Introduction
The regulatory framework for clinical trials in Brazil is meticulously structured to ensure adherence to ethical standards and scientific protocols. Governed by the National Health Surveillance Agency (ANVISA), this framework is crucial for safeguarding participant rights and maintaining the integrity of clinical research. The importance of these regulations is accentuated by the global landscape of clinical research, which continuously evolves to enhance participant protection and data reliability, as seen with the updates to the International Council for Harmonisation (ICH) Good Clinical Practice (GCP) guidelines.
Ethical considerations are a cornerstone of these guidelines, with efforts to harmonize regulations underscoring a commitment to efficiency and participant safety. In Brazil, the regulatory framework not only ensures compliance but also fosters innovation in medical research, contributing significantly to global medical advancements. By adhering to these rigorous standards, Brazil can maintain ethical and scientific integrity in its clinical trials, ultimately advancing medical knowledge on a global scale.
Regulatory Framework for Clinical Trials in Brazil
Brazil's oversight system for research studies is managed by the National Health Surveillance Agency (ANVISA). This framework is designed to ensure that medical research adheres to rigorous ethical standards and scientific protocols while safeguarding the rights and safety of participants. Particular protocols are established to oversee the behavior of research studies, which are essential for upholding regulatory adherence and enhancing medical understanding.
The significance of such frameworks is highlighted by the changing environment of global research regulations. For instance, the recent updates to the International Council for Harmonization (ICH) E6 Good Practice (GCP) guidelines emphasize the protection of participants' rights and the reliability of research results. As stated by Silvia Perez, head of quality compliance in healthcare at AstraZeneca, these updates are intended to guarantee that trials are structured with a thorough assessment of scientific aims and related risks.
