Introduction
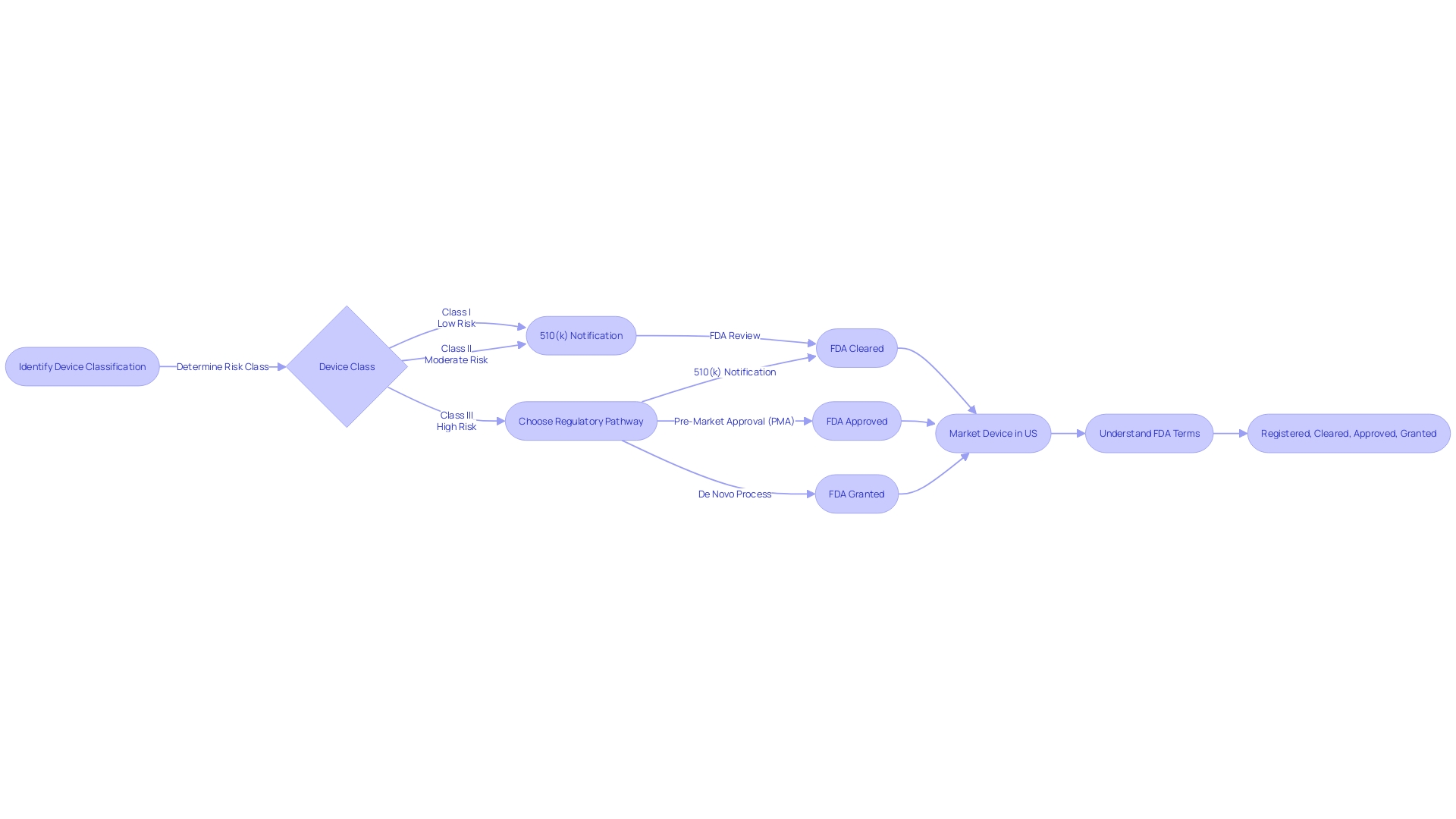
The process of bringing a medical device to market in the United States involves understanding the FDA's classification system, which categorizes devices based on the level of risk they pose to patients. There are three primary classifications: Class I (low risk), Class II (moderate risk), and Class III (high risk). To navigate the 510(k) approval process, it is crucial to accurately determine your device's classification early in the development process.
