Introduction
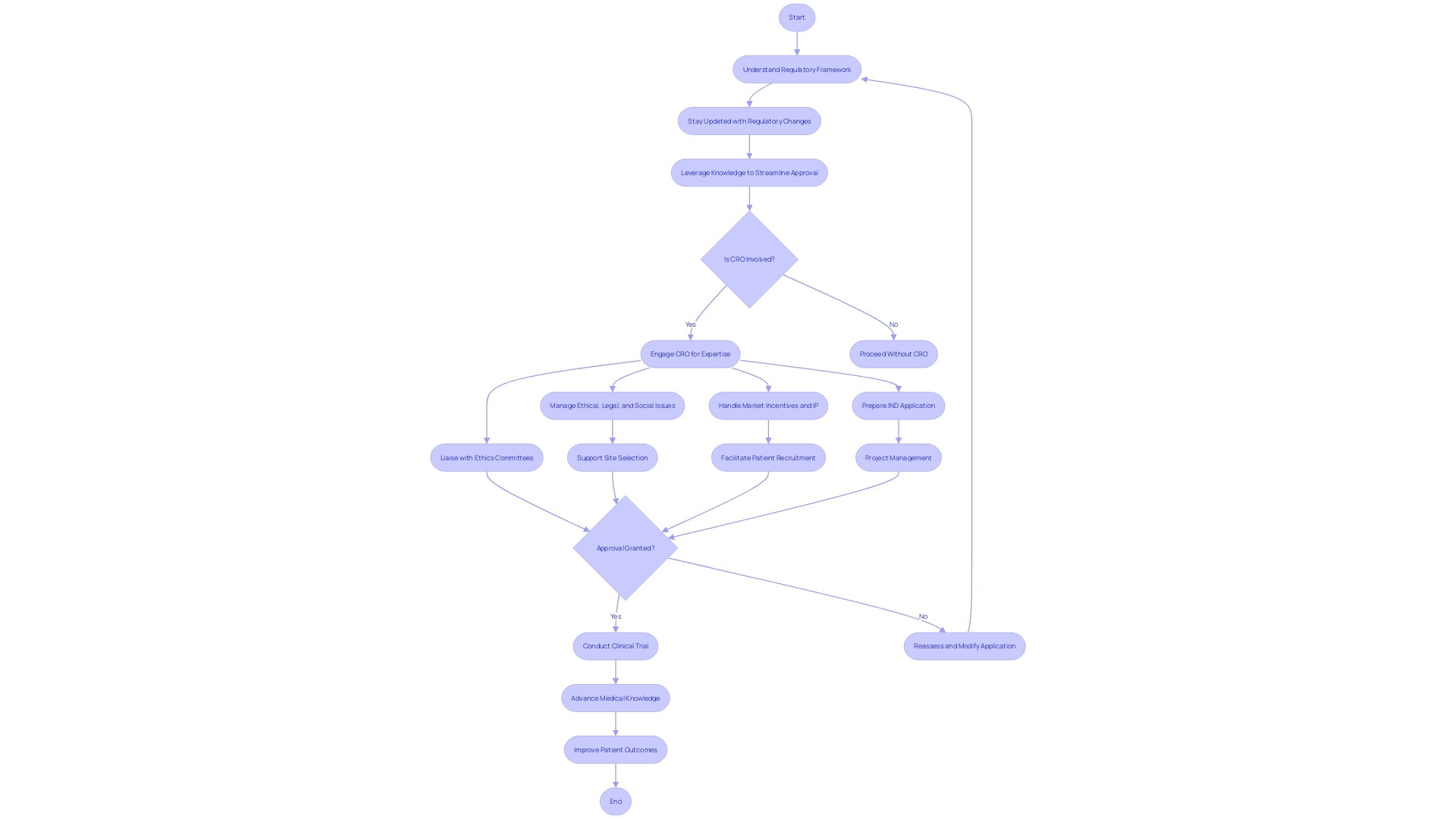
Clinical research in Latin America is subject to complex regulatory environments, especially when it comes to first-in-human (FIH) and early-feasibility studies. Navigating these regulatory landscapes requires expertise and knowledge of the existing framework as well as the ability to adapt to changing regulations. Clinical Research Organizations (CROs) play a crucial role in providing the necessary guidance and support to sponsors and investigators.
CROs possess a deep understanding of the ethical, legal, and social issues surrounding clinical research in Latin America. They help formulate robust regulatory strategies by offering insights into the multifaceted challenges faced in this region. This includes preparing essential documentation, such as Investigational New Drug (IND) applications, and liaising with Ethics Committees for successful submissions.