Additionally, ethical considerations play a significant role in shaping these guidelines. The FDA's efforts to align human subject protection regulations with the U.S. Department of Health and Human Services' Common Rule, for instance, emphasize the continuous dedication to making research more efficient while safeguarding participants. This harmonization aligns with broader ethical, legal, and social issues that emerge in medical research, ensuring that the rights, safety, and well-being of participants are prioritized.
In the context of Brazil, these governance frameworks are vital not only for compliance but also for fostering innovation and development in medical research. By following these guidelines, Brazil can aid in the worldwide progress of medical knowledge while upholding the highest standards of ethical and scientific integrity in research.
Key Regulatory Bodies and Their Roles
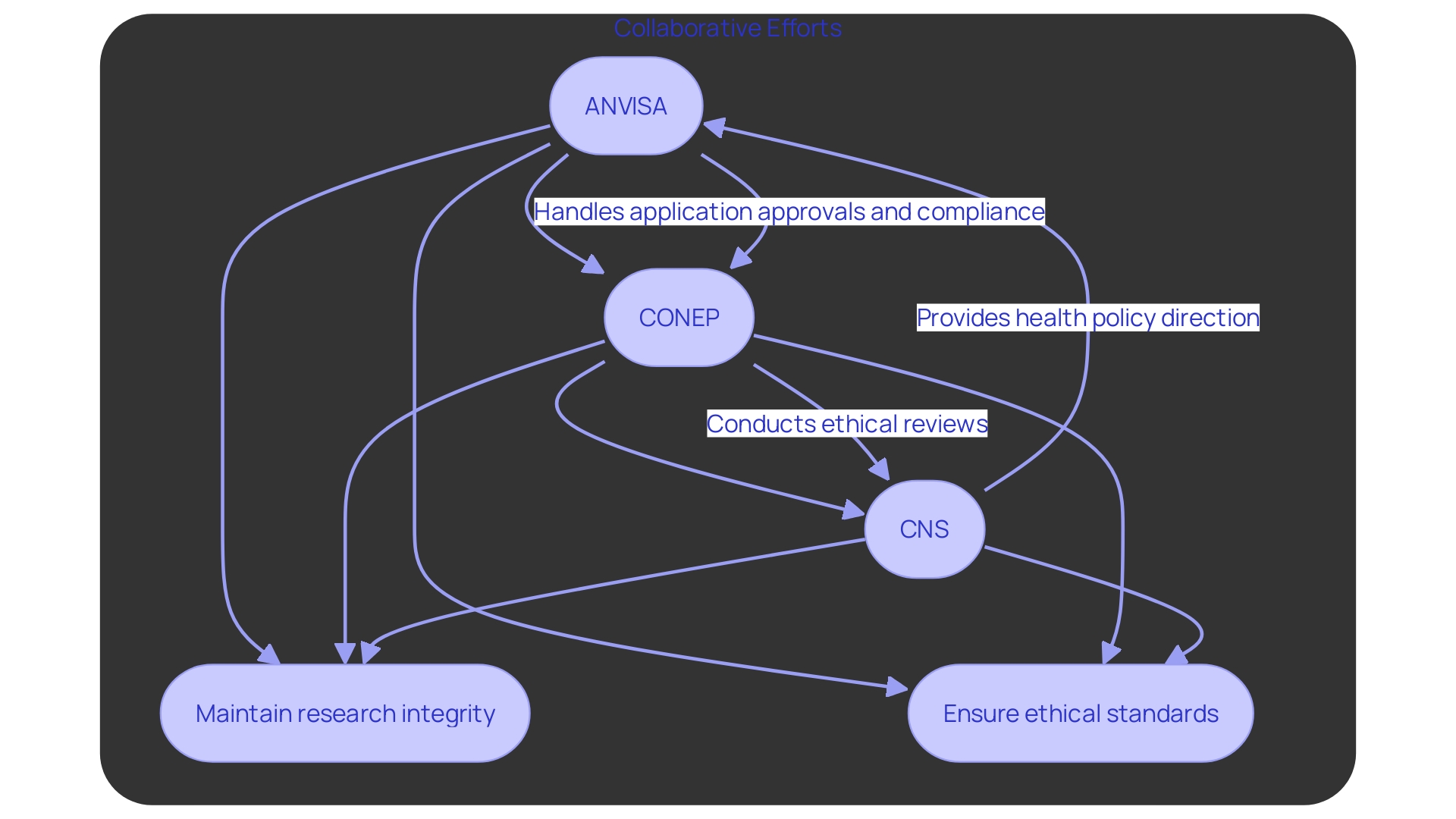
In Brazil, the main regulatory organizations supervising research studies are ANVISA, the National Commission for Ethics in Research (CONEP), and the National Health Council (CNS). ANVISA (Agência Nacional de Vigilância Sanitaria) is responsible for the approval of research study applications and ensuring compliance with health regulations, a vital role emphasized by the problems with the controlled substances database since 2021. CONEP is responsible for the ethical review of research protocols, ensuring that studies meet the necessary ethical standards. Meanwhile, CNS offers comprehensive direction on health policies and regulations that influence research activities. These organizations collaborate to uphold the integrity and ethical standards of research studies in Brazil, ensuring that they are carried out in accordance with national and international guidelines.
