Introduction
The 510(k) Premarket Notification process is a vital step for medical device manufacturers looking to enter the U.S. market. It requires companies to demonstrate that their new device is substantially equivalent to an existing, legally marketed predicate device. This article provides a comprehensive overview of the 510(k) process, highlighting the importance of understanding the device's intended users and competitive landscape.
It emphasizes the need for thorough research and analysis to craft a comparative table that showcases the similarities and differences with potential predicate devices. The article also explores the FDA's classification system for medical devices and offers insights into the FDA's guidance documents and resources. With statistics revealing that only about 10% of devices fall into the high-risk Class III category, the article discusses recent trends in streamlining the regulatory pathway.
Understanding these aspects is crucial for manufacturers aiming to compile a successful 510(k) submission and ensure a smooth entry into the medical device market.
Understanding the 510(k) Process and Its Importance
The 510(k) Premarket Notification is a vital regulatory step for medical device manufacturers aiming to enter the U.S. market. It requires companies to establish that a new device is substantially equivalent to an existing, legally marketed predicate device. A thorough understanding of the device's intended users, such as clinicians and patients, and its competitive landscape is imperative. Manufacturers must meticulously analyze research literature, clinical studies, and competitor device information to craft a comparative table that showcases the similarities and differences with potential predicate devices.
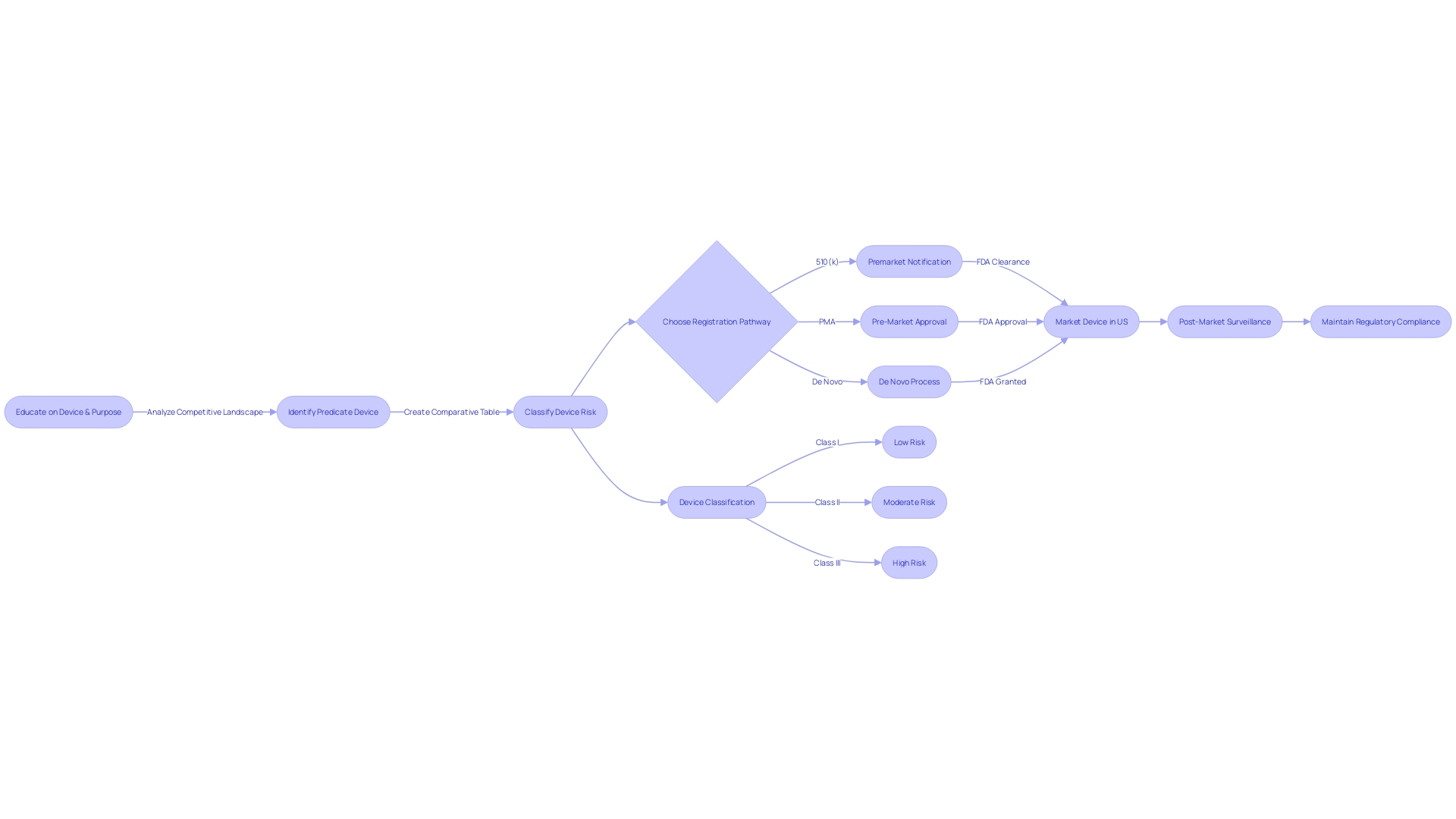
To navigate this process, it's essential to first determine the device's proper FDA classification, which ranges from low-risk Class I to high-risk Class III devices, each subject to varying regulatory controls. A Class I or II device typically undergoes the 510(k) process, while Class III devices may require a more rigorous Pre-Market Approval (PMA) or De Novo process.
The FDA maintains a repository of Summaries of Safety and Effectiveness (SSEs), which are invaluable for evaluating predicate devices. Additionally, the FDA's guidance documents offer a clear roadmap for submission, detailing the essential elements to include and the process for ensuring acceptance.
Statistics reveal that the FDA categorizes medical devices into three classes based on potential risk, with only about 10% of devices falling into the high-risk Class III category. These devices, such as life-sustaining pacemakers, undergo more stringent regulatory scrutiny. Recent trends show efforts to streamline the regulatory pathway, particularly for devices meeting urgent medical needs or in innovative areas like digital health.
Understanding these aspects is not just beneficial but crucial for manufacturers who must compile a 510(k) within a specific timeframe and meet the FDA's expectations for demonstrating substantial equivalence, thus ensuring a successful entry into the medical device market.