In addition to regulatory guidance, CROs also provide practical support in site selection, patient recruitment, and project management. This comprehensive approach is particularly valuable given the dynamic nature of Latin America's regulatory environment, where sudden changes can occur.
The proficiency of CROs in handling Latin America's specific regulatory requirements, coupled with their ability to address ethical and operational challenges, makes them invaluable partners for clinical research in the region. Their contribution ensures that studies are conducted efficiently, ethically, and in compliance with local regulations, ultimately advancing medical knowledge and improving patient outcomes.
The Role of CROs in Regulatory Environments
Clinical research in Latin America faces unique regulatory hurdles, particularly when it comes to first-in-human (FIH) and early-feasibility studies. Clinical Research Organizations (CROs) are instrumental in navigating these complex regulatory landscapes. Their expertise is not just in understanding the existing framework but also in staying abreast with the latest regulatory changes and leveraging this knowledge to streamline the approval process.
CROs possess a deep comprehension of the ethical, legal, and social issues pertaining to clinical research, which is essential in a region marked by diverse governance ecosystems. This includes managing market incentives and intellectual property issues that are pivotal in the evolution of health technologies. By offering insights into these multifaceted challenges, CROss help sponsors and investigators formulate a robust regulatory strategy.
This involves preparing essential documentation, such as Investigational New Drug (IND) applications, and liaising with Ethics Committees for successful submissions.
Furthermore, the practical support from CROss extends beyond regulatory guidance. They aid in site selection, patient recruitment, and project management, ensuring the smooth progression of clinical trials. Their comprehensive approach is particularly beneficial given the dynamic nature of Latin America's regulatory environment, where abrupt changes can occur, as seen in the recent updates to Brazil's controlled substances database.
As highlighted by the case of Brazil’s National Health Surveillance Agency (ANVISA), which faced issues with its reporting database, the role of CROss becomes even more critical in mitigating risks and maintaining compliance in such scenarios.
In conclusion, the proficiency of CROs in handling Latin America's specific regulatory requirements, coupled with their ability to address ethical and operational challenges, makes them invaluable partners for clinical research in the region. Their contribution is significant in ensuring that first-in-human and early-feasibility studies are conducted efficiently, ethically, and in compliance with local regulations, ultimately advancing medical knowledge and patient outcomes.
Services Offered by CROs in Regulatory Consulting
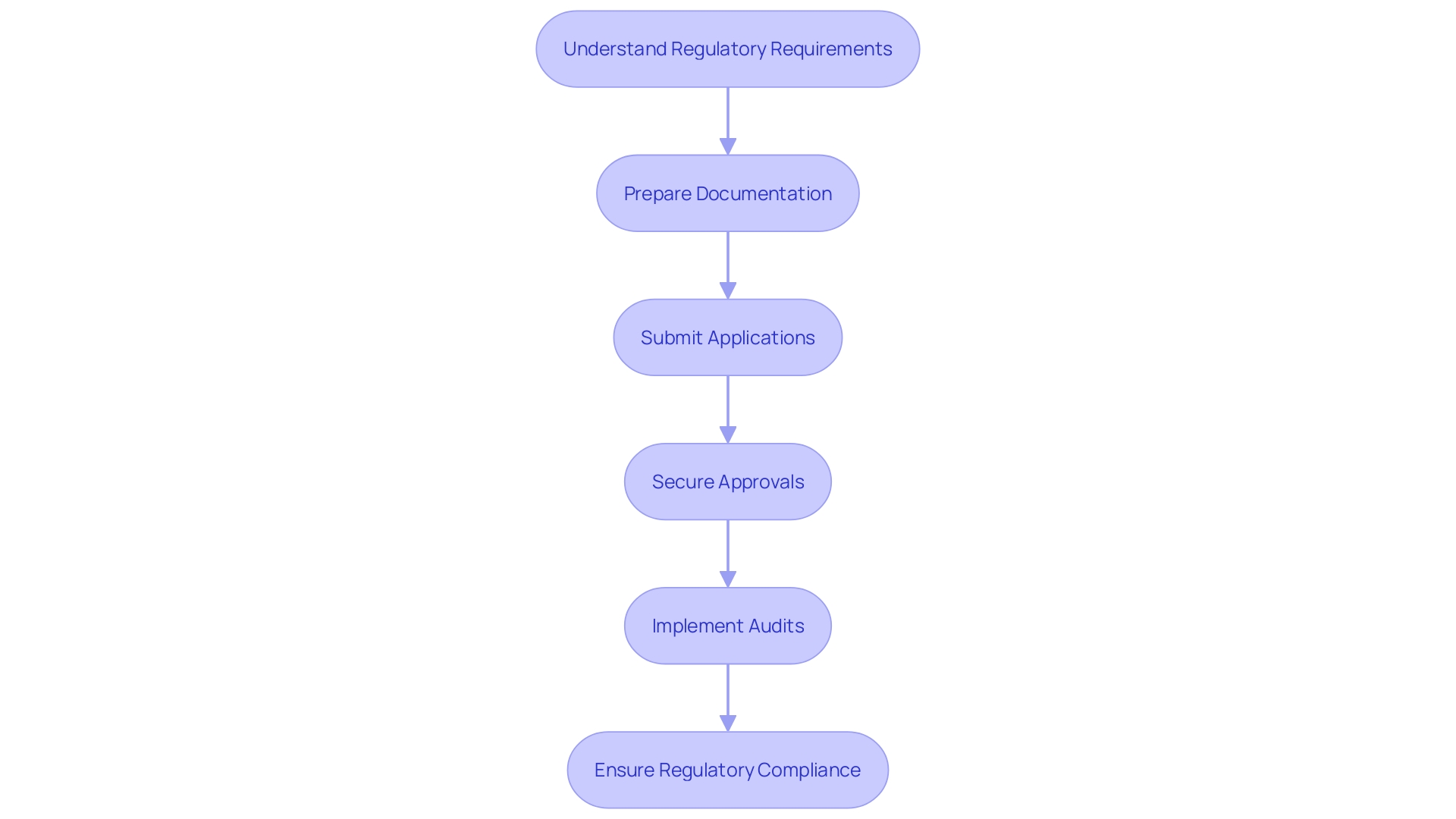