Clinical Trial Approval Process in Brazil
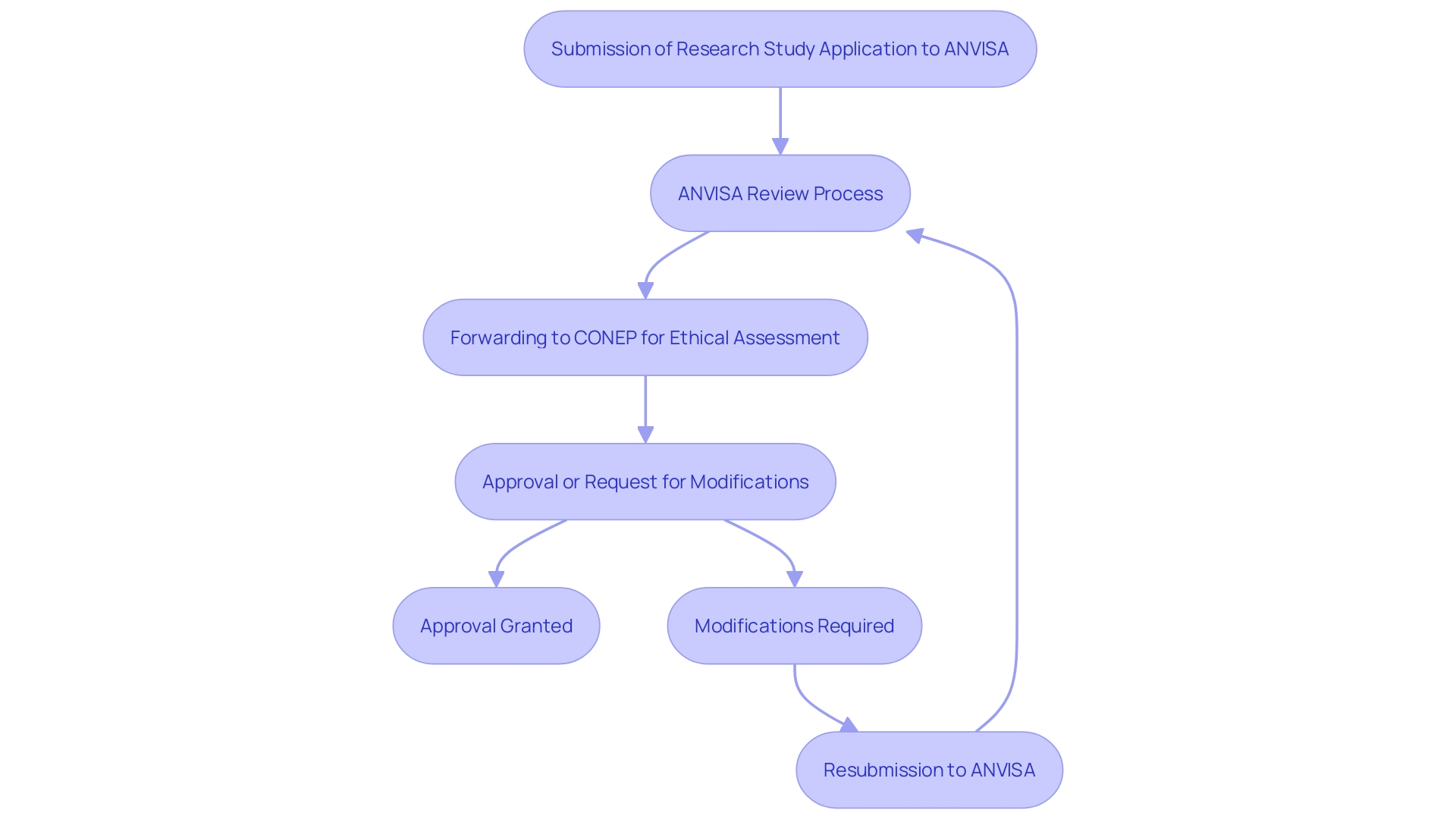
The authorization procedure for research studies in Brazil is complex, starting with the submission of a research study application to ANVISA. This application requires comprehensive information on the study design, objectives, and methodology. Following ANVISA's review, the application is then forwarded to CONEP for an ethical assessment. This entire procedure can span several months, necessitating meticulous preparation by sponsors and CROs to ensure timely approvals.
Given the complexity and duration of this process, it is crucial for sponsors to leverage data-and-analytics approaches to optimize study design and site selection, thereby streamlining the approval timelines. The increased rivalry for testing locations and the geographic variety of research studies further complicates the environment, with a 39% rise in the average number of nations involved in Phase III research. Additionally, the number of assessments in Phase 2 and 3 studies has risen from 17 to 21 between 2013 and 2020, increasing the burden on patients and potentially affecting enrollment rates.
The effect of these obstacles is substantial, with up to 80% of medical studies not finishing on schedule. This underscores the importance of thorough preparation and the adoption of innovative strategies to navigate the regulatory complexities in Brazil. As highlighted by specialists, implementing validated methods and unified systems can improve testing efficiency and site satisfaction, ultimately leading to more successful health outcomes.
