Introduction
The FDA 510(k) submission process plays a crucial role in ensuring the safety and effectiveness of medical devices entering the market. This article provides a comprehensive overview of the different types of 510(k) submissions, the process of finding a predicate device, the content of a traditional submission, and best practices for preparing and submitting a 510(k). It also explores the submission process and review timeline, acceptance and substantive review, common challenges, and best practices.
Additionally, it delves into user fees and considerations for small businesses. By understanding the nuances of the 510(k) process and following best practices, medical device manufacturers can navigate the submission pathway with confidence and optimize their chances of a successful FDA review.
Types of 510(k) Submissions
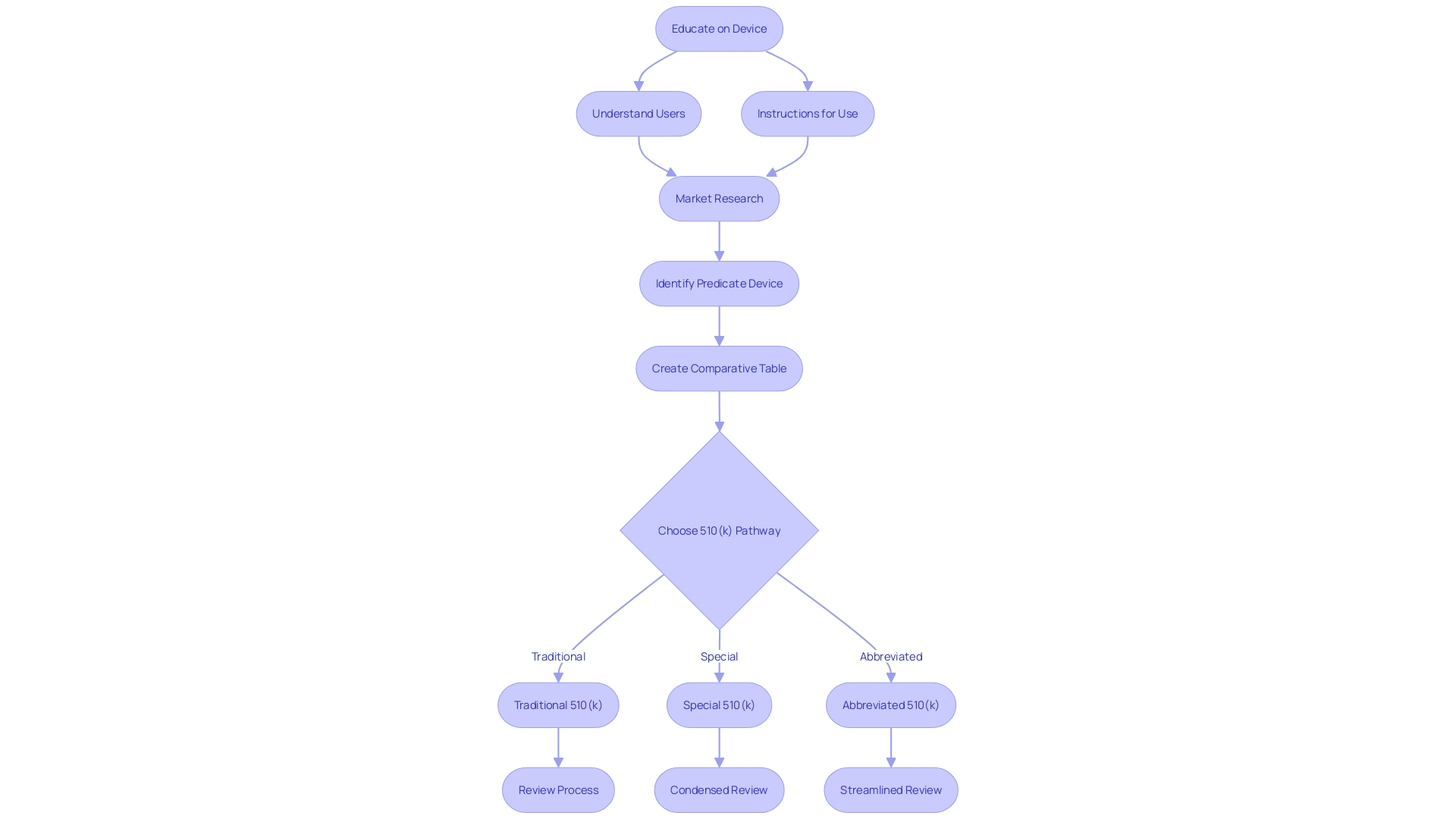
Understanding the nuances of FDA 510(k) submissions is of paramount importance for the successful clearance of medical devices. There are three distinct pathways to consider: the Traditional, Special, and Abbreviated 510(k). The Traditional 510(k) is the standard route for most submissions and requires a comprehensive set of data to demonstrate that your device is substantially equivalent to a legally marketed predicate.
The Special 510(k) allows for a potentially quicker review if the device in question involves modifications to one's own cleared device and these changes do not affect the safety and effectiveness. Lastly, the Abbreviated 510(k) leverages guidance documents, special controls, and FDA-recognized consensus standards to streamline the review process.
When preparing for submission, one must take a deep dive into understanding the intended use and technological characteristics of the subject device, and compare it against potential predicates. This involves meticulous research into clinical studies, competitor analysis, and regulatory precedents. The FDA's guidance documents and the 510(k) database serve as crucial resources in this process.
