Introduction
In the intricate landscape of medical device development, effective communication with regulatory bodies is paramount. Q-Submissions, often referred to as "Q-Sub" requests, serve as a critical conduit for sponsors to engage with the FDA on the developmental trajectory of medical products. These submissions enable early-stage discussions on pivotal issues, ultimately streamlining the regulatory pathway and enhancing the likelihood of successful market access.
This article delves into the purpose and benefits of Q-Submissions, explores the various types including pre-submissions and informational meetings, and offers best practices for managing these submissions. Additionally, it provides insights into navigating FDA feedback and review processes, ensuring that sponsors can adeptly align their development strategies with regulatory expectations.
Understanding Q-Submissions: Purpose and Benefits
Q-Submissions, or "Q-Sub" requests, are essential tools allowing sponsors to engage with the FDA on the developmental trajectory of medical products. These submissions enable early-stage conversations on crucial matters that may affect the development process, ultimately simplifying the approval pathway. The main benefits consist of gaining clarity on compliance expectations, achieving alignment on study designs, and proactively addressing potential challenges. According to the FDA, early engagement with stakeholders, including regulators and payors, is crucial for the commercial success of medical devices. Improved communication via Q-Submissions guarantees that oversight, clinical research, and reimbursement strategies are created simultaneously, thus increasing the chances of successful market access and adoption.
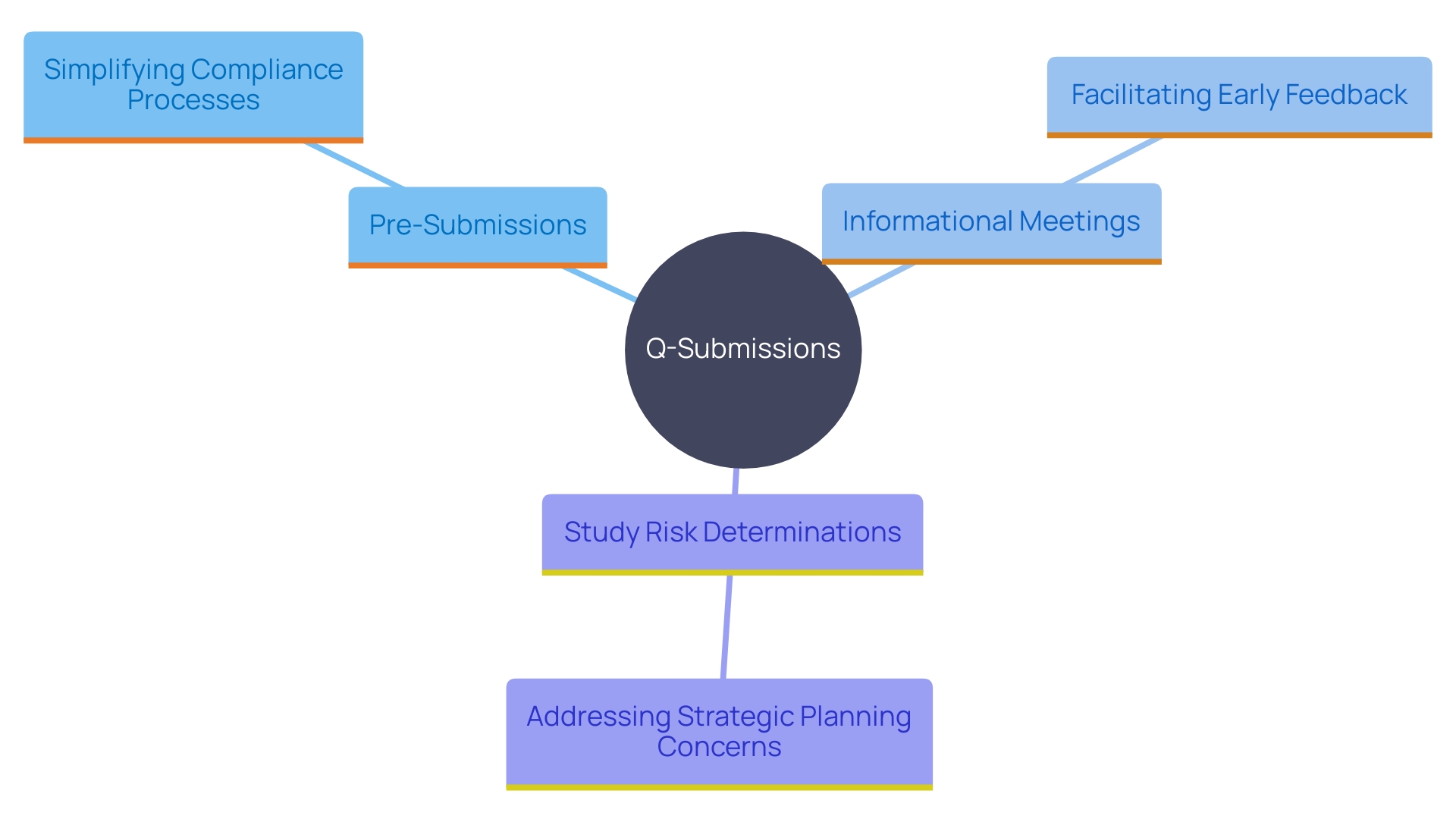
Types of Q-Submissions: Pre-Submissions, Informational Meetings, Study Risk Determinations, and More
Sponsors can engage with the FDA through various types of Q-Submissions to simplify their compliance processes. Pre-Submissions, for instance, offer a valuable opportunity to receive feedback on specific questions before submitting an Investigational New Drug (IND) or premarket application. These interactions can assist backers in clarifying requirements and addressing potential issues early in the development phase.