Educating oneself about the device, its users, and the competitive landscape is imperative. By conducting thorough research and comparing your device to similar, previously approved devices—known as predicate devices—you can create a comprehensive comparative table. It's important to comprehend the terminology used within the industry, such as 'Registered,' 'Cleared,' 'Approved,' and 'Granted.'
These terms signify different levels of FDA review and approval, and understanding the distinctions can guide you through the correct pathway, be it a 510(k) notification, a rigorous Pre-Market Approval (PMA), or the De Novo process for novel devices. Moreover, the medical technology market is witnessing a surge of growth, with the U.S. projected to generate substantial revenue in the sector. The market's expansion underscores the importance of not only getting a device to market but also ensuring it meets the highest standards of safety and effectiveness for patient care.
The FDA's commitment to facilitating access to innovative medical devices while maintaining rigorous safety controls is reflected in recent actions that classify certain devices into Class II with special controls, thereby balancing innovation with patient safety.
Understanding Medical Device Classes
The procedure of introducing a medical instrument to the market in the United States entails comprehending the FDA's classification scheme, which categorizes instruments based on the level of risk they pose to patients. There are three primary classifications: Class I (low risk), Class II (moderate risk), and Class III (high risk). To navigate the 510(k) approval process, it is crucial to accurately determine the classification of your product early in the development process. Class II products, for instance, are subject to special controls to guarantee safety and effectiveness, often requiring a 510(k) premarket notification.
Learning about the equipment, its users, and the competitive environment is crucial. By conducting extensive research and comparing your equipment to similar, previously approved devicesâknown as predicate devicesâyou can create a comprehensive comparative table. This is a crucial stage in the 510(k) application, which shows that your product is at least as secure and efficient as an already available, lawfully advertised product.
It's important to comprehend the terminology used within the industry, such as 'Registered,' 'Cleared,' 'Approved,' and 'Granted.' These terms represent various stages of FDA assessment and endorsement, and comprehending the differences can lead you through the appropriate route, whether it's a 510(k) notification, a thorough Pre-Market Approval (PMA), or the De Novo process for innovative products.
Furthermore, the healthcare technology market is experiencing a surge of growth, with the U.S. expected to generate substantial revenue in the sector. The market's growth emphasizes the significance of not only bringing a product to the market but also ensuring it meets the highest standards of safety and effectiveness for patient care. The FDA's dedication to promoting the availability of cutting-edge healthcare instruments while upholding stringent safety measures is evident in recent measures that categorize specific instruments into Class II with specialized controls, thus striking a balance between advancement and patient well-being.
What is a 510(k) Submission?
The 510(k) premarket notification is an essential process for medical equipment manufacturers seeking to market their instruments in the United States. It involves submitting comprehensive documentation to the FDA to establish that the equipment is safe and effective for its intended use. This documentation encompasses a wide range of information, including details about the equipment's design, manufacturing, intended use, and comparisons to similar, legally marketed predicate devices. By creating a comprehensive comparative table, companies demonstrate that their product is as safe and effective as an existing item on the market. It's crucial to deeply understand the gadget's users, such as clinicians and patients, and carefully consider instructions for use, including warnings and cautions. This procedure is not just a regulatory requirement but also a thorough exercise in transparency and public safety. Feedback and data provided during this procedure are disclosed to the public, guaranteeing a transparent documentation of the equipment's reliability and efficiency. The 510(k) procedure represents an essential checkpoint in introducing healthcare instruments to the market, guaranteeing that they meet the rigorous criteria established by the FDA for safeguarding patient well-being.