For instance, the Summaries of Safety and Effectiveness Data (SSED) available in the database provide invaluable insights into the FDA's decision-making for past submissions, enabling a more targeted approach for new applications.
Recent FDA draft guidance on minor labeling changes for nonprescription drugs and the eCFR's user-friendly presentation of documents reflect the Agency's ongoing commitment to clarity and accessibility, principles equally applicable to the 510(k) process. As such, it's essential to stay apprised of changes and updates to FDA policies and procedures to ensure compliance and optimize the chance for a favorable review.
In light of the documentary 'The Bleeding Edge,' which highlighted concerns about the 510(k) process, it is clear that thorough preparation and a robust understanding of the submission requirements are critical. Notably, the film underscored the fact that not all devices require clinical trials and brought to light instances of patient harm resulting from cleared devices. This underscores the gravity of the submission process and the responsibility on applicants to ensure the safety and effectiveness of their medical devices, beyond meeting regulatory benchmarks.
Finding a Predicate Device
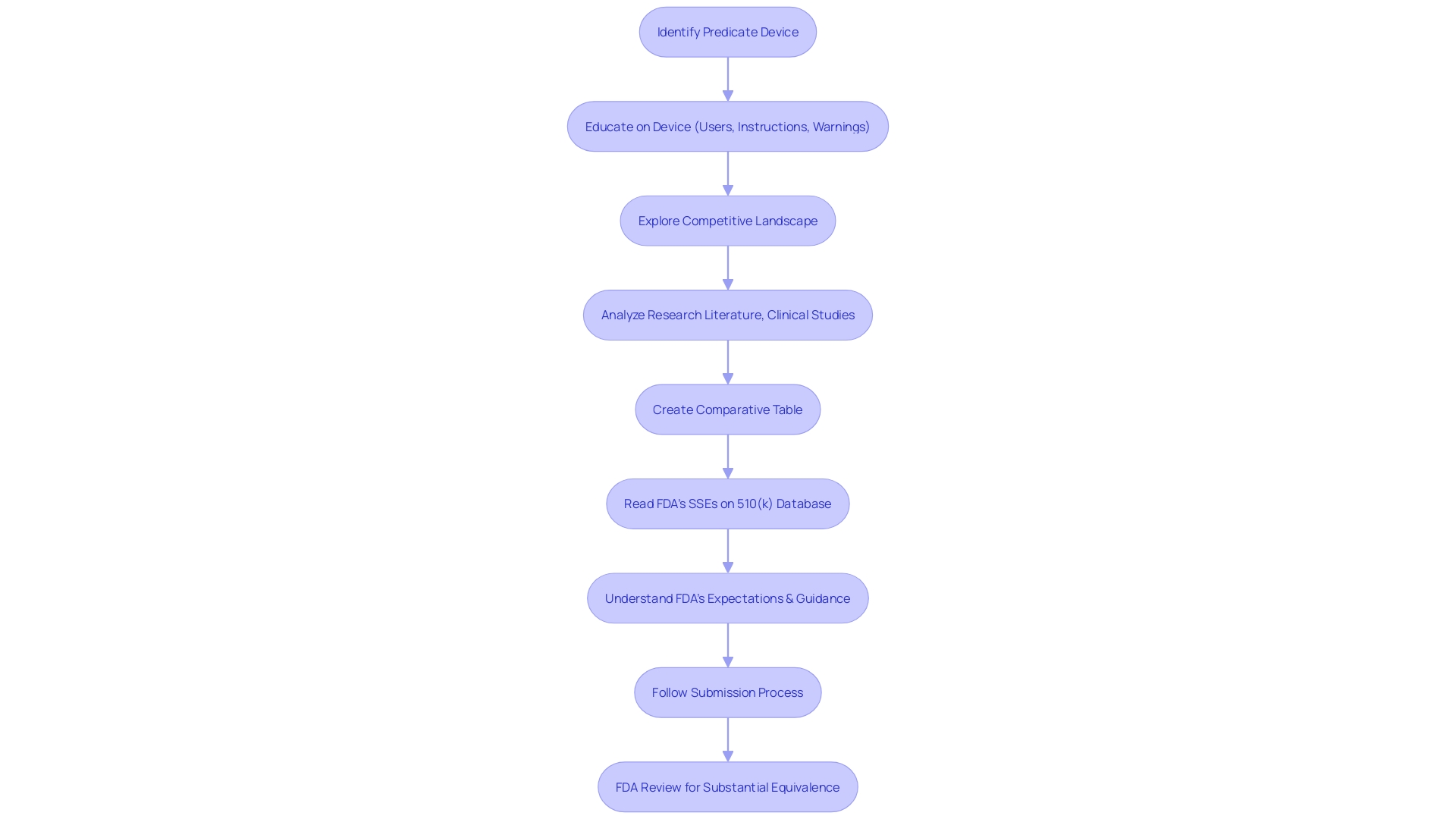
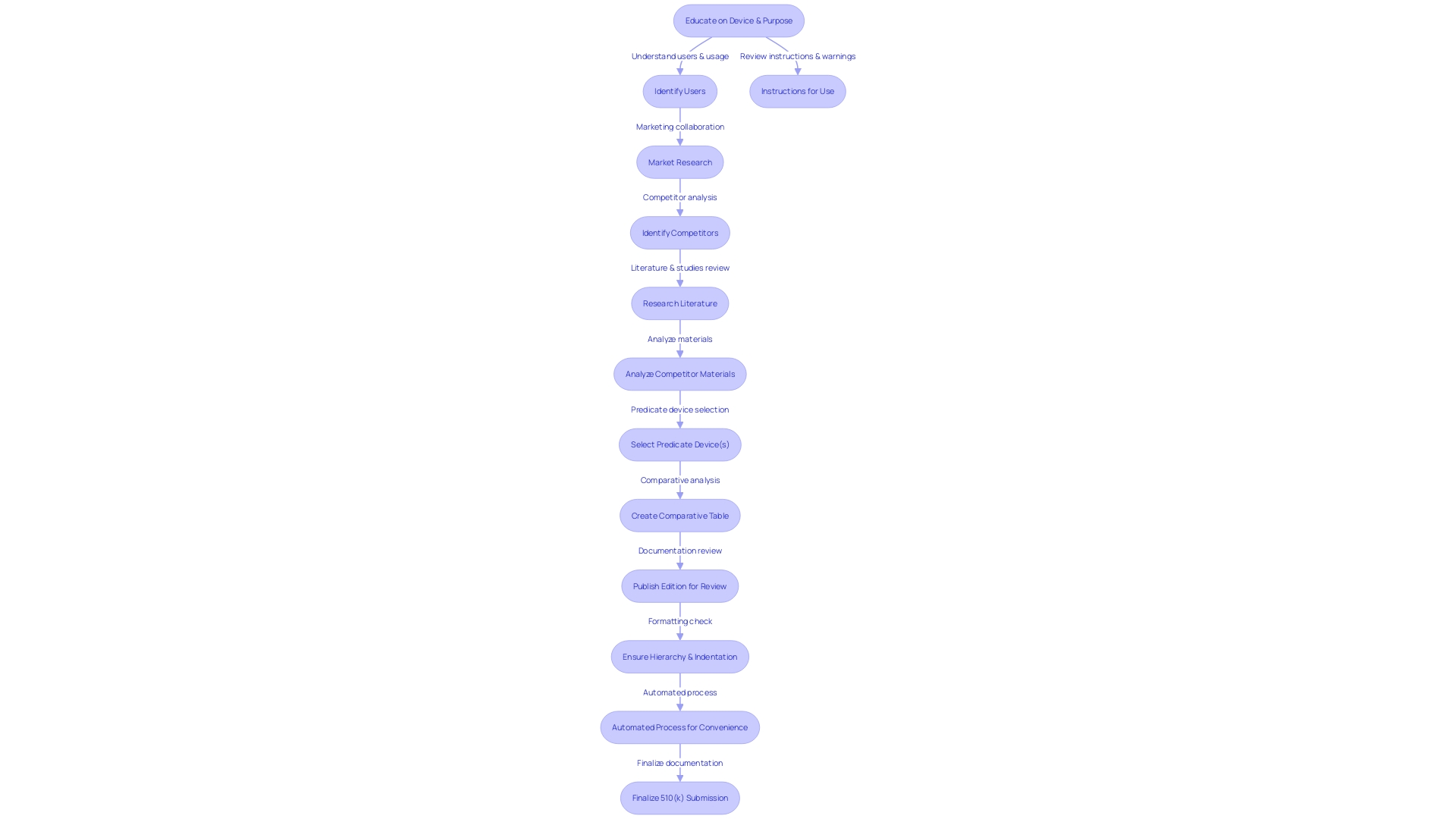
To embark on the 510(k) submission journey, the foundational step involves identifying a predicate device that is legally marketed within the United States. This predicate forms the benchmark for demonstrating that your new medical device is substantially equivalent in terms of safety and effectiveness. The process starts with an in-depth education on the device in question, including its intended users—be they clinicians, physicians, dentists, or patients—and the device's instructions for use, which encompass warnings and cautions.
Armed with knowledge, you then delve into the competitive landscape, aided by Marketing, to pinpoint competitor devices that serve as potential predicates. This involves sifting through a plethora of content such as research literature, clinical studies, and marketing materials to draw parallels between your device and existing ones. A comparative table can be an invaluable tool at this stage, listing side-by-side the intended use and technological characteristics of your device against those of the predicate.
