Introduction
Embarking on clinical trials for a new drug or biological product demands rigorous regulatory compliance to ensure the safety and efficacy of the investigational product. One pivotal step in this process is the submission of an Investigational New Drug (IND) application to the FDA. This application is mandatory before initiating any human clinical studies and is influenced by numerous factors including the nature of the product, study design, and intended use.
Historically, the FDA has emphasized the importance of demographic diversity in clinical trials, addressing the underrepresentation of women and minority groups to ensure more inclusive and applicable trial results. Recent advancements in FDA guidelines, such as the use of real-world evidence and the implementation of electronic submission portals, aim to streamline the regulatory process, expediting the development of critical medical products. The cost-intensive and time-consuming nature of drug development further highlights the significance of mechanisms like the Accelerated Approval program, which facilitates quicker market entry while maintaining stringent safety standards.
Understanding when an IND is required is crucial for sponsors aiming to navigate the complex landscape of clinical trials successfully.
When is an IND Required?
An Investigational New Drug (IND) application is essential when sponsors plan to conduct trials involving a new drug or biological product that has not yet received marketing approval. This step is mandated to ensure the safety and efficacy of the investigational product before it is tested in humans. The Hindmost be submitted to the FDA before the initiation of any research trials. The necessity for an IND is affected by numerous elements, such as the characteristics of the investigational product, the structure of the study, and the intended application within the trial.
The FDA's evolving guidance reflects a broader understanding of demographic diversity in medical trials. Historically, women and minority groups were underrepresented in medical research, which often focused on white men. This lack of diversity raised concerns about the applicability of trial results to the entire population. Notably, the 1988 Guideline for the Format and Content of the Clinical and Statistical Sections of New Drug Applications emphasized the importance of including demographic data analyses in NDA applications. Additionally, the 1993 Guideline for the Study and Evaluation of Gender Differences in the Assessment of Drugs mandated the inclusion of women in all phases of drug development.