'Informational meetings serve as a platform for broader discussions on topics of interest, allowing for the exchange of scientific and governance-related information that may impact future submissions.'. These meetings are particularly useful for addressing overarching concerns and strategic planning.
Study risk determinations are another essential type of Q-Submission, allowing developers to evaluate the risk levels linked to their study designs. This assessment is crucial for ensuring that the benefits of a study justify any potential risks involved.
Other Q-Submission types encompass inquiries for clarification or updates on current compliance matters. According to recent guidance, the FDA has provided more detailed delineation on how to obtain feedback for different types of questions, distinguishing between informal communications and more formal Pre-Submissions.
The draft guidance document, revealed in June 2023, aims to enhance the clarity and scope of Q-Submissions, ensuring that contributors can navigate the compliance landscape more effectively. This includes improved examples and mechanisms for requesting interactions with the FDA, which are essential for advancing medical device submissions.
To remain compliant and up-to-date with changes in regulations, sponsors must adjust their protocols and product development strategies accordingly. The integration of AI and digital tools in medical devices is becoming increasingly significant, and oversight frameworks are evolving to accommodate these innovations. This proactive approach helps mitigate risks while maximizing the benefits of new medical products.
Managing Q-Submissions: Best Practices for Submission and Feedback
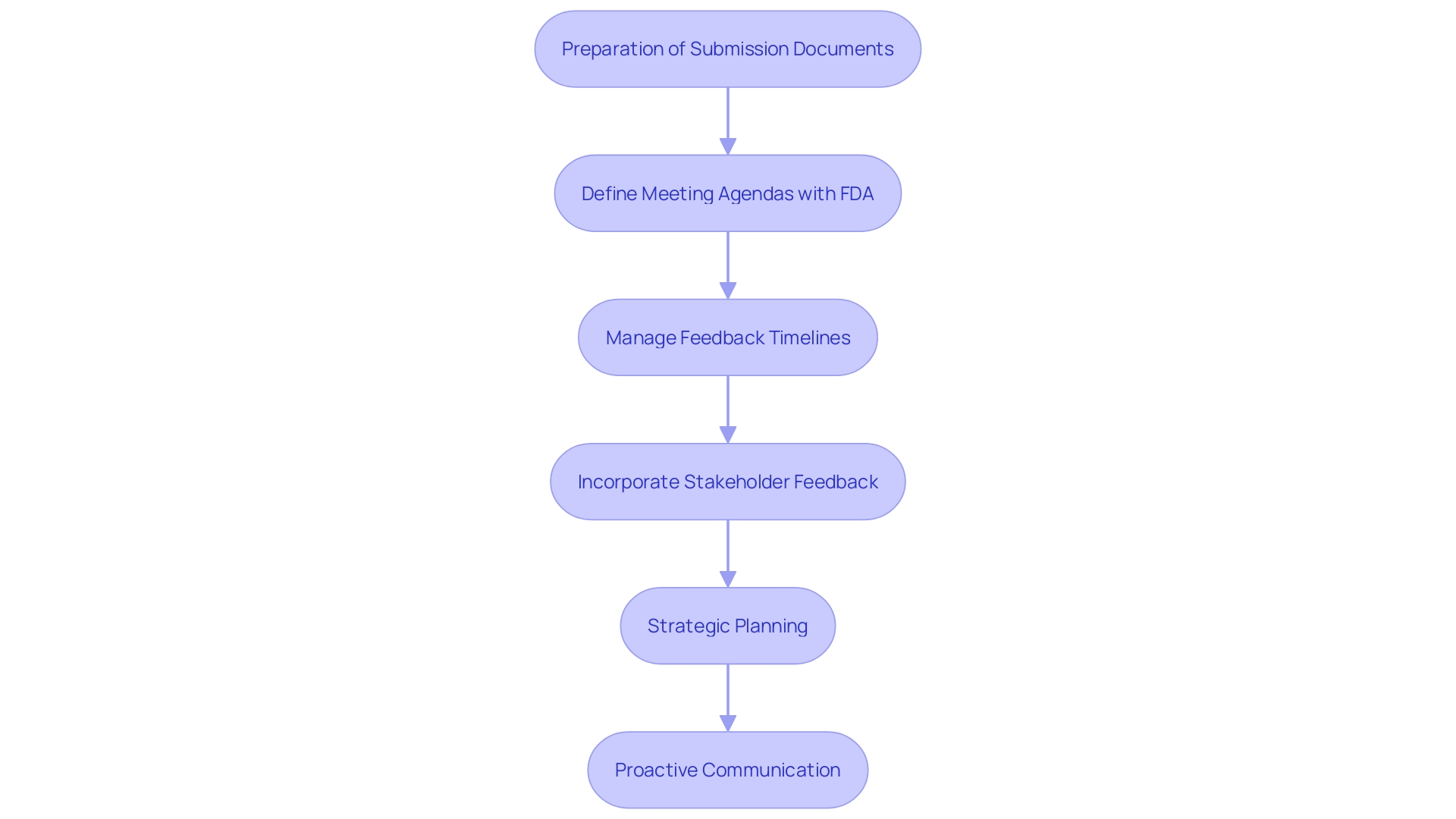
Effective management of Q-Submissions involves strategic planning and adherence to best practices, ensuring a smooth compliance pathway. Thorough preparation of submission documents and clear articulation of questions are pivotal. It's essential to define a well-structured agenda for meetings with the FDA, as highlighted in the draft guidance on the Q-Submission Program, which outlines the mechanisms for interaction with the FDA regarding medical device submissions.