The FDA's 510(k) database is a pivotal resource where you can access Summaries of Safety and Effectiveness (SSEs) of potential predicate devices. These summaries offer a clear perspective on the similarities and differences you need to consider. Remember, the comments you submit as part of this regulatory process become public record, so it is crucial to exclude confidential information unless it is submitted via a written/paper submission as per FDA guidelines.
It's important to note that not all devices require clinical trials for FDA clearance, as revealed in the 2018 documentary 'The Bleeding Edge.' The 510(k) clearance pathway allows for a more expedited review if a device is deemed substantially equivalent to an already legally marketed device. However, this process has been scrutinized due to instances of patient injuries linked to fast-tracked devices.
In summary, identifying the right predicate device is a process that demands thorough research and a strategic approach to demonstrate substantial equivalence to the FDA. Through diligent preparation and careful consideration of the regulatory landscape, you can establish a convincing case for your medical device's entry into the market.
Content of a Traditional 510(k) Submission
To navigate the intricacies of a Traditional 510(k) submission, a thorough understanding of the device in question is essential. It involves meticulously detailing the device's description, indications for use, and performance data, alongside demonstrating biocompatibility and appropriate labeling. These elements collectively form the backbone of the documentation necessary to affirm the safety and effectiveness of the device.
The Food and Drug Administration (FDA), a pivotal entity within the U.S. Department of Health and Human Services, oversees the assurance of medical device safety and effectiveness. A successful 510(k) submission results in the FDA's clearance that your medical device is safe and effective for its intended use. In light of this, it is imperative to examine relevant predicate devices—those with similar intended use and technological characteristics.