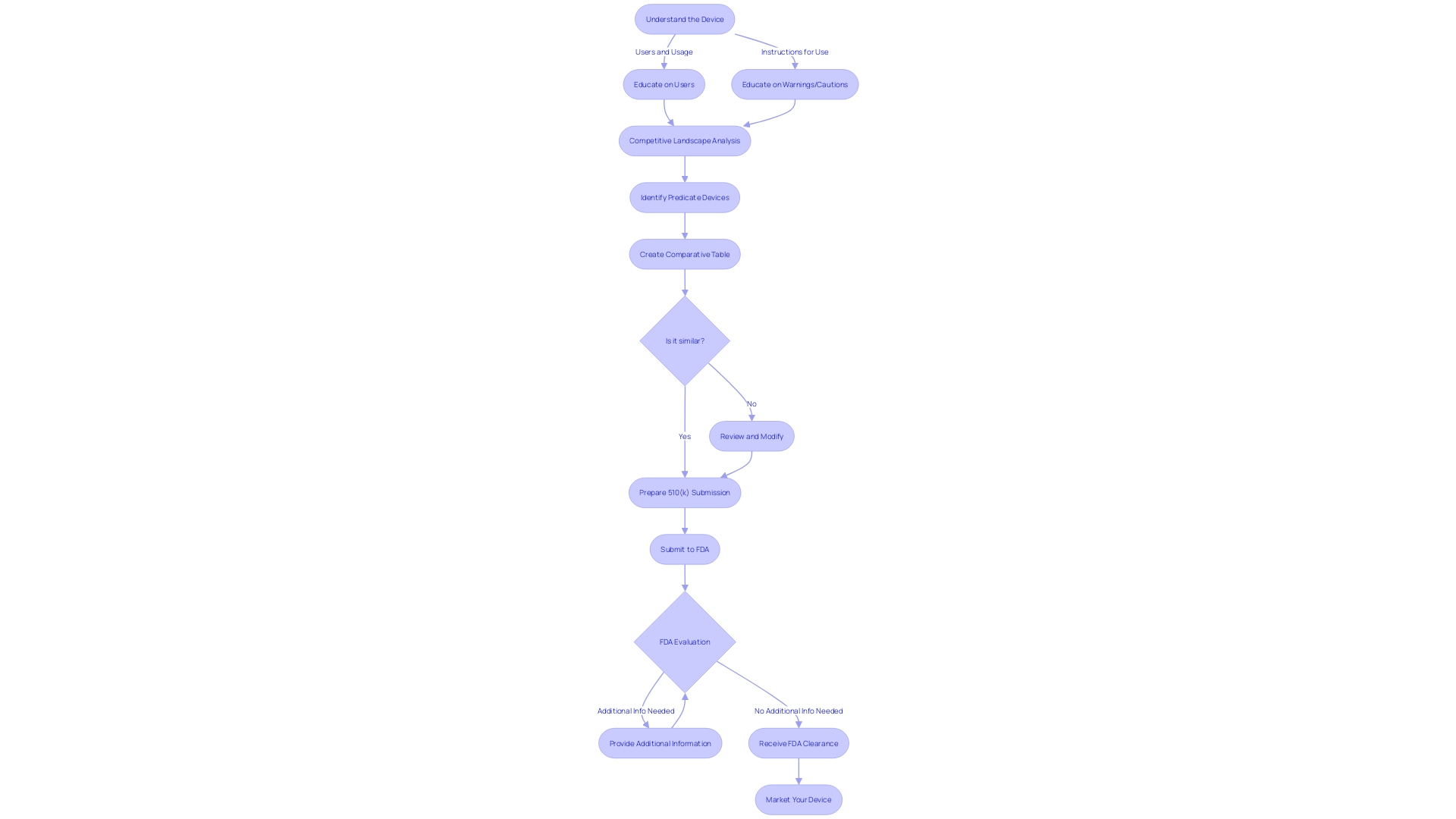
Step 1: Finding and Establishing a Predicate Device
Identifying a predicate device holds paramount significance in the 510(k) submission process, with an emphasis on ensuring the new medical device is substantially equivalent to an existing one. Initiating this process involves an in-depth analysis of the device landscape, where a meticulous comparison is made between the intended use and technological characteristics of the potential predicate and the new device.
To facilitate this, a systematic approach must be taken to gather comprehensive information about the device. This includes understanding the user demographic, such as clinicians and patients, as well as becoming familiar with the device's instructions, including any warnings and precautions. Leveraging marketing insights is also crucial to gain an understanding of the competitive environment and aid in the identification of analogous devices.
Once potential predicates are recognized, it is advisable to construct a comparative table, which serves as a visual representation of the similarities and differences between the devices. This table should be informed by thorough research, including an examination of clinical studies, regulatory summaries, and other relevant materials that contribute to a detailed understanding of the predicate device.
Moreover, it is imperative to consider the broader implications of medical devices on patient care, as underscored by the World Health Organization's definition. Medical devices, ranging from simple implements like tongue depressors to complex machinery, play a critical role in diagnosis, treatment, and improving the quality of life for patients. Therefore, the selection of an appropriate predicate device is not only a regulatory checkpoint but also a cornerstone in ensuring patient safety and efficacy of the new medical device.
In light of the significant role medical devices play, the 510(k) clearance process is designed to foster innovation while maintaining rigorous safety standards. Documentaries such as 'The Bleeding Edge' have highlighted the importance of this process, calling attention to the need for thorough evaluations without necessarily requiring clinical trials for every device. This underscores the importance of the predicate device analysis in demonstrating safety and effectiveness without compromising the speed to market.
In essence, the initial step of pinpointing a predicate device necessitates a blend of regulatory knowledge, market awareness, and commitment to patient well-being, setting the stage for a successful 510(k) submission.