Challenges in Conducting Clinical Trials in Brazil
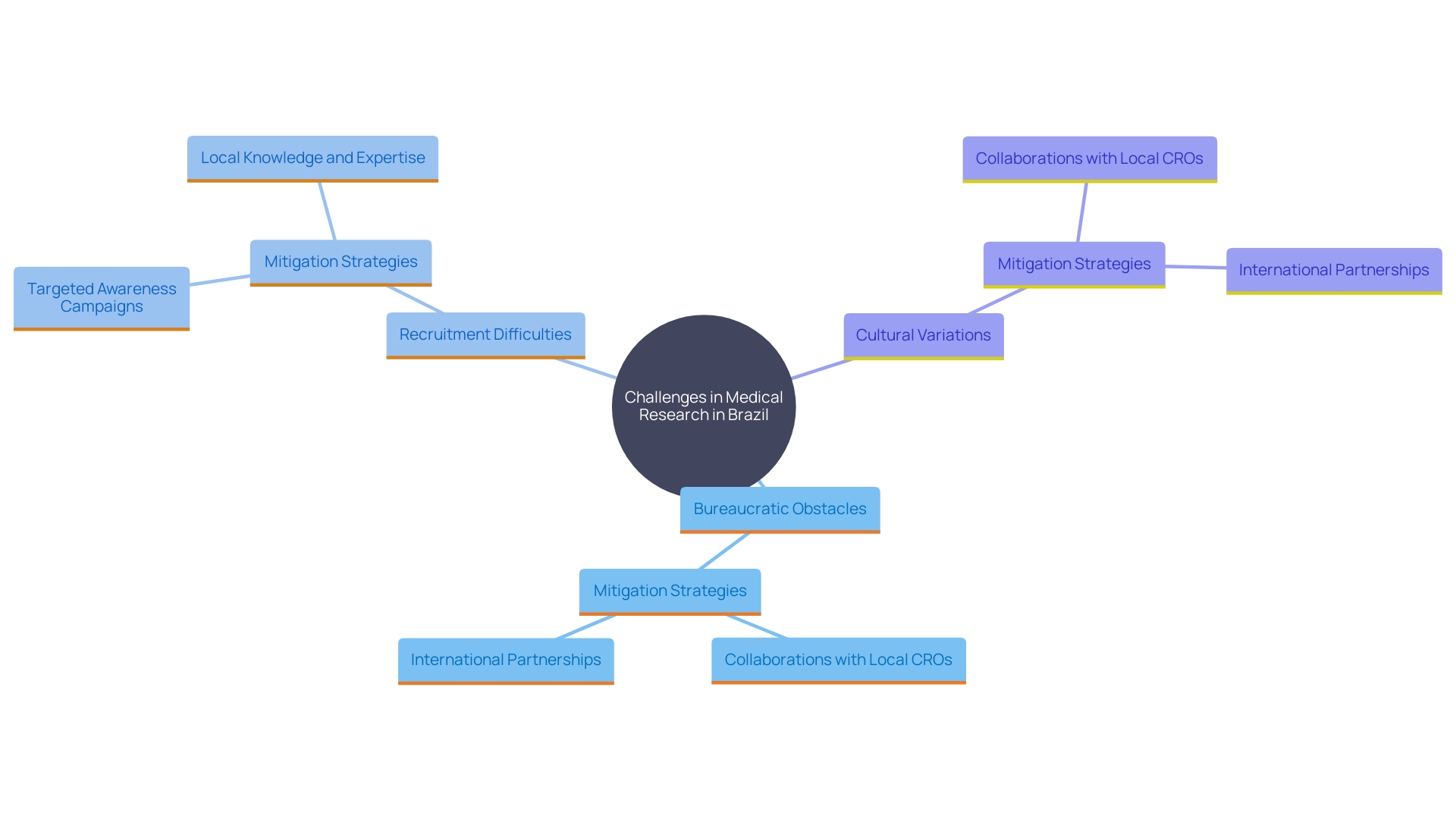
Carrying out medical experiments in Brazil poses numerous challenges, such as bureaucratic obstacles, prolonged approval timelines, and regional differences in research capability. These issues can be mitigated through strategic collaboration with local Contract Research Organizations (CROs), which possess the necessary expertise and local knowledge to navigate the complex regulatory landscape. For example, ImpulsoGov, a non-profit organization, collaborates with Brazilian municipalities to improve public health policies and has supported 150 cities across Brazil. This underscores the importance of local partnerships in enhancing research capacity and compliance.