Post-submission, managing feedback timelines is crucial. The FDA highlights the significance of prompt and recorded replies, which ought to be incorporated into continuous progress processes. This proactive strategy, which aligns with the FDA's aim to bridge the gap between clinical objectives and investigative data, helps mitigate misunderstandings and streamline the approval pathway.
Ellie Reynolds from the Global Medical Device Podcast underscores the importance of early and frequent interactions with the FDA, stating, "Interact with FDA early and often. It's what ultimately will make your submissions as seamless as possible." This emphasizes the need for ongoing and cooperative communication to align product development with compliance expectations.
'Incorporating feedback from oversight bodies and stakeholders, as suggested by the FDA, can provide invaluable insights and enhance program management.'. This collaborative effort ensures that clinical benefits and claims are accurately represented and supported by factual evidence, addressing the complexities inherent in regulatory compliance and product evaluations.
Navigating FDA Feedback and Review Processes
Once a Q-Submission is made, navigating FDA feedback is critical for maintaining compliance and progressing product innovation. Understanding the review process, including the timelines for responses and the nature of feedback provided, is essential. Sponsors should be prepared to engage in follow-up discussions and adapt their strategies based on the insights received. This iterative process fosters a collaborative relationship with the FDA and enhances the likelihood of successful outcomes.
During this iterative process, it is important to consider the latest FDA guidances, such as those related to 510(k) implant devices. As Mike Drues emphasized, meeting standards does not necessarily mean the product is safe and effective. Participating in thorough conversations with the FDA can clarify these expectations and assist sponsors in aligning their progress with compliance requirements.
Moreover, the FDA's Center for Devices and Radiological Health (CDRH) has been proactive in addressing health equity in medical devices. Their discussion paper on health equity underscores the importance of innovative technologies in reducing barriers and improving health outcomes across diverse populations. 'Sponsors should incorporate these considerations into their growth strategies to ensure their devices meet both regulatory and societal expectations.'.
In addition, the FDA's recent updates, including the addition of 191 AI/ML-enabled devices to the legally marketed list, reflect the agency's commitment to advancing medical technologies. Sponsors should stay informed about such developments to leverage new opportunities and ensure compliance with evolving standards.
To further support this process, the FDA has provided new guidance on clinical study protocols and interaction methods. This guidance is crucial for supporters to understand the non-clinical data requirements and effectively communicate with the FDA. By adhering to these guidelines, sponsors can streamline their submissions and improve the chances of regulatory approval.
Conclusion
The exploration of Q-Submissions highlights their vital role in the medical device development process. By facilitating early-stage discussions with the FDA, these submissions empower sponsors to clarify regulatory expectations, align study designs, and proactively address challenges. The various types of Q-Submissions, including Pre-Submissions, informational meetings, and study risk determinations, provide sponsors with essential avenues to engage with regulatory bodies and refine their developmental strategies.
Effective management of Q-Submissions is equally critical. Thorough preparation, clear communication, and strategic planning are necessary to navigate the complexities of the regulatory landscape. The importance of timely feedback and continuous interaction with the FDA cannot be overstated, as these practices foster collaboration and ensure that product development aligns with regulatory requirements.
Navigating FDA feedback and understanding the review processes are crucial for maintaining compliance and facilitating successful market access. By staying informed about the latest guidances and incorporating considerations such as health equity and technological advancements, sponsors can enhance their submissions and improve the likelihood of achieving regulatory approval. The proactive approach to Q-Submissions ultimately contributes to the successful launch of innovative medical devices, meeting both regulatory and societal needs.