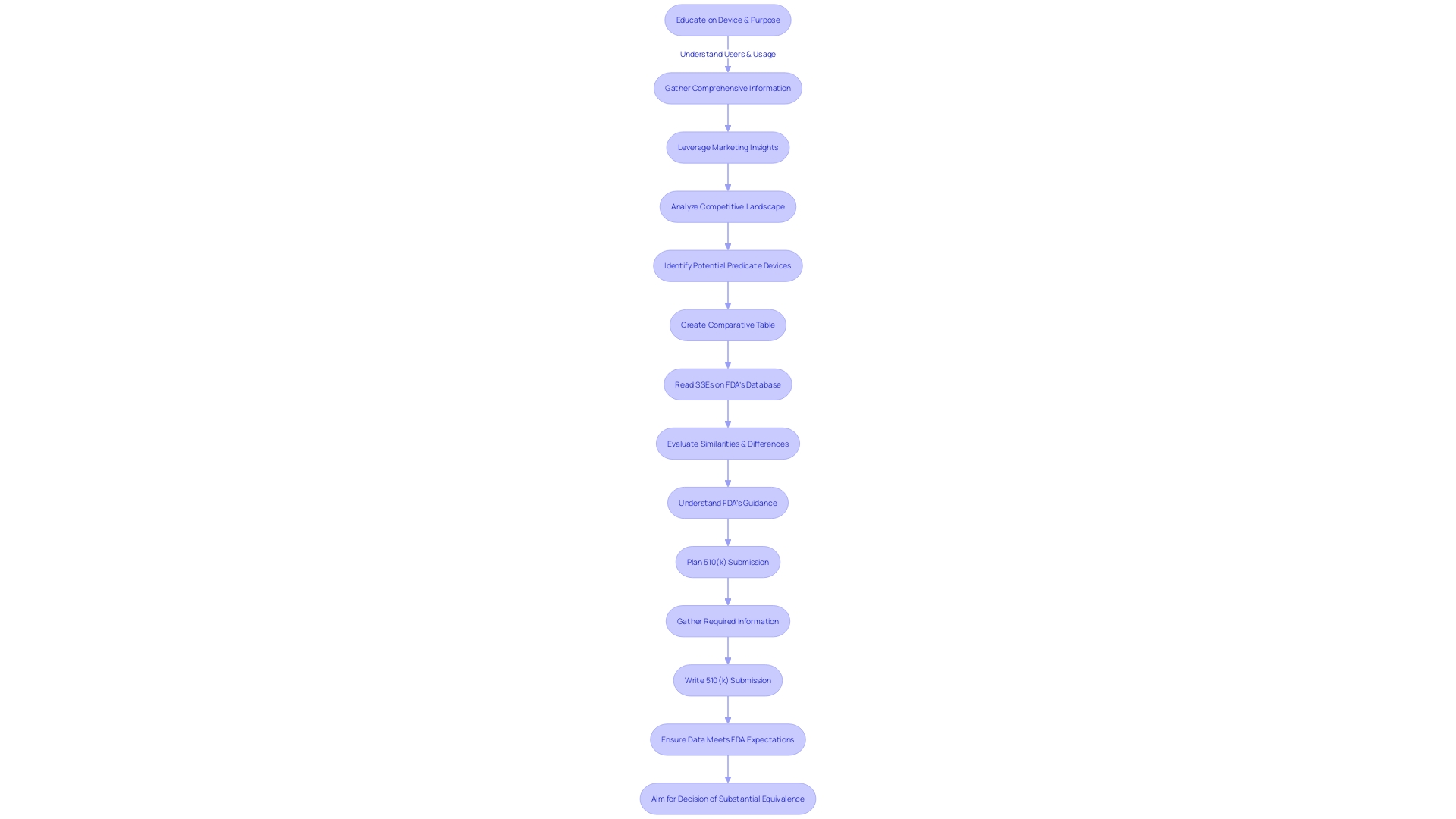
Step 2: Preparing the 510(k) Submission Documents
Identifying a predicate device is a pivotal step in the 510(k) medical device approval process, necessitating an in-depth comprehension of the subject device. To effectively prepare the submission documents, one must delve deeply into the understanding of the device's users, such as clinicians and patients, and thoroughly examine instructions for use, including all warnings and cautions. Collaboration with marketing teams can provide valuable insights into the competitive landscape, allowing for the identification of potential predicate devices that share similar intended uses and technological characteristics. It is crucial to construct a comparative table, which will serve as a foundation for the detailed description required in the submission documents.
The documents you compile should not only include a comprehensive description of the new device and its intended use, but also robust performance data and any proposed labeling changes, all of which should align with the predicate device's characteristics. This is substantiated by the Summaries of Safety and Effectiveness (SSEs) available on the FDA's 510(k) database, which is an essential resource for evaluating similarities and differences between the new device and its predicate.
The FDA's recent announcement on the draft guidance for the Q-Submission Program underscores the importance of structured interaction with the FDA during the submission process. Submitters are encouraged to provide comments on the draft guidance, which offers clarifications on obtaining feedback for medical device submissions. The guidance delineates the types of Q-Submissions and elucidates the process for informal communication versus Pre-Submission or other types. It is important to note that comments submitted to the FDA become public record, and submitters must take care not to include any confidential information.
The proper classification of the device is the initial step, as the FDA categorizes medical devices into three classes based on patient risk. Determining the correct classification guides the selection of the appropriate registration pathway, whether it be a 510(k) Premarket Notification, Pre-Market Approval (PMA), or the De Novo process. Understanding these distinctions is significant; a device must be FDA Cleared, Approved, or Granted via the De Novo process before it can be legally marketed in the U.S.
In conclusion, the preparation of submission documents for 510(k) clearance is a meticulous process that requires a thorough understanding of regulatory requirements and a strategic approach to demonstrating the safety and effectiveness of the new medical device in comparison to an existing, legally marketed predicate device.
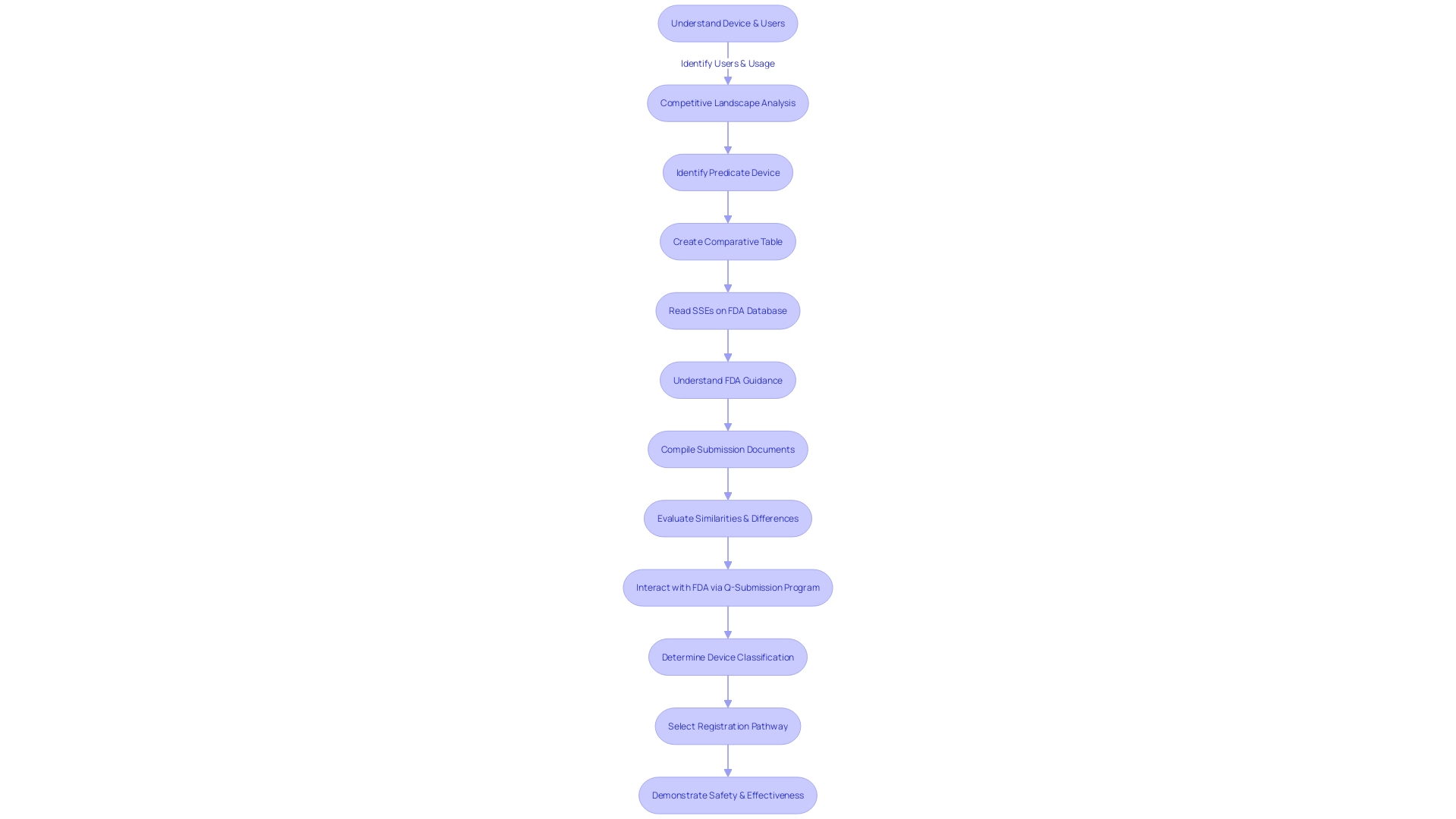
Step 3: Formatting and Submitting the 510(k) Application
To ensure a successful 510(k) submission, a meticulous approach to preparing documentation is paramount. The process begins by gaining a comprehensive understanding of the medical device in question, including its functionality, users—ranging from clinicians and physicians to dentists and patients—and the detailed instructions for use, encompassing all warnings and precautions. Collaborating with the marketing team to assess the competitive landscape is equally crucial. This involves a thorough review of various materials such as research literature, clinical studies, and marketing collateral from competitor devices, to identify a suitable predicate device that shares the intended use and technological characteristics with the new device. Subsequently, constructing a comparative table becomes an essential step.