Experts like Etienne Nichols, a seasoned Medical Device Guru and Mechanical Engineer, underscore the importance of deep diving into the product landscape. This includes understanding the users, such as clinicians and patients, and thoroughly reviewing the instructions for use, along with warnings and cautions. Additionally, collaboration with marketing teams can provide insights into competitor devices, aiding in the creation of a comparative table—a crucial step in highlighting the similarities and differences of your device relative to existing predicates.
Furthermore, in some cases where a novel medical device presents itself without a legally marketed predicate, the De Novo classification process stands as a regulatory pathway. It provides a means to classify such medical devices into class I or class II, establishing a new regulatory category and setting the device as the first predicate for future submissions.
The FDA's commitment to public health extends to comprehensive regulations, as seen in their recent final rule on direct-to-consumer prescription drug advertisements. It mandates that major statements regarding side effects and contraindications be presented in a clear, conspicuous, and neutral manner. This exemplifies the level of detail and consumer-friendly clarity that should be reflected in your 510(k) submission's documentation.
In sum, the preparation of a Traditional 510(k) submission is a meticulous process that requires a deep understanding of the device, its competitive landscape, and stringent adherence to regulatory requirements. With a comprehensive and informed approach, the chances of a successful FDA review are significantly enhanced.
Preparing and Submitting a 510(k)
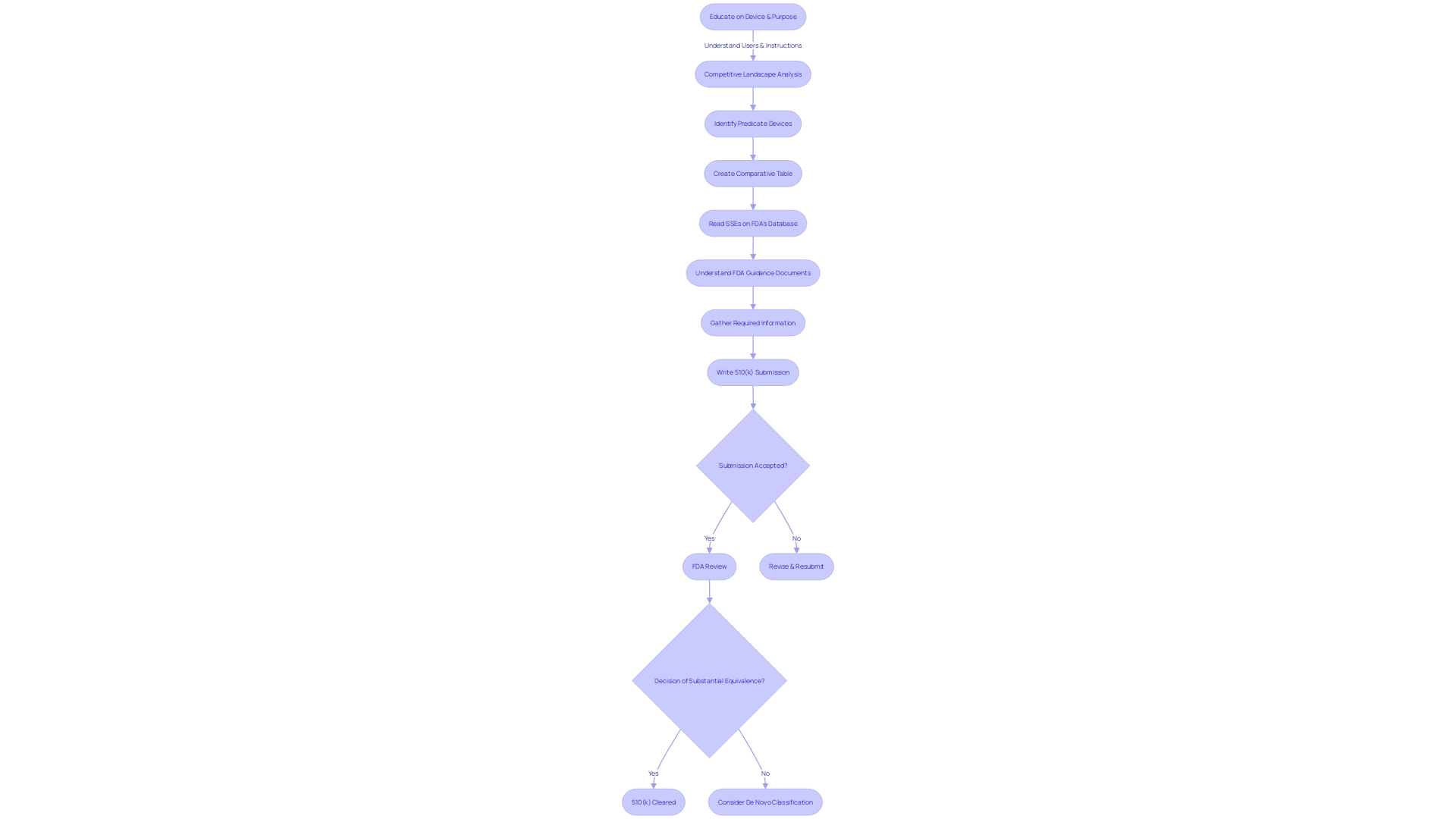
Preparing a comprehensive 510(k) submission requires a meticulous approach to compile the necessary documentation that meets the FDA's rigorous standards. The first step is to immerse yourself in understanding the device, its users, and its competitive landscape. With a focus on clinicians, physicians, dentists, and patients, it's critical to grasp the nuances of the device's usage, including any warnings and precautions.