In the dynamic landscape of clinical research, Clinical Research Organizations (CROs) have become pivotal in offering tailored regulatory consulting services to ensure the successful navigation of the regulatory environments in Latin America for first-in-human (FIH) and early-feasibility studies (EFS). The expertise of CROss in this region is instrumental in developing comprehensive regulatory strategies that align with the intricate guidelines of local authorities. These strategies are meticulously crafted to include the identification of necessary documentation, timelines, and milestones to streamline the regulatory process.
The proficiency of CROs is especially evident when facilitating the preparation and submission of regulatory applications such as Investigational New Drug (IND) applications and Ethics Committee (EC) submissions. Their intricate understanding of Latin American regulatory frameworks enables them to ensure that all documentation is both complete and precise, thereby optimizing the efficiency of the submission process.
Moreover, CROs provide indispensable regulatory compliance support throughout the clinical trial lifecycle. Through regular audits and quality assessments, they identify compliance gaps and proactively recommend improvements to mitigate risks related to regulatory reviews.
Beyond these core offerings, CROs extend their support to encompass site selection, patient recruitment, and project management. These additional services are integral to a holistic approach for sponsors and investigators, facilitating a seamless journey through the regulatory terrains of Latin America.
Given the complexities of the regulatory systems, such as those managed by ANVISA in Brazil, which oversees the safety and quality of food and health-related products, and the landmark Law # 6360 for sanitary surveillance, the role of CROss is ever more critical. They are adept at navigating the multifaceted regulations that govern pharmaceuticals, cosmetics, dietary products, and medical devices, ensuring safety, efficacy, and compliance.
The impact of professional regulatory consulting by CROs cannot be overstated, as it paves the way for the successful execution of clinical trials, ultimately driving advancements in healthcare and patient outcomes in Latin America.
Case Study: Successful Regulatory Consulting by a CRO
A noteworthy example of regulatory consulting success within the Clinical Research Organization (CRO) sector in Latin America is underscored by a partnership between a pharmaceutical company and a CRO, which facilitated the launch of a first-in-human (FIH) study for an innovative medication. Initially, the pharmaceutical firm faced a daunting challenge due to their unfamiliarity with the specific regulatory requirements prevalent in the region. Enlisting the expertise of a specialized CRO, the pharmaceutical company was able to gain a comprehensive understanding of the regulatory scenario, ensuring a bespoke strategy that was in line with their study's unique requirements.
The CRO's role was pivotal in preparing the necessary documentation, including the Investigational New Drug (IND) application and Ethics Committee (EC) submissions, ensuring accuracy, completeness, and regulatory alignment. Additionally, the CRO played a critical part in securing the regulatory approvals and permits by effectively communicating with the local regulatory bodies.