A predicate device's Summaries of Safety and Effectiveness (SSEs) available on the FDA’s 510(k) database are invaluable resources to discern the parallels and distinctions between devices. It’s also important to be mindful of confidentiality when submitting comments to the FDA, taking care to exclude any private information such as medical records or Social Security numbers that should not be made public.
The FDA has been working on modernizing the 510(k) process, with a focus on creating best practices for selecting predicates, communicated through draft guidance documents. This is part of the FDA's broader mission to assure public health by confirming the safety and efficacy of medical devices. As the agency continues to implement measures to present information in a clear and consumer-friendly manner, applicants must adhere to these evolving standards, ensuring documents are formatted correctly and that the major statement in advertisements is presented in a clear, conspicuous, and neutral manner.
After a thorough preparation of the submission, the documentation must be formatted following the FDA's stringent guidelines. This includes dividing the information into designated sections, labeling each clearly, and including all requisite supporting documentation. Once formatted appropriately, the application is ready to be submitted to the FDA for a comprehensive review.
Acceptance Review Process
The initial step in the 510(k) Medical Device Approval Process involves a meticulous acceptance review by the U.S. Food and Drug Administration (FDA), an essential procedure designed to confirm the thoroughness and compliance of the submission. During this phase, the FDA rigorously checks that the application encapsulates all required forms, documents, and that the submission fee is duly processed. The objective is to ascertain that the application adheres to the stringent regulatory framework and is equipped with the necessary information for further assessment. The FDA's role is pivotal in ensuring the safety and efficacy of medical devices, which ultimately impacts public health and patient safety. The acceptance review is but a preliminary yet crucial step in the intricate journey of a medical device from conception to market, navigating through the regulatory pathways that safeguard our health system's integrity.

Substantive Review and Interactive Review
A meticulous review of a 510(k) submission, known as the substantive review, is conducted by the FDA to determine the safety and efficacy of a medical device. The agency scrutinizes the submitted data, contrasting it with an already approved device, termed the predicate. This evaluation is not merely a checkbox exercise but an interactive dialogue, where the FDA may request further information or clarifications from the manufacturer to ensure thorough understanding and compliance.
During the review, the FDA's aim is to ensure that the new device is at least as safe and effective as the predicate. The manufacturer's role in this phase is critical; they must provide comprehensive evidence, which may include clinical or nonclinical data, to demonstrate equivalence. The FDA's recent focus group studies underscore the importance of understanding user perspectives, which can be a critical aspect of the review process. This is particularly relevant as the FDA emphasizes the role of qualitative research in capturing user attitudes and beliefs towards FDA-regulated products.
Furthermore, the FDA's Compliance Program Manual advises manufacturers to be prepared to respond swiftly to any FDA Form 483 issued post-inspection, with a turnaround time of 15 business days for a written response. This response should detail corrective actions taken or planned, with a specified timeline for implementation. The recent case where a firm's response to complaint coding issues was deemed inadequate by the FDA highlights the necessity for manufacturers to not only propose systemic corrective actions but also to provide evidence of outcomes and effectiveness of these actions.
Manufacturers are cautioned to manage the confidentiality of information responsibly when engaging with the FDA. Any public comments or documents submitted electronically become part of the public record, and manufacturers must be vigilant not to disclose sensitive data such as proprietary manufacturing processes or personal medical information.
Overall, the substantive review is an integral part of the FDA's mission to safeguard public health by ensuring medical devices meet stringent safety and effectiveness criteria before reaching the market.
