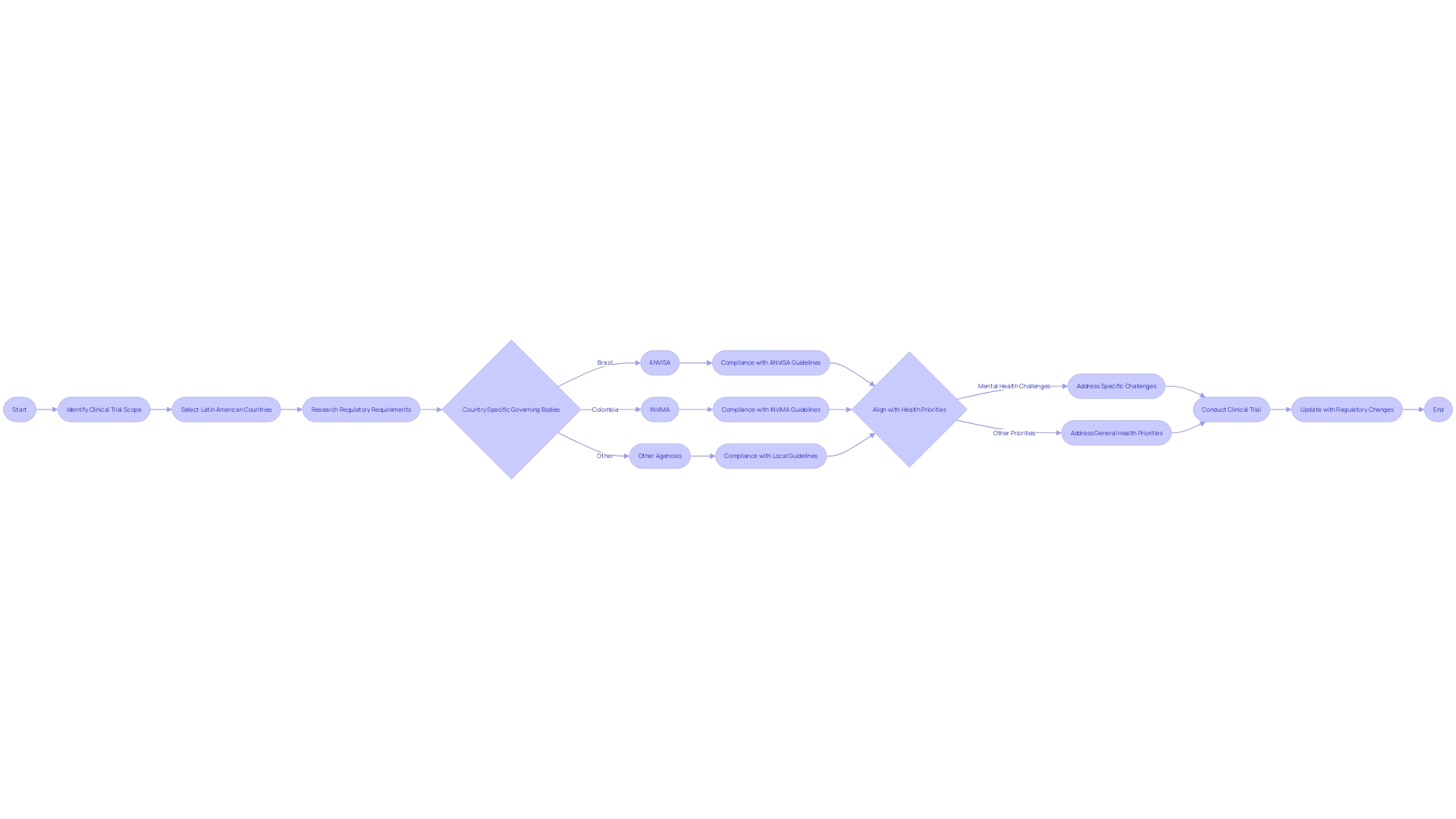
Navigating the regulatory intricacies of Latin America for first-in-human (FIH) and early-feasibility studies (EFS) requires a thorough understanding of submission requirements, timelines, and key considerations for successful approval. With regulatory landscapes varying significantly across the region, it is essential to be conversant with the specificities of each country's regulatory body and procedures. For instance, in Brazil, ANVISA, alongside the Ministry of Agriculture, Livestock, and Supply (MAPA), and the National Institute of Metrology, Quality, and Technology (INMETRO), play critical roles in ensuring product safety and health compliance, particularly in the food sector.