To maintain regulatory compliance, the CRO implemented continuous audits and quality checks, promptly identifying and addressing any gaps, which greatly contributed to the study's adherence to the regional regulatory expectations.
The culmination of these efforts led to the successful approval and initiation of the FIH study. The CRO's profound regulatory insight and proactive support were instrumental in navigating the complex regulatory framework, which culminated in achieving the study's objectives in accordance with local guidelines.
The strategic partnership between the pharmaceutical company and the CRO exemplifies the value of specialized regulatory consulting in Latin America. For sponsors aiming to conduct clinical trials within this region, the collaboration with a knowledgeable CRO is invaluable in overcoming regulatory barriers and ensuring the successful execution of clinical studies.
Challenges and Solutions in Navigating Regulatory Environments
When undertaking first-in-human (FIH) and early-feasibility studies (EFS) in Latin America, researchers and sponsors may find the regulatory environment to be a complex maze of evolving rules and standards. A prevalent obstacle faced is the unfamiliarity with the nuances of local regulatory frameworks, which can vary considerably across the region. To circumvent this, enlisting the services of a Clinical Research Organization (CRO) that specializes in regulatory consulting is instrumental.
These organizations bring a wealth of localized knowledge, capable of navigating the specific guidelines and expectations set by Latin American regulatory authorities, thus smoothing the path to compliance.
Another notable challenge lies in the prolonged nature of the regulatory review process, which can introduce significant delays to study initiation. Strategically partnering with a CRO to devise a clear regulatory strategy, inclusive of realistic timelines, can be a pivotal step. This forward planning is essential to mitigate lengthy approval times, allowing for efficient study progression.
Compliance is yet another cornerstone in the successful execution of clinical studies in the region. Any deviation from local regulations may result in detrimental consequences, ranging from study delays to outright termination. Collaborative efforts with a CRO that provides robust regulatory compliance support, including regular audits and quality assurance checks, can identify and rectify compliance gaps, thereby ensuring seamless adherence to local mandates.
The Latin American healthcare landscape is rapidly transforming, marked by an increasing demand for quality systems that can deliver results in diverse settings. The Quality Evidence for The Transformation of Health Systems for Latin America and the Caribbean (Quest LAC) network exemplifies such an initiative, aiming to enhance healthcare quality across the region. In the context of mental health—a sector that carries a significant burden, particularly in low- and middle-income countries—the Vida Plena project serves as a case study.
It highlights the critical need for improved mental health services and the potential impact of additional funding opportunities to address this public health challenge.
In summary, while Latin America presents a unique set of regulatory challenges for FISH and EFS, the strategic engagement of specialized CROss, comprehensive planning, and stringent compliance checks are key strategies that can facilitate successful navigation through this intricate landscape.
Conclusion
Clinical Research Organizations (CROs) play a crucial role in navigating the complex regulatory landscapes of clinical research in Latin America. Their deep understanding of the ethical, legal, and social issues surrounding clinical research allows them to formulate robust regulatory strategies and provide practical support in site selection, patient recruitment, and project management.
Latin America's dynamic regulatory environment requires expertise and adaptability, which CROs possess. Their proficiency in handling the region's specific regulatory requirements, coupled with their ability to address ethical and operational challenges, makes them invaluable partners for clinical research. They ensure studies are conducted efficiently, ethically, and in compliance with local regulations, advancing medical knowledge and improving patient outcomes.
CROs offer tailored regulatory consulting services, including comprehensive regulatory strategies, preparation of necessary documentation, and regulatory compliance support throughout the clinical trial lifecycle. Their expertise in navigating the specific guidelines and expectations set by Latin American regulatory authorities optimizes the efficiency of the submission process and ensures compliance.
A notable case study exemplifies the success of regulatory consulting by a CRO in Latin America. By partnering with a pharmaceutical company, the CRO facilitated the launch of a first-in-human study, providing comprehensive regulatory understanding and ensuring accurate and complete documentation. Their regulatory insight and proactive support were instrumental in navigating the complex regulatory framework, leading to successful study approval and initiation.
Despite the unique regulatory challenges in Latin America, the strategic engagement of specialized CROs, comprehensive planning, and stringent compliance checks are key to successful navigation. Collaborative efforts with CROs that provide robust regulatory compliance support ensure seamless adherence to local mandates and mitigate compliance gaps.
In summary, CROs' expertise in regulatory consulting is essential for navigating the complex regulatory environments in Latin America. Their comprehensive approach and ability to address challenges ensure efficient and compliant clinical research. By leveraging their knowledge and experience, CROs contribute to advancing medical knowledge and improving patient outcomes in the region.