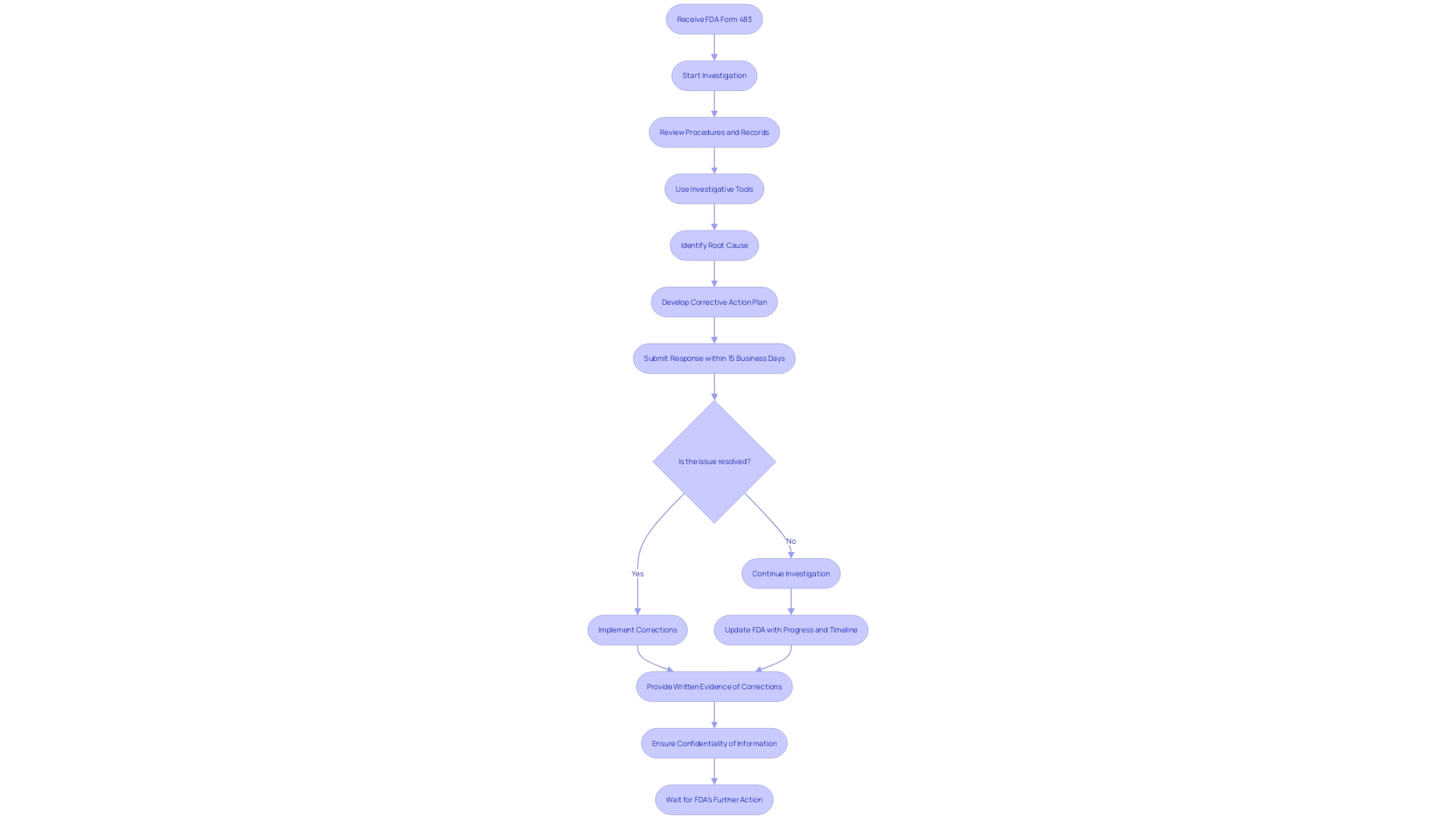
Addressing Deficiencies and Additional Information Requests
Manufacturers must be diligent in responding to FDA inquiries during the medical device review process. If deficiencies are identified or additional information is requested, manufacturers should thoroughly investigate the issue, using methods such as employee interviews, procedure reviews, and analytical tools like 5-whys or fishbone diagrams. Detailing the steps of the investigation, its findings, and the planned corrective actions, along with a clear timeline, is essential to address the FDA's concerns effectively.
It is important to recognize that FDA's role is to assess the safety and effectiveness of medical devices for U.S. use. Post-approval, coverage and reimbursement decisions are made by payors and healthcare providers, and there may be discrepancies between the data submitted for FDA approval and the data required by payors, potentially leading to delays in device availability to patients.
Navigating the complexity of FDA's regulatory databases and ensuring compliance can be challenging for manufacturers, as no single source of truth exists for medical product approval status. The FDA acknowledges this complexity and has introduced the 'Q-Submission Program' to facilitate better communication between manufacturers and the agency, offering clarity on the submission process.
Clinical trials are not always a prerequisite for medical device approval under the FDA's 510(k) clearance process, as highlighted by the 2018 documentary 'The Bleeding Edge'. Nevertheless, the 510(k) process has come under scrutiny due to instances of device-related injuries. The FDA is actively working to enhance oversight and safety measures, addressing funding and patient identification challenges to improve post-market surveillance as part of its ongoing commitment to public health protection.
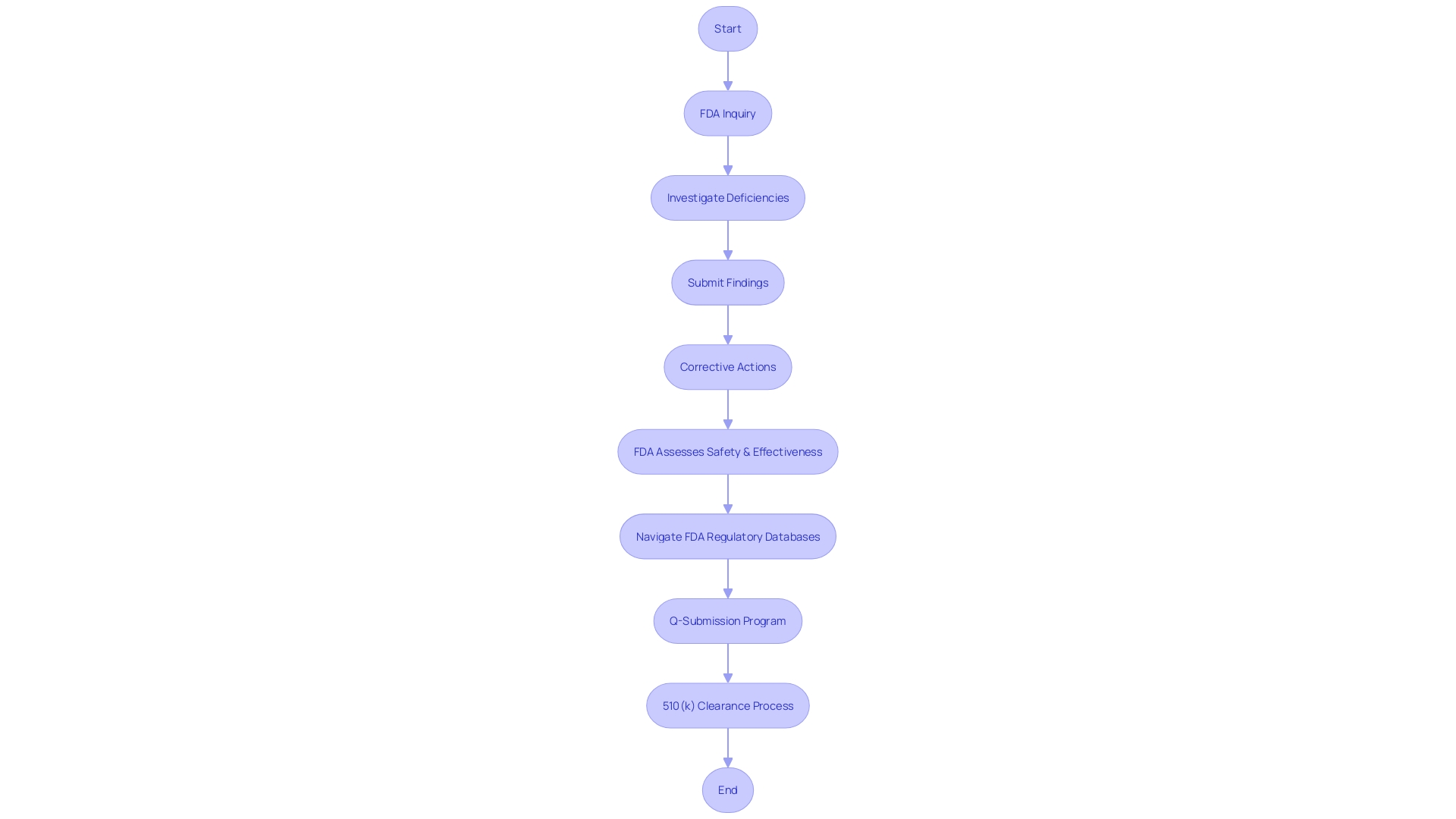
Post-Submission Activities and Clearance
Once the FDA addresses any outstanding queries or concerns regarding a medical device, a critical decision is made regarding its clearance. Following a successful review, manufacturers gain the authorization to market the device within the U.S., an essential step in making the device widely available. However, this is not the end of the regulatory process. Manufacturers are obliged to adhere to an array of post-clearance obligations, including updates to product labeling and rigorous post-market monitoring. This ensures ongoing compliance and the continued safety of the device for users.