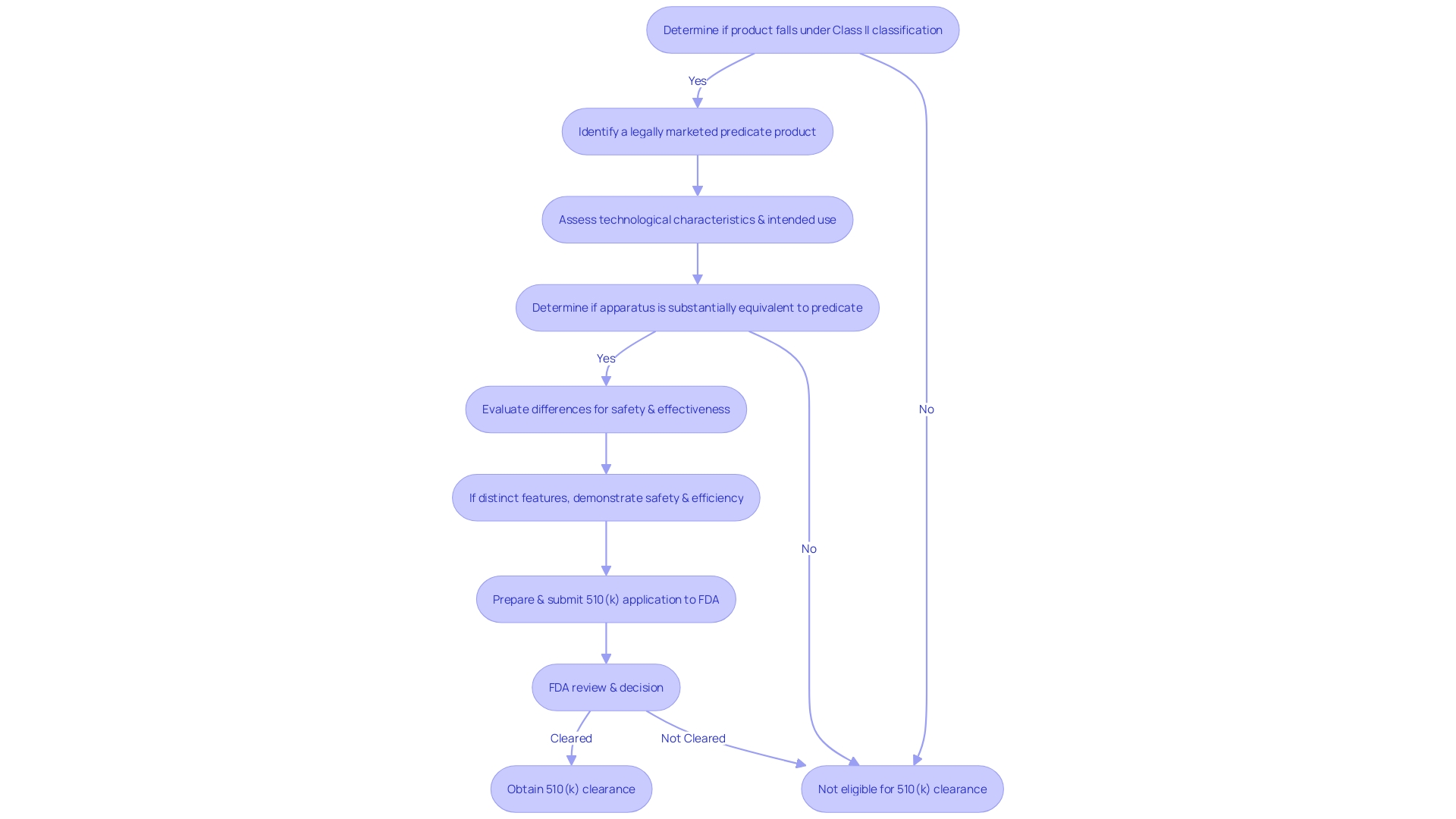
Eligibility Criteria for 510(k) Clearance
Obtaining 510(k) clearance is a vital stage for medical equipment created for the U.S. market. This process, overseen by the FDA, requires that an item be substantially equivalent to a legally marketed product that is not subject to Premarket Approval (PMA). Substantial equivalence means that the new apparatus must have the same intended use as the predicate and that any differences in technological characteristics do not raise new questions of safety and effectiveness. Moreover, if the apparatus possesses distinct technological features, it must be shown that it is equally safe and efficient compared to the reference apparatus.