The FDA's efforts to enhance regulatory decision-making are ongoing, as seen in their recent draft guidance on the use of real-world evidence for medical devices. This guidance aims to clarify how real-world data can be utilized to support regulatory submissions, ultimately expediting the development process for critical medical products. Additionally, the introduction of new electronic submission portals is part of the FDA's strategy to streamline the submission and tracking processes, improving user experience and transparency.
Statistical information shows that the drug development process is both expensive and requires a significant amount of time, with many candidates not advancing through trial phases. This underscores the importance of mechanisms like the Accelerated Approval (AA) program, which allows for earlier market entry by utilizing surrogate endpoints instead of waiting for clinical benefits. The AA program offers a significant advantage by reducing the time and cost associated with bringing new drugs to market while providing the same statutory exclusivities as traditionally approved drugs.
Key Components of an IND Application
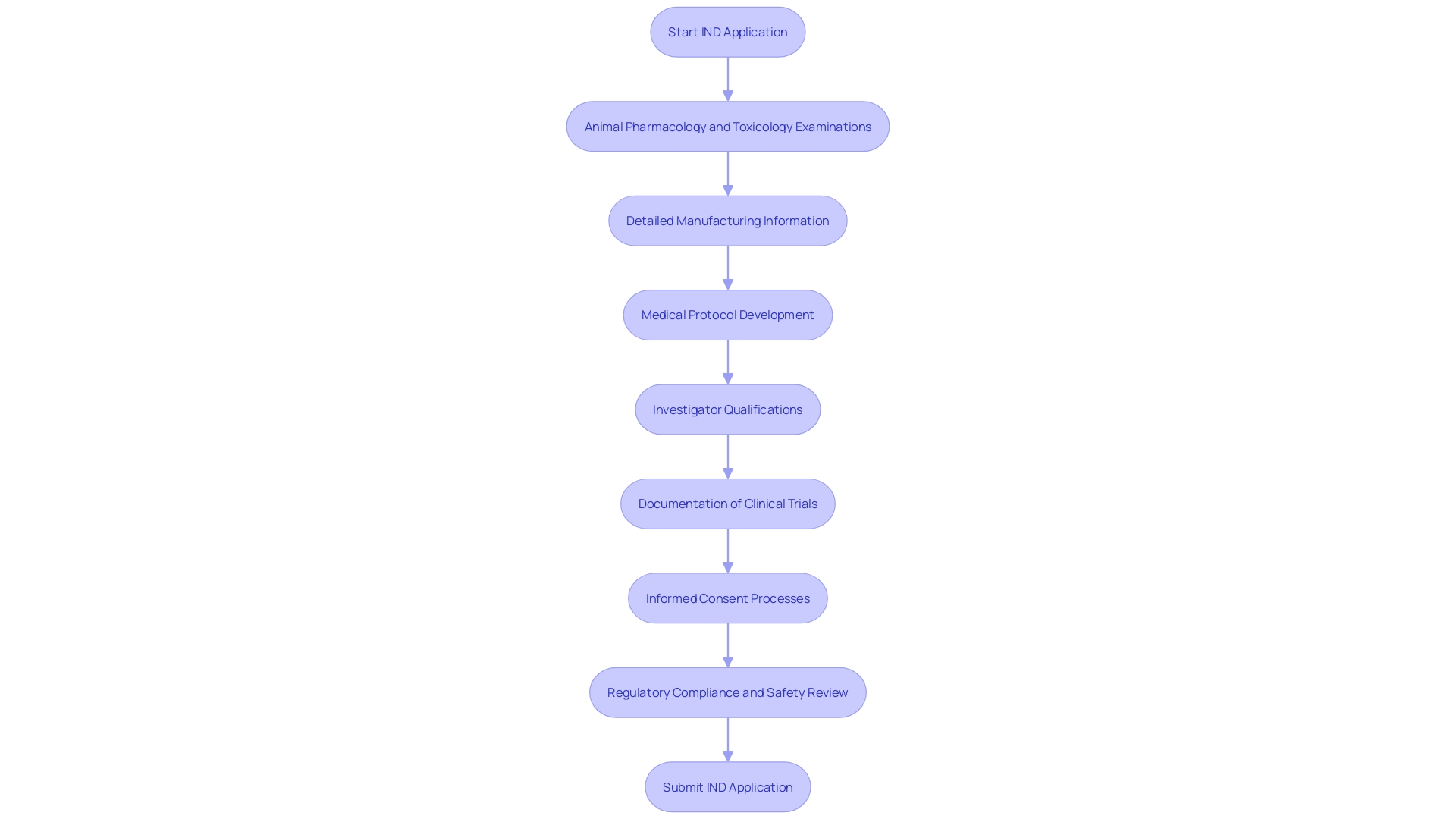
An IND application comprises several crucial components to ensure the investigational product's safety and efficacy before human trials. Firstly, animal pharmacology and toxicology examinations are conducted to evaluate the drug's safety profile. 'These investigations are essential, particularly when the drug is designed for use in food-producing animals, as complete records of the examination must be maintained for two years post-shipment or delivery.'.
Secondly, detailed manufacturing information outlines how the drug is produced, ensuring consistency and compliance with regulatory standards. This encompasses non medical laboratory examinations and trial information not previously disclosed, which are essential for preserving the integrity of the investigational process.
The medical protocol is another essential element, detailing the study design, objectives, and methodology. This protocol must tackle safety issues in the development of medical products, as emphasized in a researcher training course aimed at participants in the trial enterprise.
Investigator's information, including their qualifications and experience, is also required. This ensures that the personnel involved are adequately trained and capable of managing the investigational product's complexities. Reports of clinical trials, adverse drug experiences, and product defects must be meticulously documented and submitted to the FDA as specified.
Finally, informed consent documents for research participants are mandatory. These documents protect participants' rights and ensure they are fully informed about the nature of the research and potential risks. Each of these components plays a pivotal role in facilitating a comprehensive review process, ultimately promoting the development of safe and effective medical products.
