Introduction
Selecting the right site for a clinical trial is essential for ensuring its success. A well-chosen site can enhance participant recruitment, ensure adherence to trial protocols, and facilitate timely data collection, ultimately accelerating the path to regulatory approval and patient access. However, site selection is a multifaceted process that involves considering factors such as infrastructure, data strategy, treatment methodology, and patient logistics.
In this article, we will delve into the key factors and best practices for site selection in clinical trials, as well as explore case studies that highlight innovative strategies in this area. By understanding the importance of site selection and adopting effective strategies, clinical research directors can maximize the likelihood of successful trial outcomes while advancing medical research and protecting participant rights.
Understanding Site Selection for Clinical Trials
Selecting the right site for a clinical trial is not just a necessary step; it’s a definitive factor that can determine the trajectory of a trial's success. A well-chosen site can enhance participant recruitment, ensure adherence to trial protocols, and facilitate timely data collection, ultimately accelerating the path to regulatory approval and patient access.
Clinical trial sites must be equipped with the technology and infrastructure capable of supporting today’s digital data strategies. Implementation of electronic health records (EHR) and the utilization of real-world data (RWD) are essential for ensuring quality data collection and analysis. For example, Flatiron’s research emphasizes the importance of data relevance and reliability, underscoring the necessity of establishing a robust framework for quality data collection from the outset.
Equally important is the trial site’s ability to navigate the complexities of treatment allocation methodologies, such as Equal Randomisation (ER) or Thompson Sampling (TS), to bolster statistical power and improve overall trial efficiency.
Furthermore, considering the patient's journey, as mentioned in a quote regarding a hypothetical scenario with a patient traveling from rural Pennsylvania to Turkey, highlights the need for trial sites to address logistical barriers as well as to ensure patients' familiarity and comfort with the trial process.
Selecting a trial site in Latin America, for instance, presents its own set of opportunities and challenges. Protocol design must incorporate an understanding of local regulations and ethical standards, while also taking into account regional nuances that can impact the trial's execution and outcome.
In summary, the rigor of site selection hinges on a multifaceted approach that includes infrastructure, data strategy, treatment methodology, and patient logistics – all aiming to foster a beneficial ecosystem for both clinical research and patient welfare.
Key Factors in Site Selection
Identifying the optimal location for clinical trials necessitates a thorough evaluation of multiple pivotal aspects, such as the presence of an adequate patient population, resources, and a solid infrastructure that supports medical care, encompassing a spectrum from clinical expertise to advanced diagnostics and treatment abilities. Furthermore, the expertise and credibility of investigators, as well as their team's proficiency, are crucial in conducting research effectively. However, merely having these elements available is not enough; active engagement and participation from the site are also essential for the fruition of a successful clinical trial.
The integration of electronic health records (EHR) in clinical trials exemplifies an innovative approach that may pose questions about the preparedness of existing infrastructure to meet study objectives. By identifying and rectifying EHR integration challenges, clinical sites can bolster their capability to manage complex trials effectively. On the international front, as evidenced by the recent partnership between Mount Sinai School of Medicine and the Brazilian Clinical Research Institute, there is a growing recognition of the need to extend research efforts internationally to tackle global health challenges like cardiovascular disease, calling for the design of more efficient trials.