In collaboration with your marketing team, analyze the competition by examining research literature, clinical studies, and promotional materials. This will help you identify a predicate device with the same intended use and technological characteristics, which is an integral part of the submission. Creating a comparative table to document these similarities and differences is a valuable tool in this process.
Furthermore, it is essential to thoroughly review the Summaries of Safety and Effectiveness (SSEs) available on the FDA's 510(k) database. This will provide insight into the agency's evaluation criteria and assist you in ensuring your device demonstrates substantial equivalence.
Keeping in mind the importance of confidentiality, any public comments or submissions should be carefully reviewed to exclude sensitive information such as medical data or personal identifiers. The FDA's guidelines on electronic submissions offer detailed instructions on safeguarding private information.
The challenge lies in gathering and organizing the substantial amount of information required within the given timeframe, ensuring that it aligns with the FDA's expectations for a successful determination of substantial equivalence. Articulating your methods and conclusions clearly, as recommended by experts, will facilitate a smoother review process. Additionally, conducting a thorough literature review prior to writing your submission will not only showcase your field expertise but also allow you to position your research within the broader context, enhancing the strength of your submission.
Finally, while gathering and formatting the documentation, consider the overall demands on the courts and regulatory bodies, as they handle a constant flux of cases and submissions. An awareness of these demands can inform a strategic approach to your 510(k) preparation, ultimately contributing to a more efficient and effective submission process.
Submission Process and Review Timeline
Navigating the intricacies of FDA 510(k) submissions is a strategic process requiring a comprehensive understanding of the regulatory landscape. The De Novo pathway, established to classify novel devices, has evolved since its inception in 1997, allowing sponsors to bypass a 510(k) when no predicate exists. As of 2012, the FDA targets a review time of 120 days for De Novo requests, streamlining the journey for low to moderate risk devices.
Once granted, a De Novo submission yields a decision summary and classification order, which are public documents that guide subsequent 510(k) submissions by delineating the special controls and evidence required to demonstrate substantial equivalence.
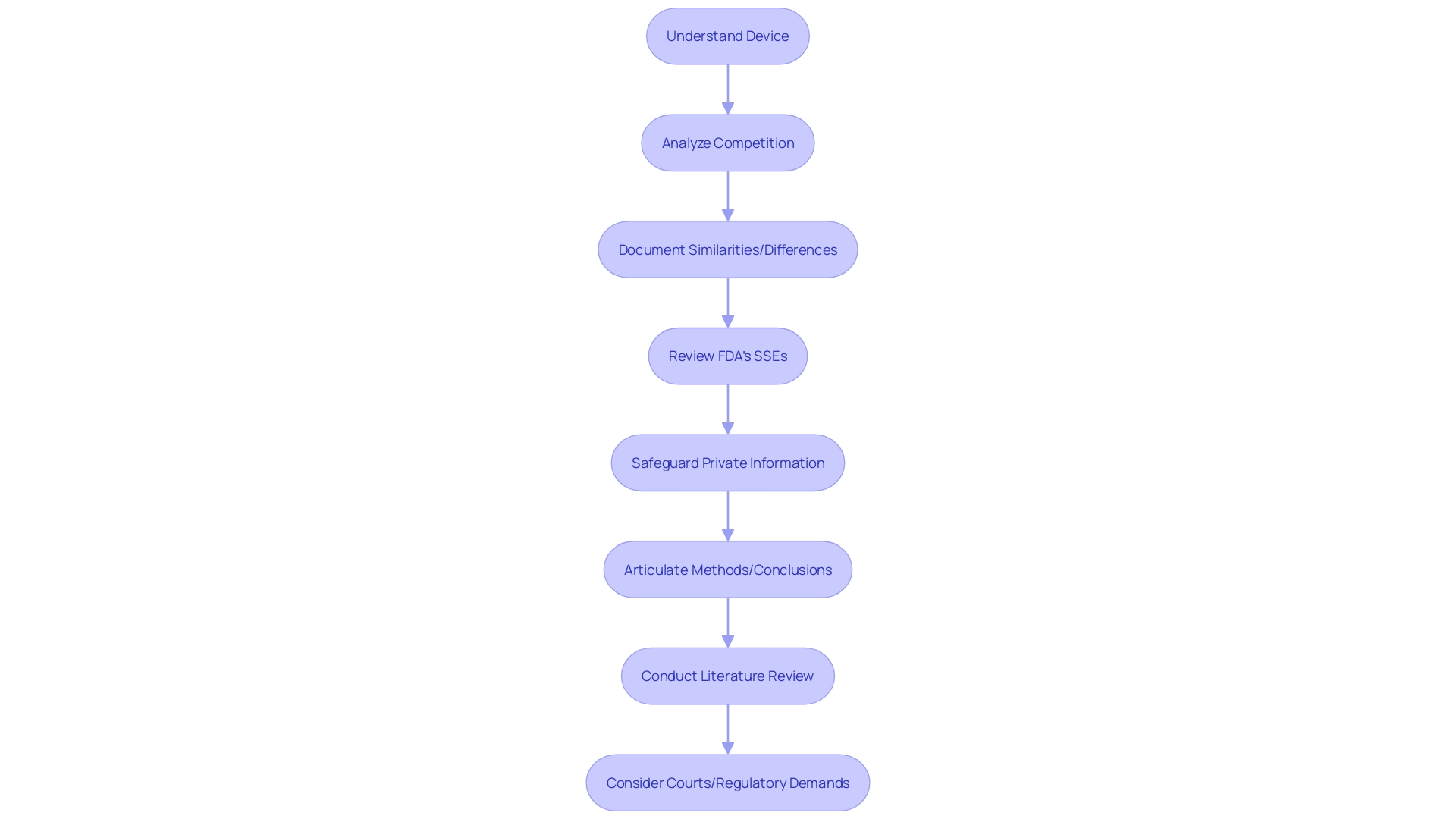
For a successful FDA review, it is imperative to thoroughly educate oneself on the medical device in question. This involves understanding the device's users, usage instructions, and the competitive landscape, along with identifying potential predicate devices. A comparative table detailing similarities and differences between the subject device and its predicate can greatly facilitate the process.