IRB Review Process for IND Studies
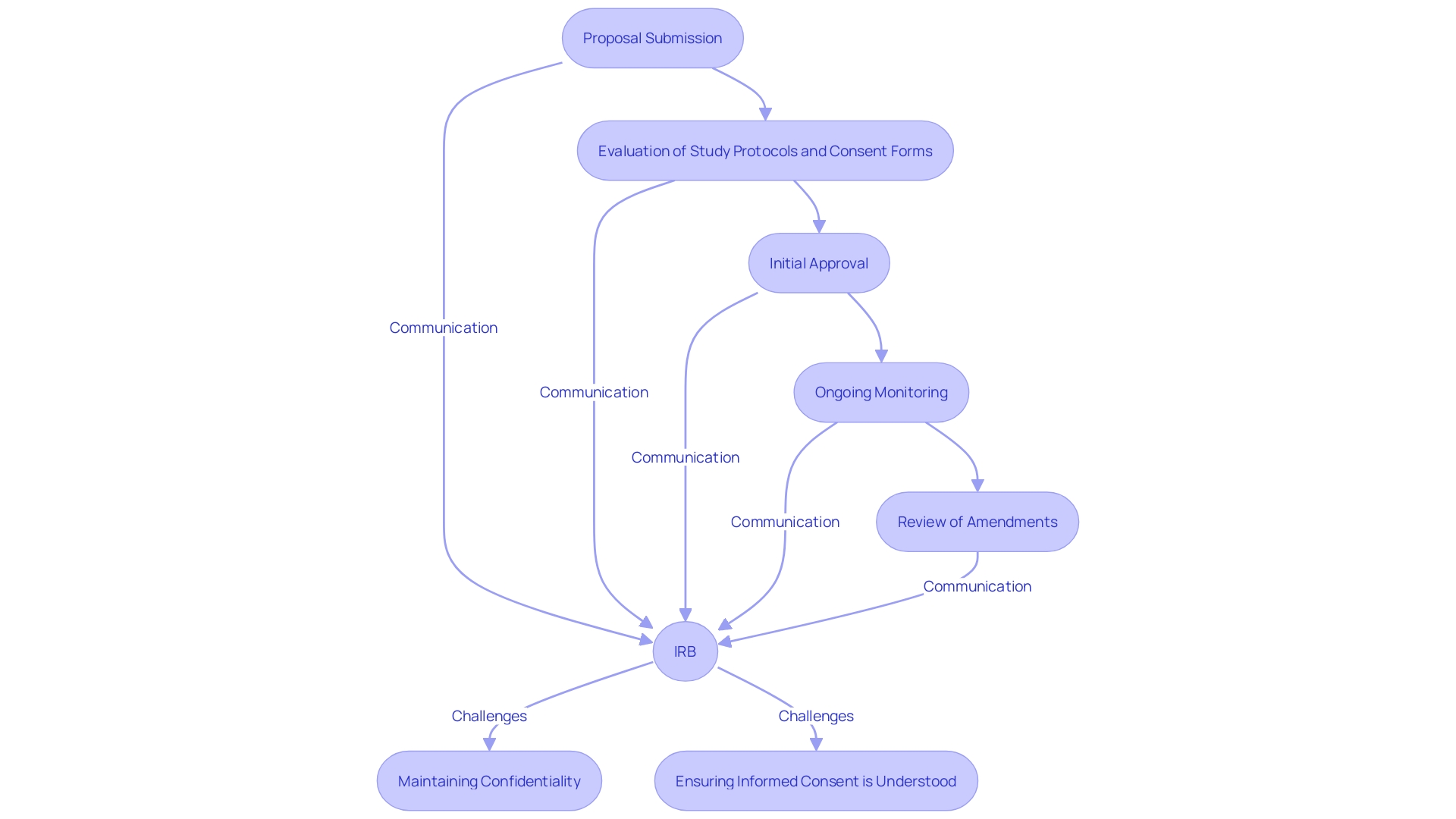
The Institutional Review Board (IRB) review procedure for Investigational New Drug (IND) research is essential in safeguarding the rights and welfare of participants. The IRB meticulously evaluates the study protocol, informed consent forms, and any potential risks associated with the study. This evaluation process encompasses initial approval, ongoing monitoring, and the review of any amendments to ensure compliance with ethical standards.