For instance, the Impella Connect System, comprising both hardware and software components, was meticulously assessed for its technological characteristics and intended use to ensure compliance with 510(k) requirements. The system's capability to offer remote monitoring and crucial alarm notifications exemplifies the technological advancements discussed during the 510(k) evaluation.
Moreover, the market for surgical sutures, like surgical stitches, is expanding considerably. As reported by GlobalData, this market is projected to grow annually by 3.4%, reaching a value of $4.5 billion by 2033. With such growth, the importance of understanding and navigating the 510(k) process becomes even more paramount.
The FDA categorizes healthcare equipment into three classifications according to the level of regulation required to ensure the safety and efficacy of the equipment. 'Class I products are subject to the least regulatory control and Class III products the most.'. Devices that require 510(k) clearance typically fall under Class II, where general controls alone are insufficient to assure safety and effectiveness.
It's crucial for professionals in the medical equipment industry to understand the various FDA terms such as 'Registered,' 'Cleared,' 'Approved,' and 'Granted,' and their respective implications for marketing a medical product. For instance, a 'Limited equipment' refers to one for which sales, distribution, or usage is restricted by the FDA, emphasizing the different layers of regulatory oversight that may apply to a tool.
In summary, obtaining 510(k) clearance involves demonstrating substantial equivalence to a predicate product, understanding the product's classification, and selecting the appropriate registration pathway. This procedure guarantees that items used in healthcare are secure and capable of delivering the intended therapeutic or diagnostic impact while maintaining patient safety.
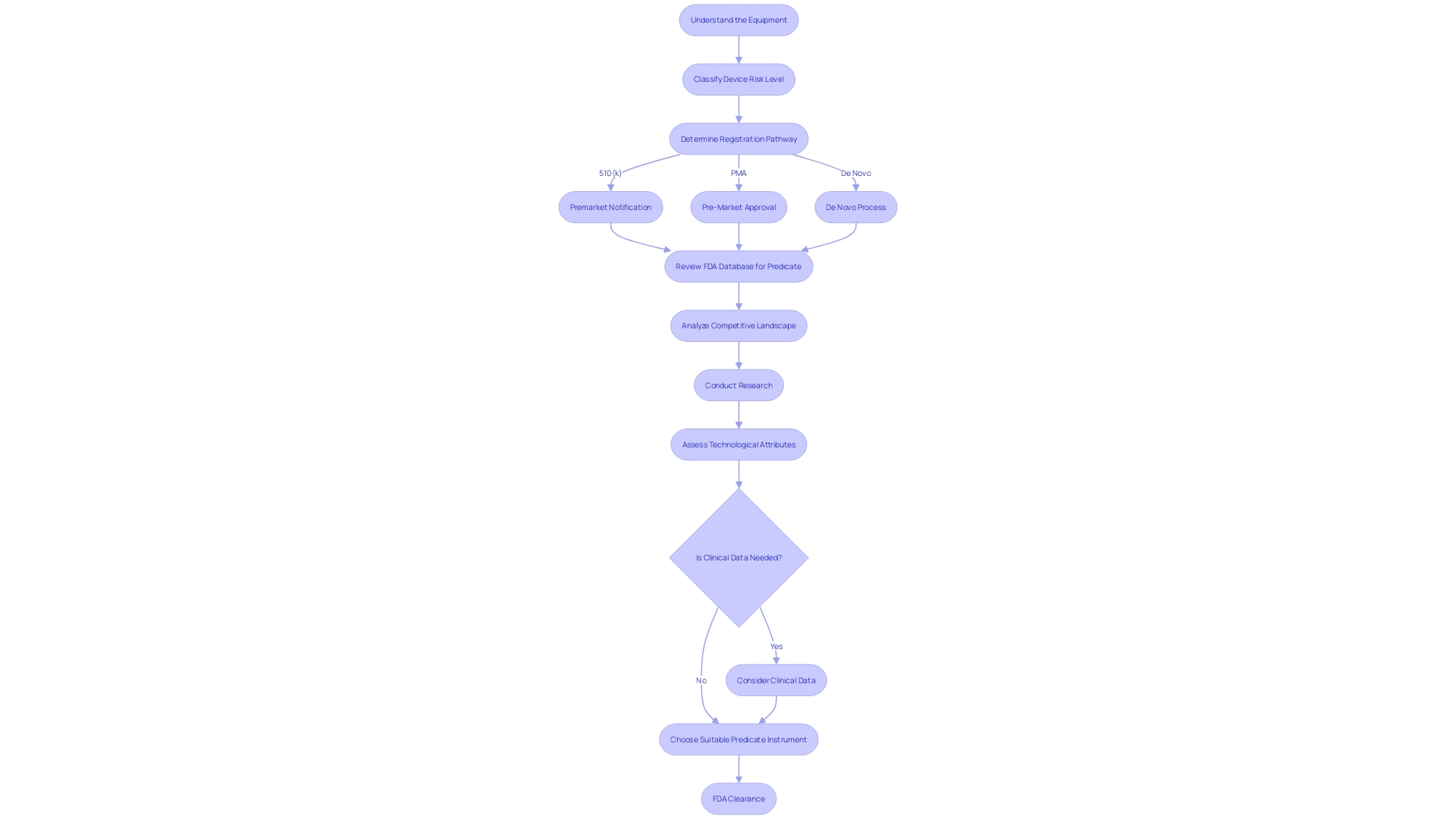
Finding a Predicate Device
Choosing the appropriate predicate instrument is a fundamental aspect of the 510(k) clearance pathway for medical devices. To guarantee a precise comparison, it is crucial to have a thorough understanding of the equipment in question, including its intended users—whether they are clinicians, physicians, dentists, or patients—and the subtleties of its usage, along with any warnings and precautions. Working together with the marketing team to comprehend the competitive landscape can uncover insights into potential predicate objects that have the same purpose and technological characteristics.