In light of the FDA's responsibility to ensure the safety, effectiveness, and security of medical devices, the submission process demands careful attention to detail. Submissions and comments should be made with the awareness that they become part of the public record. Confidential information must be judiciously protected to avoid unintended disclosure.
With medical devices segmented into three risk-based classes, each with distinct regulatory controls, the depth of review varies. Class I and II devices, considered lower risk, typically undergo the 510(k) clearance process, which involves comparing the new device to an already cleared predicate. Class III devices, which sustain or support life, require more rigorous scrutiny due to their high-risk nature and significance in healthcare.
Understanding regulatory timelines is crucial for manufacturers. The FDA's review process can be lengthy, especially for high-risk Class III devices. Industry stakeholders and regulatory agencies have been working to refine regulatory pathways, a movement accelerated by the COVID-19 pandemic, to expedite approvals for devices that meet critical health needs.
Manufacturers must manage expectations realistically, focusing on launching a device that is safe and effective, without overburdening the initial launch. The initial goal is to establish a manufacturing process that can be scaled up if market demand validates the product. The overarching objective is to navigate the FDA's regulatory framework efficiently, ensuring timely communication with the agency, thereby optimizing the pathway to market for innovative medical devices.
Acceptance and Substantive Review
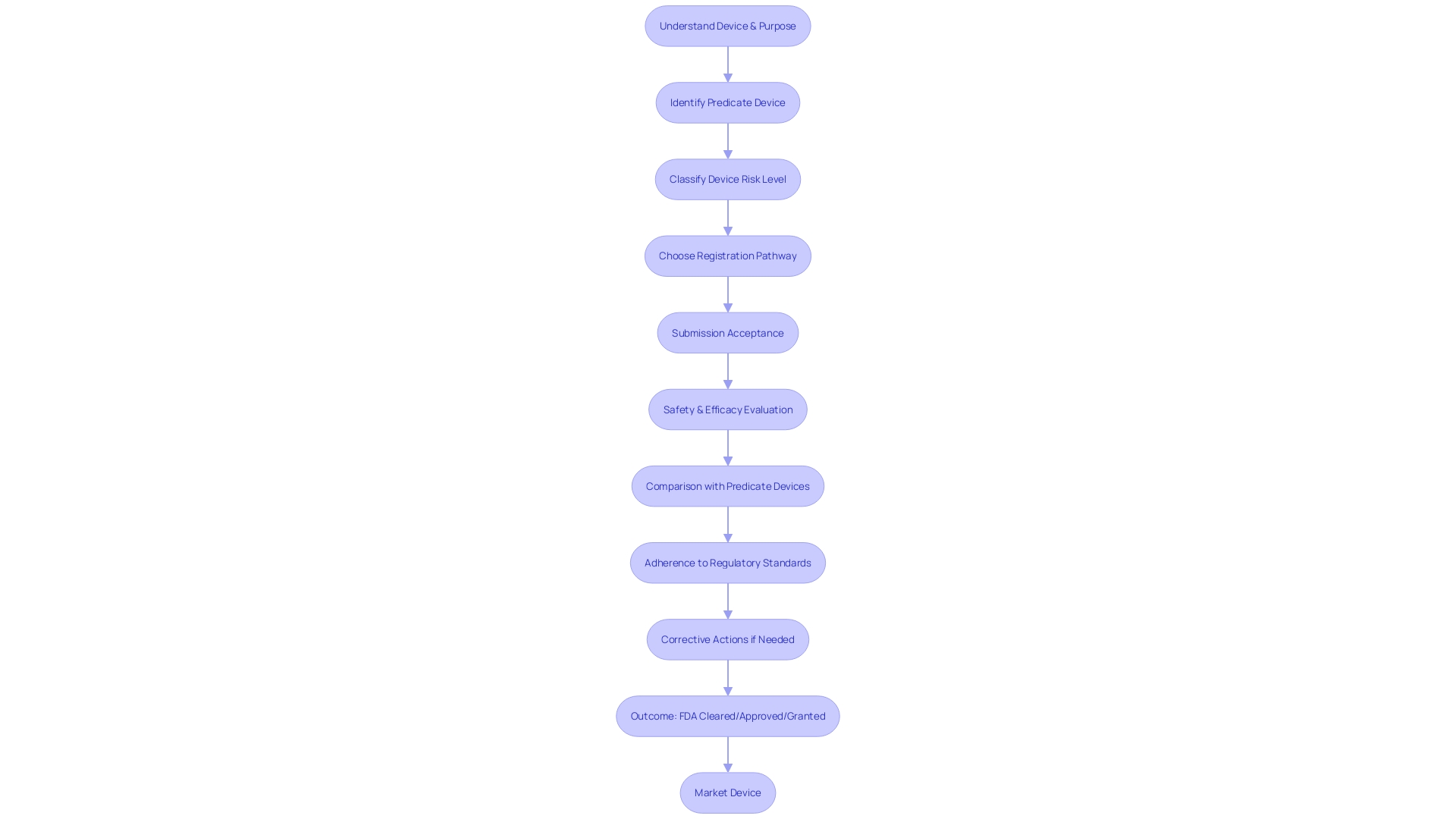
The FDA's 510(k) review process is a critical pathway for bringing medical devices to market. After a 510(k) submission has been accepted, the FDA assesses whether the device is as safe and effective as a legally marketed predicate. A thorough evaluation involves several layers of scrutiny.