Understanding the classification of medical devices is pivotal as it dictates the regulatory pathway and the associated risk to patients. Devices are categorized into three classes, each reflecting a different level of risk, and determining the correct class is essential for selecting the appropriate FDA submission pathway—be it a 510(k) Premarket Notification, Pre-Market Approval (PMA), or De Novo process. The terms 'Registered,' 'Cleared,' 'Approved,' and 'Granted' are often used interchangeably in the industry, but they have distinct meanings that are important to grasp.
For instance, the Impella Connect System, a sophisticated device offering critical cardiac support through remote monitoring, underwent a rigorous premarket analysis due to its software functions that provide vital patient-specific information and generate urgent notifications for healthcare providers. Such devices underscore the importance of compliance with FDA requirements. In another case, the FDA's response to a firm's corrective actions for complaint coding and quality data analysis illustrates the ongoing nature of regulatory compliance, even after initial clearance.
Moreover, the FDA's role extends beyond device clearance, influencing various aspects of public health. This includes ensuring the safety and efficacy of drugs, biological products, and medical devices, as well as overseeing food safety, cosmetics, dietary supplements, products emitting electronic radiation, and tobacco regulation. Recent initiatives, such as the 'Direct-to-Consumer Prescription Drug Advertisements' final rule, demonstrate the agency's commitment to transparent communication by mandating clear and accessible presentation of major side effects in drug advertisements.
It is crucial for manufacturers to navigate these regulatory waters with precision to prevent delays or denial in device coverage and reimbursement, which can occur if the data submitted for FDA clearance does not align with the information required by payors such as CMS and private health plans. A strategic approach to this multi-faceted approval process is imperative for the successful introduction of medical devices to the market.
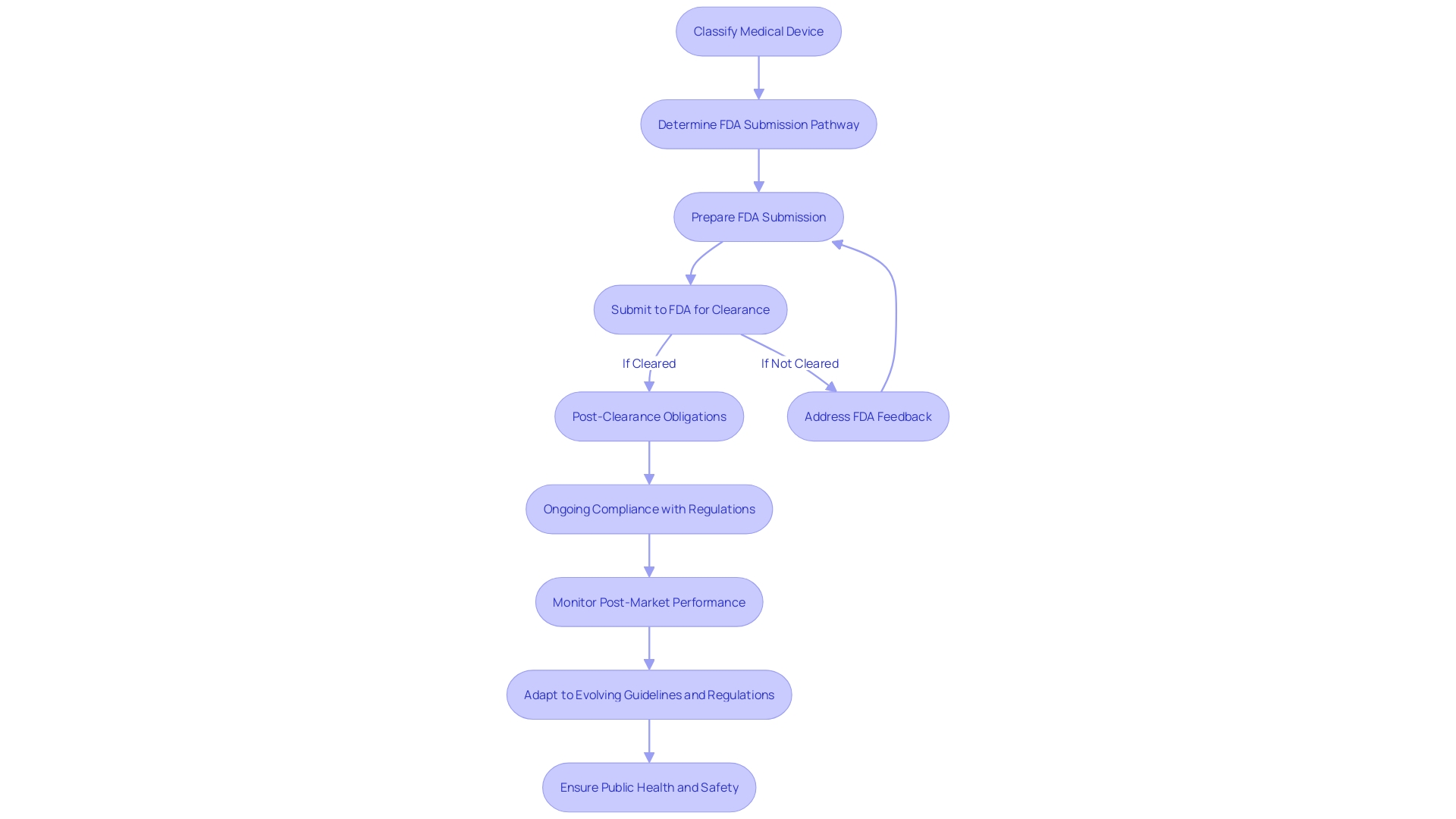
Best Practices for a Successful 510(k) Submission
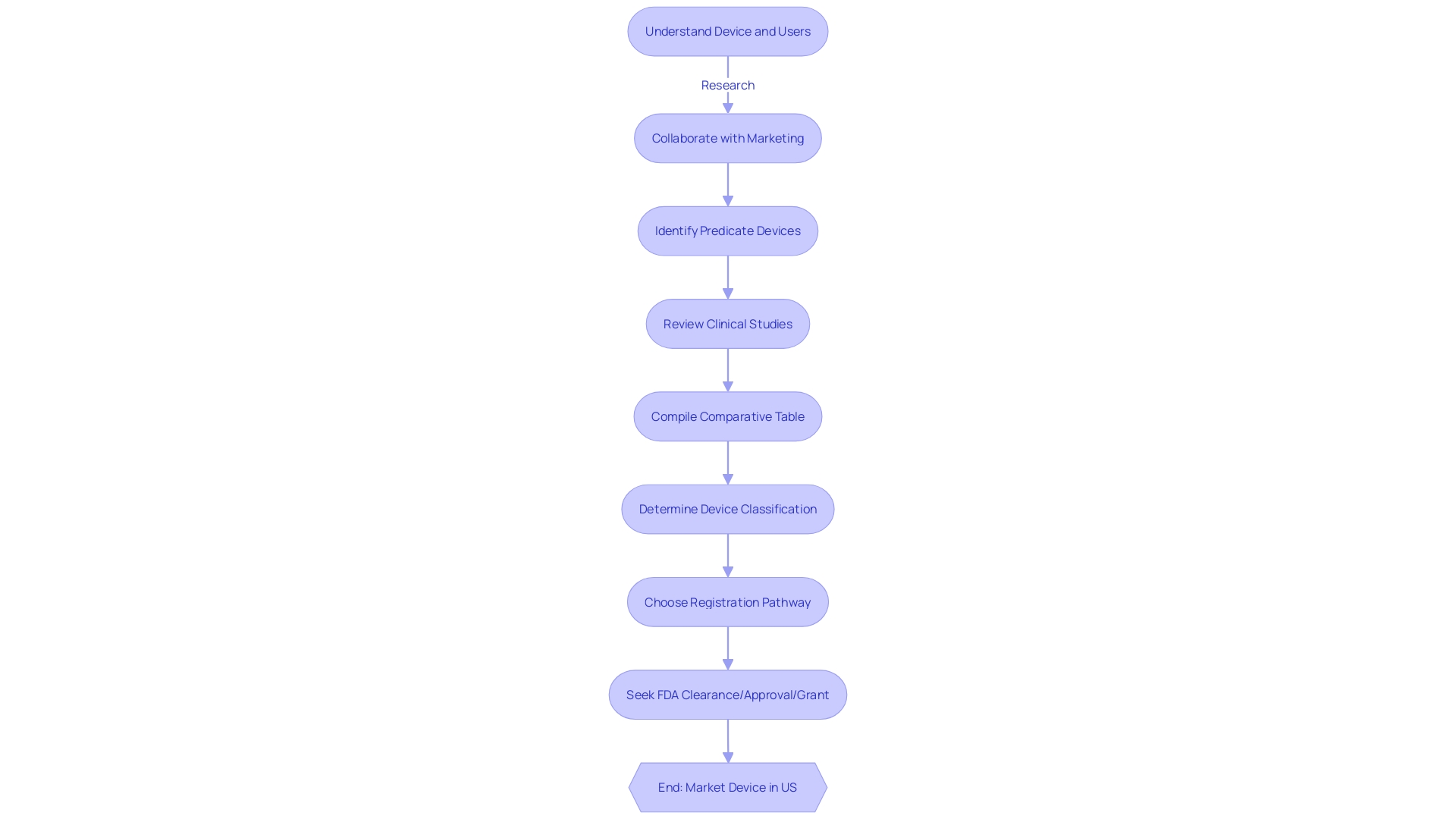