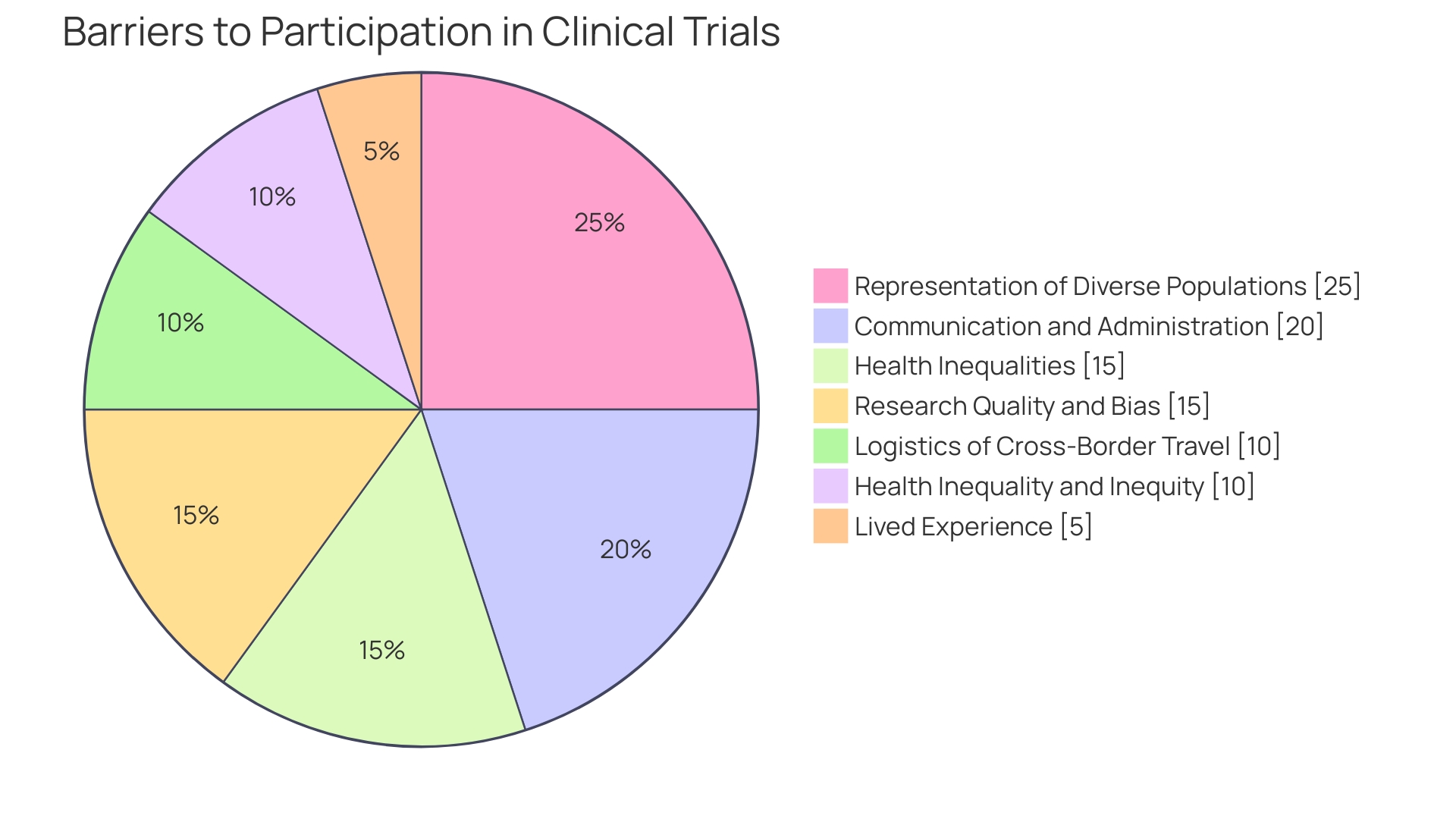
Furthermore, diversifying trial locations is increasingly acknowledged, not just for broader scientific representation but also for practical and ethical reasons. This is underscored by challenges noted in delivering clinical trial materials and navigating the complex regulatory landscape, particularly in continents like Africa with higher disease burdens. Meanwhile, clinical trials face significant stumbling blocks with patient recruitment and retention, where an alarming 80% do not finish as planned and 20% face over six-month delays – primarily due to insufficient participant numbers and retention.
This necessitates a reevaluation of trial management, where strategies such as Early Access Programs (EAPs) emerge as a viable pathway for patients who are ineligible for conventional clinical trials. These programs provide critical access to investigational medicinal products, bridging the gap between trial participation and treatment authorization. Ultimately, clinical trials continue to serve as a pivotal axis in the medical research domain, testing new prescription methodologies, surgical devices, and other unique approaches to healthcare, with each designed to adhere to specified eligibility criteria to guarantee participant safety and the validity of trial outcomes.
Best Practices for Site Selection
Effective site selection is a cornerstone of clinical trial success, especially when navigating the complex regulatory environments of first-in-human (FIH) and early-feasibility studies (EFS) in Latin America. To optimize this process, it's crucial to engage in comprehensive site feasibility assessments. This entails not only evaluating each potential site's performance history and compliance with regulatory standards but also their prior involvement with similar research endeavors.
For instance, the utilization of electronic health records (EHR) to ascertain patient data in trials, known as EHR-sourced trials, has shown significant promise. However, these trials often face uncertainties around the operationalization of study goals within existing site infrastructures. Such insights underscore the importance of adapting site selection strategies to accommodate modern trial designs, including the pragmatic integration of EHR data.