Firstly, the FDA will examine the safety and efficacy of the device through a comprehensive analysis of performance data. It's imperative to present a detailed comparison with a predicate device, highlighting the similarities in intended use and technological characteristics. This comparison is vital to establishing substantial equivalence—a key deciding factor in the FDA's determination.
Moreover, the review process also extends beyond the technicalities of the device itself to include the manufacturer's adherence to regulatory standards. For instance, the FDA requires companies to establish and maintain design validation procedures, such as those stipulated under 21 CFR 820.30(g), to ensure proper risk analysis. A case where a firm's procedure for 'Health Hazard Evaluation' was found inadequately implemented serves as a cautionary tale; such oversights can delay or derail the approval process.
The FDA's commitment to public health is evident in its rigorous review process, which aims to protect consumers by ensuring medical devices meet stringent safety and effectiveness criteria before they can be marketed. In the context of recent updates, such as the FDA's clear and neutral presentation requirements for direct-to-consumer prescription drug advertisements, it's clear that transparency and consumer understanding are top priorities.
It's crucial to note that the FDA's process is dynamic, with the potential for ongoing corrective actions, like those outlined in a firm's response regarding complaint coding and quality data analysis. These actions may include retrospective reviews, procedural revisions, and staff training. However, the outcomes of these corrective measures may influence the FDA's final decision, as the agency requires concrete evidence of their effectiveness.
In summary, the FDA's review process is exhaustive and multifaceted, encompassing device analysis, comparison with predicates, and compliance with regulatory requirements. The potential outcomes range from clearance—with or without limitations—to requests for additional data. Staying informed and prepared for each step can significantly impact the success of your submission.
Common Challenges and Best Practices
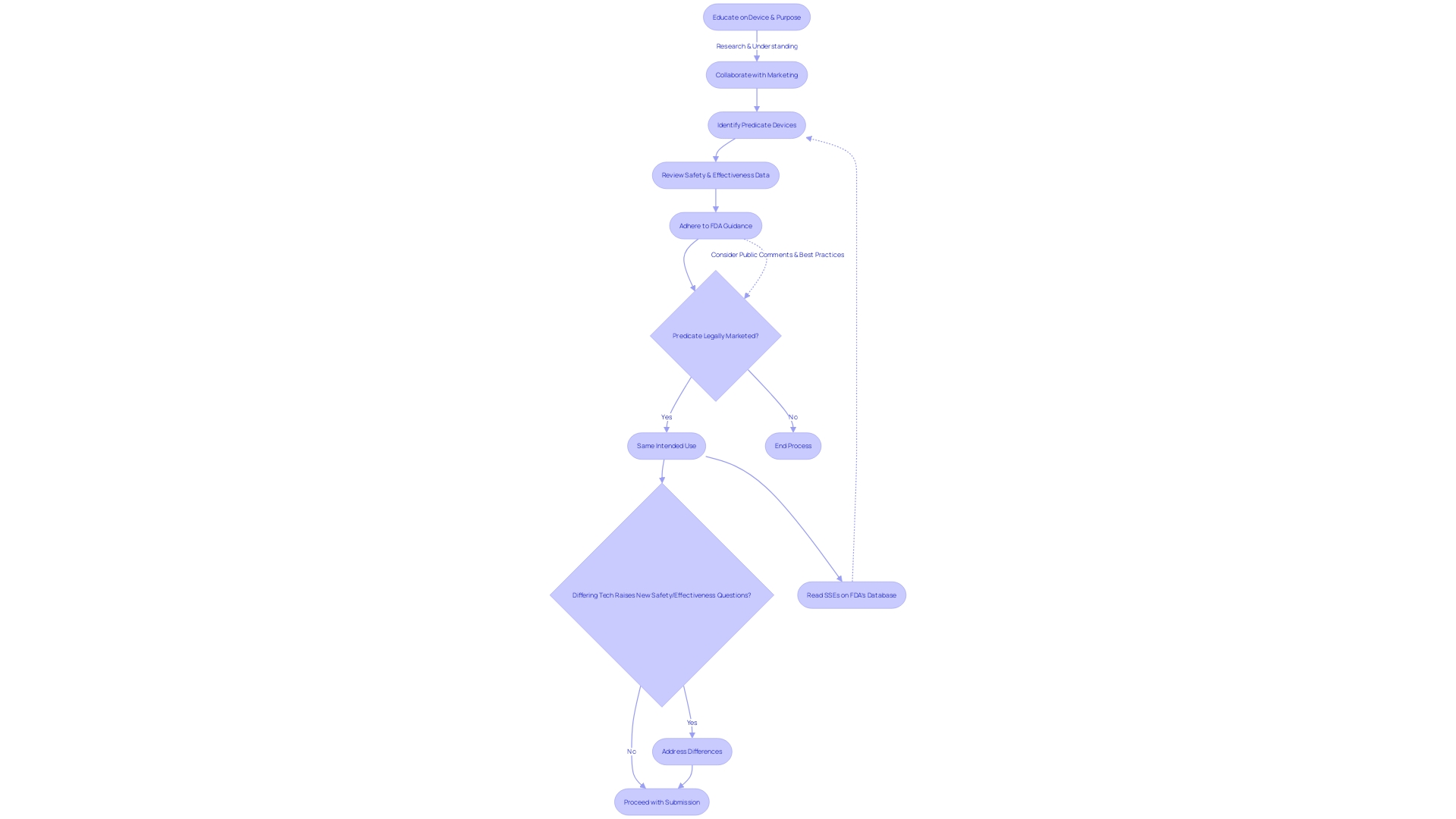
To enhance the probability of a successful FDA 510(k) submission, it is essential to engage in comprehensive research on the medical device in question, focusing on a thorough understanding of its users—ranging from clinicians and physicians to patients—and its usage instructions, inclusive of warnings and precautions. Collaboration with Marketing is crucial to gain insights into the competitive landscape and identify predicate devices that share the same intended use and technological characteristics. This process involves reviewing research literature, clinical studies, and marketing materials to construct a comparative table.