Moreover, sponsors often face challenges in attracting participants because of cultural variations and differing levels of knowledge about research studies. As stated by the National Institutes of Health (NIH), factors like study burden, distrust of researchers, and a lack of understanding of the medical process contribute to recruitment challenges. This highlights the necessity of targeted awareness campaigns and education to improve participation rates.
Effective strategies include raising awareness of local health services, training professionals, and validating data. For instance, the collaboration between Oncoclinicas and Dana-Farber Cancer Institute aims to enhance oncology research and treatment in Brazil, demonstrating the potential impact of international partnerships. These initiatives pave the way for more inclusive and effective health studies, ultimately advancing medical knowledge and enhancing patient outcomes.
Role of a Local Clinical Research Organization (CRO)
Local Contract Research Organizations (CROs) are crucial in advancing research studies in Brazil by providing specialized expertise in regulatory compliance, site management, and patient recruitment. Their deep understanding of the Brazilian healthcare landscape allows them to guide sponsors through the approval process with greater efficiency. This collaboration significantly increases the likelihood of success by leveraging their familiarity with regional regulations and market dynamics.
For instance, the partnership between Oncoclínicas and Dana-Farber Cancer Institute exemplifies the impact of local expertise combined with international collaboration. This alliance has not only improved medical education and training programs but also placed Brazil at the forefront of oncology research and treatment. As Bruno Ferrari, CEO of Oncoclinicas, noted, this collaboration is a unique opportunity to expand the reach and impact of oncology research and treatment, positioning Brazil as a global leader in cancer care.
Moreover, Brazil's extensive public health infrastructure offers a strong basis for research initiatives. With over 90% of the country's 57,000 primary care clinics utilizing electronic medical records, data standardization facilitates scalable solutions and effective patient monitoring. The involvement of more than 500,000 public health professionals, including community health agents and nurses, ensures that interventions are seamlessly integrated into the existing healthcare system without incurring additional staffing costs.
By merging local CRO expertise with Brazil's extensive healthcare resources and global partnerships, research studies in the country can achieve greater efficiency, improved patient results, and make a substantial contribution to worldwide medical progress.
Quality Management and Investigational Product Handling
Quality management is essential in research studies, particularly in managing investigational products. Local CROss enforce rigorous quality assurance protocols to ensure that all products are stored, transported, and administered in compliance with regulatory guidelines. This encompasses keeping detailed records and following proper medical practice (GCP) standards to safeguard the integrity of research information.
Regulatory organizations such as the EU MDR stress that medical investigations must prioritize the safety, dignity, and well-being of participants while ensuring the produced information is scientifically sound. For example, studies for high-risk devices offer crucial information demonstrating their safety and effectiveness, an important element for regulatory submissions. Roughly 10-15% of successful 510(k) applications for Class II devices in the US depend on research study findings, while all Class III devices necessitate comprehensive trials.
Moreover, there is frequently a gap between cutting-edge safety and performance goals and the information gathered. This discrepancy underscores the necessity for well-defined clinical endpoints and accurate information gathering methods. Efficient information management is essential, as the information constitutes the foundation of compliance submissions and substantiates product safety and effectiveness. To tackle these challenges, CROss must ensure comprehensive risk evaluations, supported by reliable information, and remain informed about compliance changes to adjust protocols and product development as needed.