By scrutinizing research literature, clinical studies, and marketing materials such as websites, brochures, and labels, one can begin to assemble a comparative table. This table should concentrate on identifying gadgets that are not only technically similar but also share the same intended use as the new gadget. Further investigation into the FDA's database to review Summaries of Safety and Effectiveness can shed light on the similarities and differences that are critical in establishing a suitable predicate.
The FDA's modernization efforts, including a draft guidance released on September 7, 2023, emphasize the importance of choosing predicates that are legally marketed and currently registered with the FDA. The chosen predicate should be assessed for technological attributes that align with the new equipment without raising new safety or effectiveness concerns.
The agency's approach to advancing the efficiency of the 510(k) review process has highlighted the value of using older predicates due to their long-term safety data. Nevertheless, the FDA also suggests choosing ancestors cleared using well-established methods such as FDA-recognized consensus standards, guidance documents, or qualified tools for the development of health-related equipment.
In the evolving landscape of medical equipment approval, the FDA has outlined scenarios in recent guidance where clinical data may be necessary to affirm substantial equivalence. These scenarios involve variations in indications for use, technological characteristics, non-determinable substantial equivalence through non-clinical testing, and new or increased risks identified for the predicate product. These criteria help ensure that the selected predicate instrument is the most suitable point of reference for the new apparatus seeking clearance, maintaining the FDA's dedication to protecting public health by ensuring safety and effectiveness.
Building a Quality Management System (QMS)
Implementing a strong Quality Management System (QMS) is not only a regulatory obligation, but a fundamental aspect of guaranteeing medical products fulfill the utmost criteria of safety and effectiveness. With the emergence of digital technologies, the incorporation of a digital QMS can align existing systems and technologies, optimizing workflows and enhancing product quality.