Achieving a successful 510(k) submission entails a strategic approach that involves meticulous research and a collaborative effort with the FDA. Initially, it is crucial to gain an in-depth understanding of the medical device at hand, delving into its use, the end-users (which include clinicians, physicians, dentists, and patients), and comprehensive instructions for use, paying particular attention to any warnings and cautions.
Through collaboration with marketing teams, it is essential to explore the competitive environment, examining competitor devices and assessing a wide array of resources including research literature, clinical studies, and marketing materials such as brochures and sell-sheets. This research is pivotal in identifying a suitable predicate device that shares the same intended use and technological characteristics as the subject device, and establishing a comparative analysis.
In light of the FDA's efforts to modernize the 510(k) process, they have released a draft guidance document on September 7, 2023, outlining best practices for selecting an appropriate predicate. This includes confirming the potential predicate device is currently marketed and registered with the FDA and ensuring it has the same intended use. Moreover, it is imperative to assess whether the technological differences do not pose new safety and effectiveness questions.
The FDA's role in safeguarding public health by ensuring the safety and efficacy of medical devices underscores the importance of adhering strictly to their guidelines. Submitting comprehensive documentation, following the FDA's formatting directives, and fostering open lines of communication with the agency are all fundamental to navigating the review process effectively. This includes responding formally in writing to any communication from the FDA and addressing all specified points to ensure official acknowledgment.
The narrative of companies like Masimo, which has significantly impacted patient outcomes through their technology, illustrates the potential success that awaits those who meticulously adhere to the FDA's standards. Masimo's SET Measure-through Motion and Low Perfusion™ pulse oximetry technology, for instance, is a testament to the effectiveness of rigorous clinical validation and the importance of continuous innovation and monitoring within healthcare settings.
In summary, a comprehensive approach that combines a deep understanding of the subject device, strategic identification of a predicate device, and adherence to the FDA's procedural guidelines will significantly enhance the prospects of a successful 510(k) submission.