Proactive communication and ongoing collaboration with potential sites are also essential for streamlining this process. Clinical trials design, like those explored in discussions around Equal Randomization (ER) and Thompson Sampling (TS), stresses that patient assignment methods should not only be powerful but also practical for the chosen sites. Furthermore, when considering global trials, issues arise around the need for participants to navigate cross-border travel and language barriers, which can significantly impact site selection and trial participation.
Thus, aligning site capabilities with the unique demands of multinational trials is vital for a successful study outcome.
Additionally, in aligning with new regulations such as the FDA's efforts to harmonize human subject protection regulations, site selection must reflect an adherence to these evolving standards. Medical product developers are now being guided to submit diverse action plans (DAPs) that outline enrollment goals and the procedures for meeting them. This requirement has substantial implications for site selection regarding the diversity of trial participants and the ability to conduct multinational studies.
By integrating these best practices into the site selection strategy, clinical research directors can enhance their trial's likelihood of success while facilitating medical advancements and protecting participant rights.
Strategies for Effective Site Selection
In the realm of clinical trial execution, particularly in Latin America, establishing criteria for site selection is a pillar of success. This involves considering the study objectives, target patient demographics, and projected enrollment metrics. To achieve optimal site selection, it is critical to harness the vast data pool from electronic health records (EHR) and other digital data sources.
These EHR-sourced trials harness a treasure trove of data, offering a nuanced approach to operationalizing study goals. For instance, using this data allows for creating an objective site evaluation process; a centralized site selection committee, extolled for ensuring consistency and objectivity, heralds a more standardized evaluation methodology.
The integration of cutting-edge technology and data analytics further empowers the site selection realm. Data quantity alone cannot drive the decision-making process; it is the quality of data and the strategic extraction of actionable insights that truly augment the evaluation of potential study sites and their performance metrics. By taking into account the diversity of participants and the specifics at the disease level, we can create inclusive clinical trials that are reflective of the population's needs.
Modern trial designs such as Equal Randomisation (ER) and Thompson Sampling (TS) can bolster the statistical power of studies and ensure a more equitable treatment assignment. Such innovative approaches offer alternative methods to retain high levels of statistical power, beyond the traditional ER method.
It is essential to think critically about the study design and existing infrastructure. Leveraging a wealth of industry data can redefine participant recruitment and site selection strategies, ensuring adherence to the latest regulatory standards. By focusing on the core question a study aims to answer, we can outline our methodology and garner deeper, more focused insights into patient behavior and treatment efficacy.
This well-considered approach to selecting trial sites is fundamental in upholding the integrity of the research and enhancing the potential for clinical advancement.
Case Studies and Examples
Pioneering strategies in clinical site selection can increase the chances of successful trial outcomes. Raman et al. (2023) illustrate the potential of using electronic health records (EHR) to enhance traditional clinical trial models, emphasizing the value of data quality.
By incorporating EHR data, researchers can better operationalize study goals and make informed decisions about site selection. Similarly, exploring different randomization methods, such as Equal Randomization and Thompson Sampling, illuminates the intricate balance between statistical power and implementation simplicity in trials. These innovative approaches, combined with insights on quality frameworks for real-world data as articulated by Flatiron Health and regulatory guidance from the FDA, serve to refine site selection practices.
Moreover, with the advent of 'digital twins,' as proposed by Karen Willcox, and their application in predicting clinical outcomes, the horizon for site selection and trial design continues to expand, offering promising avenues to enhance research efficacy.
Conclusion
In conclusion, the site selection process is crucial for the success of clinical trials. A well-chosen site enhances participant recruitment, ensures adherence to trial protocols, and facilitates timely data collection. Factors such as infrastructure, data strategy, treatment methodology, and patient logistics must be carefully considered.
The incorporation of electronic health records (EHR) and real-world data (RWD) is essential for quality data collection and analysis. Treatment allocation methods like Equal Randomization (ER) and Thompson Sampling (TS) can improve trial efficiency and statistical power.
Effective site selection requires comprehensive feasibility assessments and proactive communication with potential sites. Adapting to evolving standards and aligning site capabilities with the demands of multinational trials is crucial.
Innovative strategies such as utilizing EHR data and exploring alternative randomization methods can significantly increase the likelihood of successful trial outcomes. By adopting best practices and understanding key factors, clinical research directors can maximize the success of trials, contribute to medical advancements, and protect participant rights.