Moreover, the cornerstone Law # 6360 underpins sanitary surveillance for public health risk products in Brazil, setting a precedent for pharmaceuticals, cosmetics, and other health-related items that mandate registration before entering the market.
Amid these regulations, recent news highlights the dynamic nature of the Latin American regulatory environment. In Colombia, the unexpected withdrawal of a leading candidate for the head of INVIMA underscores the complexities and delays that can occur within the approval process. Brazil's ANVISA has experienced challenges with its controlled substances database, pointing to the need for robust and stable reporting systems.
Proactive measures are also evident, as seen with ANVISA's initiative to digitize information access for medication labels and Honduras's ARSA agency increasing inspection actions and pharmacovigilance training.
The variability in technology adoption and the necessity for enhanced regulations become more pronounced when considering the protection of biometric data. The urgency to bolster regulations is palpable, with studies contrasting Latin America's current frameworks against the more advanced European Union standards. This comparison reveals significant gaps, particularly in the face of burgeoning AI integration, emphasizing the need for encryption, multi-factor authentication, and tailored recommendations to strengthen data protection.
To successfully navigate this complex regulatory terrain, it is imperative to adopt a strategic approach that aligns with regional best practices and anticipates potential obstacles. As innovative drugs and biological products enter the market, the insights from FDA's Center for Drug Evaluation and Research (CDER) on study design and application data are invaluable, demonstrating the importance of scientific understanding to facilitate approvals. As new therapeutic options emerge, the commitment to quality, safety, and efficacy remains paramount, with each regulatory step paving the way for advancements in health care and patient treatment options.
Conclusion
In conclusion, navigating the intricate regulatory landscape of clinical research in Latin America is crucial for ensuring high-quality healthcare outcomes and the wellbeing of communities. Understanding the specific frameworks governing first-in-human (FIH) and early-feasibility studies (EFS) is vital in meeting public expectations and delivering tailored care.
The dynamic nature of the regulatory scene in Latin America is underscored by recent developments in health policy and regulatory leadership. Networks like QuEST LAC are emerging to support research and advance health systems in the region. Staying updated with regulatory updates is essential for researchers to navigate this evolving landscape.
Ethics and informed consent are of utmost importance in the diverse and evolving Latin American context. The linguistic and cultural heterogeneity of the population necessitates careful consideration in research practices. Successfully navigating the approval process for FIH and EFS studies requires a deep understanding of submission requirements and key considerations.
Clinical trial leaders must understand the nuances of regulatory environments in Latin America, as each country has its own governing bodies and guidelines. Compliance with these regulations is essential for conducting successful trials. Prominent regulatory agencies, such as ANVISA in Brazil and INVIMA in Colombia, ensure ethical and safe conduct of trials.
Staying informed about regulatory updates is crucial, as changes in leadership and increased vigilance from agencies like Honduras's ARSA can impact the regulatory landscape. Upholding ethical principles and ensuring informed consent are indispensable in the diverse Latin American context. Clear communication and culturally appropriate consent processes are essential in respecting participants' rights and diversity.
Navigating the regulatory intricacies of Latin America for FIH and EFS studies requires a comprehensive understanding of submission requirements and timelines. Researchers must align with regional best practices and anticipate potential obstacles. Emphasizing quality, safety, and efficacy standards contributes to advancements in healthcare and patient treatment options.
In summary, researchers must navigate the complex regulatory landscape in Latin America, stay updated with regulatory changes, and uphold ethics and informed consent. Understanding submission requirements and aligning with regional best practices are key to conducting successful clinical trials that contribute to advancements in healthcare and patient outcomes.