Prompt communication between the IRB and the study team is essential to address any concerns. As noted, "All human studies are reviewed by an IRB — investigators submit proposals detailing the purpose of the investigation, the procedures, the risks and benefits, consent forms, and more.". The committee decides whether or not the proposal is acceptable and may ask for revisions, which can take weeks or months depending on how often a given IRB convenes.”
Additionally, the U.S. Department of Health and Human Services is working to harmonize human subject protection regulations with the Common Rule, aiming to make clinical research more efficient while safeguarding participants. This harmonization effort includes a final rule by the FDA that allows exceptions to obtaining informed consent when studies pose minimal risk, provided there are appropriate safeguards to protect participant rights, safety, and welfare.
IRBs must also navigate challenges such as maintaining the confidentiality of peer review materials and ensuring that informed consent is presented in a way that facilitates understanding. Miscommunication and ethical breaches can occur if IRBs are not designed to approve studies on behalf of the communities being examined. Instead, incorporating community members into ethics committees, despite barriers, can help address these issues.
By supporting a wide variety of experimental instruments and promoting cooperation, especially for multisite trials, institutions can further improve the integrity and efficiency of the IRB process. This commitment to rigorous and reliable data ultimately strengthens the trust in clinical research and promotes advancements in medical knowledge.
Best Practices for Navigating IND and IRB Requirements
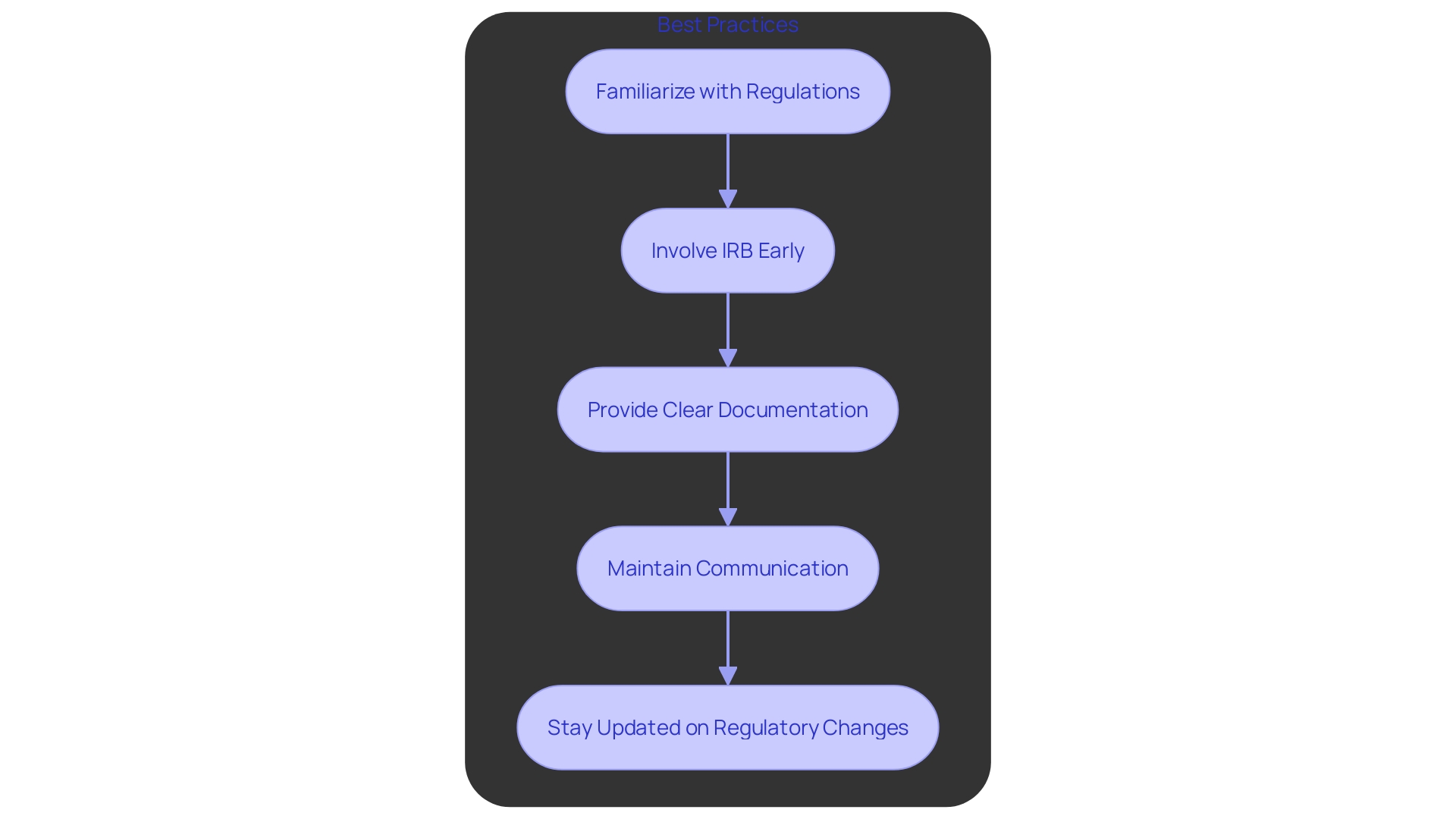
To effectively navigate IND and IRB requirements, sponsors and researchers should adopt several best practices. First, familiarize themselves with FDA regulations and IRB policies to ensure compliance and avoid potential pitfalls. Involving the IRB early in the research design process is essential to address any concerns swiftly. Providing comprehensive and clear documentation to the IRB facilitates a smoother review process, which is critical for timely approvals. Maintaining open lines of communication with both the IRB and the FDA throughout the study lifecycle ensures that any arising issues are managed efficiently.