After selecting a potential predicate device, applicants should delve into the Summaries of Safety and Effectiveness Data (SSEs) available on the FDA's 510(k) database. This enables a thorough comparison of similarities and differences, ensuring that the chosen predicate justifies the claim of substantial equivalence. Moreover, the FDA's modernization efforts for the 510(k) process emphasize the importance of selecting appropriate predicate devices.
In particular, the draft guidance document released on September 7, 2023, outlines best practices for predicate selection, including verifying that the device is legally marketed and assessing whether technological differences may pose new safety and effectiveness questions.
Recent guidelines from the FDA underscore the necessity for manufacturers to carefully choose predicates, particularly avoiding those with design-related recalls or unresolved safety issues. Manufacturers are encouraged to provide detailed justifications for their choices, using flow charts if necessary. This transparency in the selection process is intended to mitigate the risks associated with predicate devices and ensure the safety and efficacy of new medical devices entering the market.
In summary, a successful 510(k) submission hinges on meticulous preparation, informed predicate device selection, and adherence to FDA guidance. By thoroughly understanding the device and its context in the market, as well as following the FDA's evolving recommendations, applicants can navigate the submission pathway with greater confidence and efficiency.
User Fees and Small Business Considerations
Understanding the intricacies of FDA 510(k) user fees is critical for medical device companies approaching the submission process. User fees differ based on the submission category and the financial scale of the submitting entity. For small businesses, defined by the FDA as entities with $100 million or less in gross receipts, these fees can be particularly impactful.
In fact, businesses with gross receipts of $1 million or less are eligible for a registration fee waiver, an opportunity that is highly beneficial yet narrowly applicable due to stringent criteria, such as a bankruptcy filing requirement.
The FDA's 510(k) database is a valuable resource for comprehensive comparative analysis, where you can access Summaries of Safety and Effectiveness to discern the nuances between your device and similar market offerings. This research is a vital preparatory step that informs not only your submission but also the financial planning regarding user fees. It is important to bear in mind that any confidential information should be carefully handled to avoid unintentional disclosure during the submission process.
The FDA's approach to user fees, including the criteria for reduced fees and waivers, reflects a balance between facilitating market entry for smaller innovators and maintaining a rigorous oversight process. With the average fee for certain submissions, such as Color Additive Petitions, ranging from $1,600 to $3,000, companies must budget accordingly and stay abreast of the latest FDA policies and thresholds to navigate the 510(k) process successfully.
Conclusion
In conclusion, the FDA 510(k) submission process is a complex journey that requires thorough research, meticulous preparation, and adherence to regulatory standards. This article has provided a comprehensive overview of the different types of 510(k) submissions, the process of finding a predicate device, the content of a traditional submission, and best practices for preparing and submitting a 510(k).
Understanding the intended use and technological characteristics of the device, comparing it to potential predicates, and utilizing resources like the FDA's guidance documents and the 510(k) database are crucial steps in the submission process. Thoroughly educating oneself on the device, its users, and the competitive landscape is essential for a successful submission.
Preparing a comprehensive 510(k) submission involves attention to detail, clear articulation of methods and conclusions, and adherence to regulatory guidelines. It is important to gather and organize the necessary documentation, conduct a thorough literature review, and consider the demands on regulatory bodies during the submission process.
Navigating the submission process and review timeline requires a comprehensive understanding of the regulatory landscape and the different risk-based classes of medical devices. Managing expectations, focusing on safety and effectiveness, and maintaining effective communication with the FDA are key factors in optimizing the pathway to market.
The acceptance and substantive review of a 510(k) submission involve a thorough evaluation of the device's safety, efficacy, and compliance with regulatory standards. Manufacturers must be prepared for ongoing corrective actions and provide concrete evidence of their effectiveness.
By following best practices, conducting thorough research, and staying informed about FDA policies and guidelines, medical device manufacturers can navigate the 510(k) submission pathway with confidence and enhance their chances of a successful FDA review. With careful preparation and adherence to regulatory requirements, medical device manufacturers can optimize their chances of obtaining FDA clearance and bringing safe and effective devices to market.