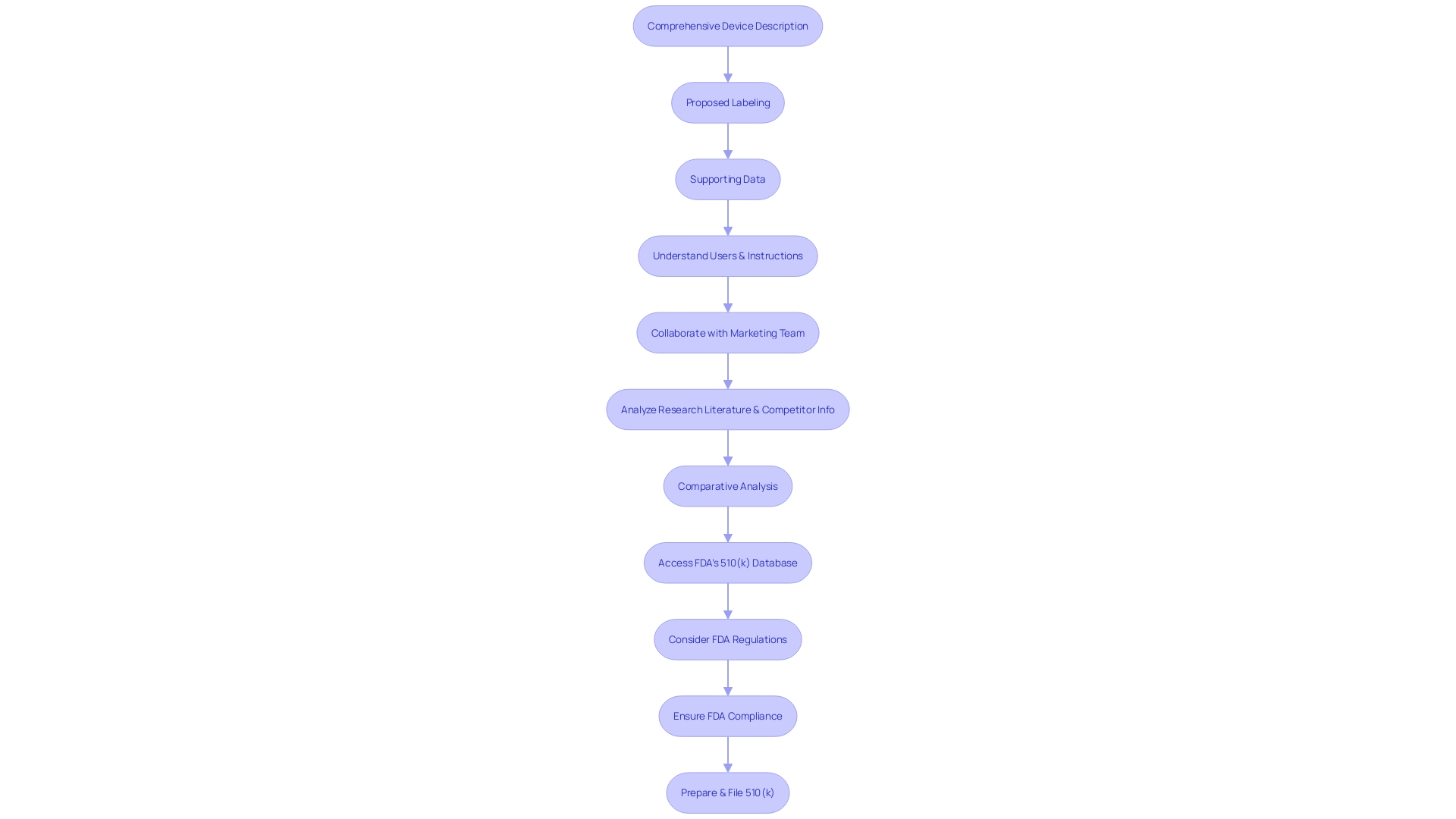
Common Pitfalls and How to Avoid Them
Navigating the 510(k) approval process requires a thorough understanding of both the regulatory requirements and the complex ecosystem of medical device development. A key step in this process is identifying an appropriate predicate device, which involves extensive research and a deep understanding of the clinical landscape. By examining research literature, clinical studies, and other relevant materials, manufacturers can create a comparative table that clearly demonstrates the substantial equivalence of their device to an already approved one.
However, it's not just about finding a similar device; the submission must be meticulously prepared, adhering to FDA's stringent formatting requirements. Etienne Nichols, a seasoned Medical Device Guru, emphasizes that a submission is more than just a formality—it's a comprehensive package that includes non-clinical data such as bench performance testing, sterilization and shelf-life testing, and biocompatibility testing, as well as potential clinical data. The FDA's recent draft guidance sheds light on when clinical data may be necessary, outlining scenarios such as differences in indications for use or technological characteristics that cannot be resolved through non-clinical testing.
Moreover, communication with the FDA is paramount. It's not simply about submitting documents but engaging in a dialogue to ensure all concerns are addressed. This sentiment is echoed in the innovative work of Art, founded by Varun and Josh, who recognize the complexities of regulatory submissions and advocate for a proactive approach in compiling and formatting information.
The regulatory landscape is evolving, with the FDA encouraging modernization and clarity in submissions. Medtech companies must adapt to new methods, such as predetermined change control plans, to streamline their compliance while fostering innovation. In doing so, they can avoid common pitfalls such as inadequate research, incomplete submissions, and poor communication, which can significantly impact the time to market for life-saving medical devices.
Seeking Expert Help and Regulatory Consulting
The 510(k) approval process for medical devices is a rigorous and intricate pathway, one that demands comprehensive knowledge of the device in question. To enhance the chances of a successful submission, it is crucial to delve deeply into understanding the device's users—be they clinicians, physicians, dentists, or patients—and the specific instructions for its use, along with any warnings and precautions. Collaboration with marketing teams is also vital in order to gain insight into the competitive landscape and to pinpoint competitor devices that could serve as potential predicate devices, which have the same intended use and similar technological characteristics.
Creating a comparative table that contrasts your medical device with these predicate devices is a foundational step. This involves extensive research, reviewing clinical studies, and examining materials such as brochures and labels. Additionally, reading through the Summaries of Safety and Effectiveness (SSEs) available in the FDA's 510(k) database is essential for assessing similarities and differences that will be critical in your submission.
The World Health Organization defines medical devices broadly, ranging from simple tools like tongue depressors to complex technology such as implants. The design and regulation of these devices are paramount, with the FDA categorizing them into classes based on potential risk. For classes one and two, a 510(k) clearance is sought, which requires showing substantial equivalence to a predicate device.
Understanding the expectations and recommendations outlined in FDA guidance documents is imperative for a successful 510(k) submission. These documents detail the necessary information and steps to ensure acceptance by the FDA. However, the challenge lies in compiling the required information within the set timeframe and ensuring it meets FDA standards.
In this landscape of ever-increasing regulatory demands, seeking expert assistance could prove invaluable. Industry professionals, well-versed in medical device regulations, can offer critical support and guidance throughout the entire 510(k) process. As quoted by industry experts, selecting the right partner for medical device design and regulatory consulting is essential to navigate this complex environment and increase the likelihood of a successful outcome.
Conclusion
The 510(k) Premarket Notification process is vital for medical device manufacturers entering the U.S. market. Thorough research and analysis are necessary to demonstrate substantial equivalence to an existing predicate device. Understanding FDA classification and utilizing their guidance documents are crucial.
Recent trends aim to streamline the regulatory pathway.
Identifying a suitable predicate device involves thorough analysis of the device landscape and constructing a comparative table. Collaboration with marketing teams is important for insights into the competitive environment. Proper classification guides the selection of the registration pathway.
Preparing the 510(k) submission documents requires understanding the subject device, adhering to FDA formatting guidelines, and collaboration. Compliance and open communication with the FDA are crucial. Responding to inquiries and addressing deficiencies is essential.
A successful submission entails meticulous attention to documentation, understanding the device, and constructing a comparative table. The FDA's acceptance and substantive reviews confirm compliance and safety. Post-clearance obligations ensure ongoing compliance and device safety.
Navigating the process requires understanding regulatory requirements and avoiding pitfalls like inadequate research and poor communication. Seeking expert help and regulatory consulting can provide valuable support.
In conclusion, a strategic approach combining research, collaboration, adherence to FDA guidelines, and expert assistance increases the likelihood of success. The 510(k) process ensures device safety while fostering innovation. Precise navigation leads to a smooth market entry and improved patient care.