To ensure a smooth digital transition, manufacturers should start by clearly defining their objectives and setting realistic goals that align with their business vision. A phased roadmap and implementation plan, which incorporates lessons from earlier stages of digital adoption, can lay a strong foundation for future advancements. It's not just about embracing the latest technologies like AI, but choosing solutions that truly resonate with the company's specific needs and contribute to long-term success.
Medical product manufacturers face unique challenges when adopting digital quality, such as resistance to change and a workforce that may lack necessary technology proficiency. Addressing these issues head-on with a comprehensive Change Management Plan and frequent communication can facilitate better adoption. Investing in employee training, including self-paced and formal education, ensures that the entire team is proficient with the new digital tools.
It's important to consider the regulatory environment when establishing a QMS. For example, the FDA's Title 21 Code of Federal Regulations Part 820 outlines the Quality System Regulation (QSR), which requires a traceable and auditable system, incorporating current Good Manufacturing Practices (cGMP). These regulations provide guidance to manufacturers on techniques, facilities, and controls employed in the creation to implementation of healthcare equipment, emphasizing the significance of validation of procedures.
As Eliot Dratch, a seasoned quality consultant, has suggested, the aim is to measurably improve productivity, safety, and profitability. His insights underscore the value of a QMS in supporting U.S. manufacturing excellence. Moreover, recent advancements in Michigan emphasize the sector's expansion and the requirement for establishments that can adjust examination techniques to the changing requirements of manufacturers, while minimizing impurities and guaranteeing adherence to regulatory guidelines.
In the end, a QMS is a collection of procedures and responsibilities customized to each organization, aimed at enhancing consumer satisfaction, promoting a company's growth, and improving performance. The importance of a QMS cannot be overstated—it underpins brand reputation and is instrumental in an organization's journey to excellence.
Device Testing and Validation
To guarantee the safety and efficacy of medical equipment, the FDA mandates a comprehensive validation process that includes multiple testing phases. This includes bench testing, in which the equipment is tested in a laboratory setting to assess its basic functionality and durability. Animal testing may also be conducted to evaluate the biocompatibility and potential systemic effects before reaching clinical trials with human subjects. The clinical trials are the critical final step to establish the safety and performance of the instrument in clinical settings.
Validation studies are a necessary component of this procedure to verify that the device will consistently generate the anticipated outcomes and adhere to all quality criteria. This is particularly important when introducing new product lines or when changes are made to existing products or procedures. It's not just about the product itself but also about the systems used in manufacturing, such as software for barcode label design and printing. Each element of the process, from installation to operation, must be documented and tested to ensure it meets stringent regulatory requirements.
These steps are vital because, ultimately, they impact patient safety. For example, inaccurate test results from laboratory tests could have life-threatening consequences for patients. Hence, the FDA has a strong interest in ensuring that the instruments used in healthcare are thoroughly assessed and validated. This includes both the equipment and any associated software, which must be re-validated following any changes to ensure it continues to perform as expected and does not introduce new risks.
Staying up to date with the swift progress of technology in the field of healthcare equipment, facilities such as UL Solutions' laboratory in Rochester Hills, Michigan, are enhancing their capacities for testing medical devices. They can adapt to various manufacturers' methods and specifications, which is essential for addressing potential risks such as quality, safety, and cybersecurity, while also fostering innovation and protecting end-users.