Record Keeping and Transparency Requirements
Record keeping and transparency are foundational elements in clinical research, ensuring integrity and reliability in trial information. Brazil's governance framework stipulates that all trial-related documents must be preserved for a specific duration to allow thorough auditing and review. CROss play a crucial role in this process by implementing advanced information management systems designed to maintain accurate records and ensure transparency with regulatory authorities and stakeholders.
For example, research studies that have received substantial references since 2018 have highlighted the significance of openness. A systematic evaluation revealed that trials registered on platforms such as the Open Science Framework prior to information extraction had greater compliance with transparency standards. 'This practice aligns with the global need for robust clinical evidence, as highlighted by the World Health Organization, which underscores the necessity of meticulous information management to safeguard participant safety and derive accurate conclusions.'.
Furthermore, advancements in information infrastructure, such as the widespread adoption of electronic medical records in over 90% of Brazil's primary care clinics, facilitate scalable solutions for information management. These systems, standardized and reported to federal entities, enable precise identification and monitoring of patients without additional primary information collection efforts. As noted by experts, good clinical data management is crucial because it ensures that the vast amount of data generated in studies is accurately captured, managed, and accessible for future use, ultimately supporting the development of effective medical interventions.
Selecting the Right CRO for Medical Device Trials in Brazil
Choosing the best CRO for medical device studies requires a comprehensive assessment of various essential elements. Firstly, the CRO’s experience with similar studies is paramount. Hiring a CRO with extensive knowledge of local regulations and strong patient recruitment capabilities can significantly simplify the study process. For example, Carine Cochereau, Vice President of Regulatory International at Integra Life Sciences, highlighted the necessity for CROs to have established research hubs and trained support personnel to address regional challenges. Moreover, the significance of robust relationships with governing organizations cannot be emphasized enough, as these connections enable easier navigation through compliance requirements.
Assessing the quality management systems and resources of a CRO is also essential. Based on a thorough evaluation of the most referenced studies released after 2018, clear and well-documented protocols are essential for successful results. This includes having detailed documentation of the study protocol, patient information inclusion and exclusion criteria, and a clear scope of work. As highlighted by industry experts, providing comprehensive information about the proposed study and device to the CRO ahead of time can enhance collaboration and efficiency.
In summary, selecting the appropriate CRO involves not only assessing their experience and understanding of regulations but also ensuring they have the necessary quality management systems to maintain compliance and achieve successful outcomes. Leveraging industry expertise and fostering strong regulatory relationships are key to optimizing clinical trials for medical devices.
Conclusion
Brazil's regulatory framework for clinical trials, overseen by the National Health Surveillance Agency (ANVISA) and supported by organizations such as CONEP and CNS, plays a critical role in ensuring ethical standards and scientific integrity. The comprehensive guidelines established not only protect participant rights but also foster innovation within the medical research landscape. As global clinical research regulations evolve, Brazil's adherence to these standards positions it as a significant contributor to international medical advancements.
The approval process for clinical trials in Brazil is intricate and can be prolonged, requiring meticulous preparation from sponsors and CROs. Challenges such as bureaucratic hurdles, recruitment difficulties, and regional disparities necessitate strategic collaborations with local CROs, which can enhance compliance and streamline the approval process. Local expertise is indispensable in navigating the regulatory landscape, ensuring that trials are conducted efficiently and ethically.
Moreover, the emphasis on quality management, transparency, and robust record-keeping is vital for maintaining trial integrity and reliability. By leveraging advanced data management systems and ensuring thorough documentation, Brazil can uphold high standards in clinical research. The selection of an appropriate CRO is paramount, as their experience and understanding of local regulations can significantly impact trial success.
In conclusion, Brazil's commitment to maintaining a rigorous regulatory framework for clinical trials not only safeguards participant rights but also enhances the overall quality and efficiency of clinical research. By fostering collaboration, adhering to ethical standards, and embracing innovative strategies, Brazil can continue to advance medical knowledge and improve patient outcomes on a global scale.