Staying updated on regulatory changes is equally important, as new guidelines can significantly impact the IND application or IRB review. For example, the FDA's alignment of human subject protection regulations with the HHS Common Rule seeks to enhance studies while protecting participant rights. The implementation of this harmonization is expected to result in cost savings, with an estimated net present value of approximately $1.7 million over ten years.
Additionally, the FDA's final rule on minimal risk studies and consent waivers provides an exception from obtaining informed consent under specific conditions, further streamlining the process without compromising participant safety. These regulatory advancements underscore the importance of staying informed and adapting to new guidelines to enhance the efficiency and effectiveness of clinical research.
Conclusion
The Investigational New Drug (IND) application is essential for sponsors initiating clinical trials of new drugs or biological products, serving as a safeguard for safety and efficacy before human testing. The requirement for an IND is influenced by various factors, including the product's characteristics and study design. The FDA's focus on demographic diversity in clinical trials addresses historical underrepresentation, enhancing the applicability of trial results across different populations.
Key components of an IND application include animal studies, manufacturing details, clinical protocols, and informed consent documents, all designed to protect participant rights and ensure ethical development. The Institutional Review Board (IRB) is integral to this process, evaluating study protocols to uphold participant welfare.
To navigate IND and IRB requirements effectively, sponsors and researchers should engage with regulatory bodies early, provide clear documentation, and stay informed about evolving guidelines. The FDA's initiatives, such as electronic submission portals and the Accelerated Approval program, aim to streamline the approval process while upholding safety standards.
In summary, understanding the IND application and IRB review process is crucial for successful clinical research. By following regulatory requirements and best practices, the development of safe and effective medical products can be achieved more efficiently, ultimately benefiting public health and advancing medical knowledge.