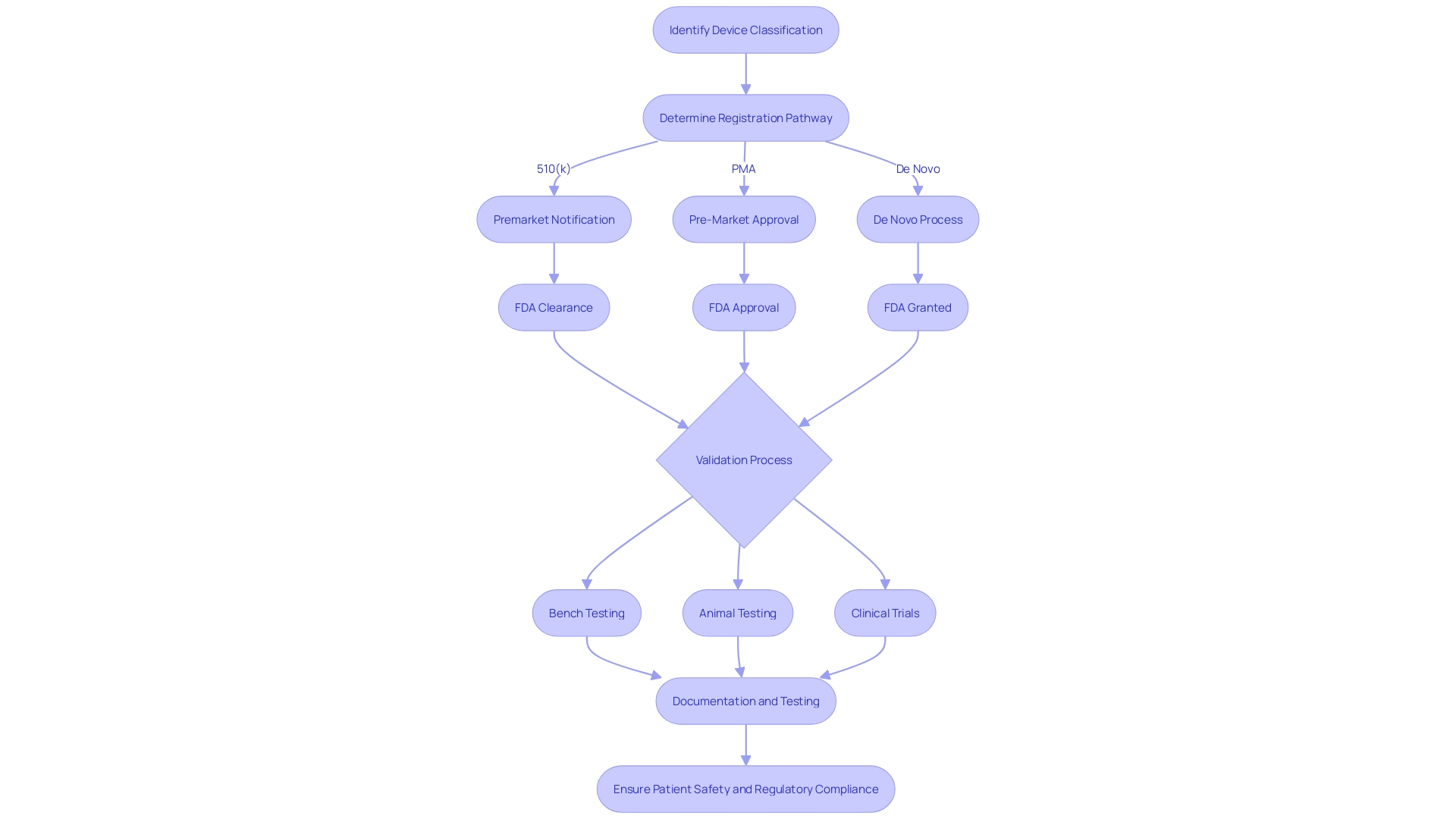
Preparing the 510(k) Submission
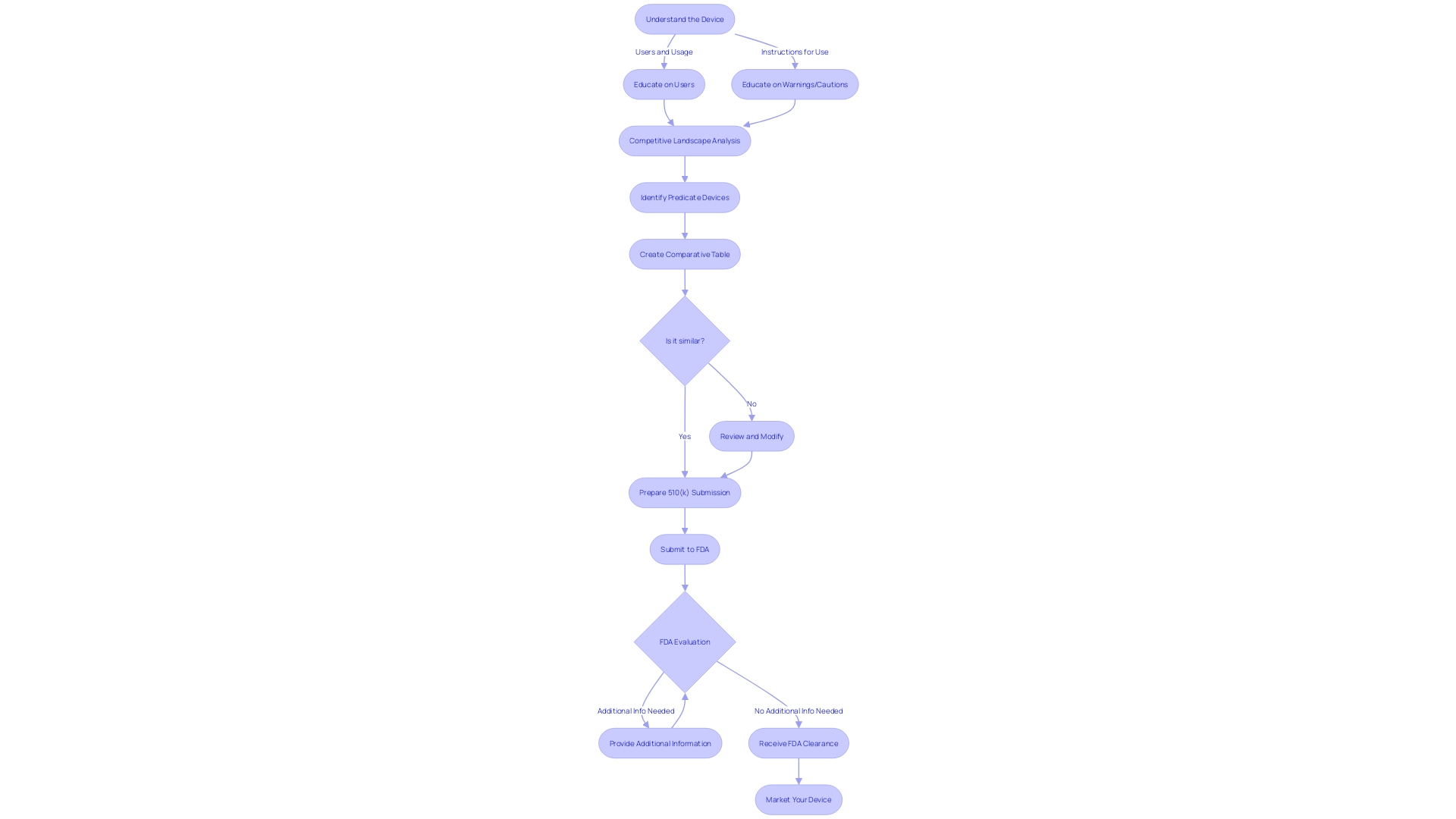
Developing a 510(k) submission is a meticulous process that requires a comprehensive understanding of the medical equipment, its intended use, and the regulatory landscape. To start, it is essential to fully engage in the instrument's particulars and the setting in which it will be utilized. This involves comprehending the needs of users such as clinicians, physicians, dentists, and patients, and digesting the instructions for use, which includes any warnings and cautions. It's also recommended to work together with marketing teams to obtain insights into the competitive environment and identify potential predicate products with equivalent intended uses and technological characteristics.
The next step is to construct a comparative table informed by a variety of sources including research literature, clinical studies, and marketing materials to clearly delineate the similarities and differences between your device and the identified predicate. This table is a cornerstone of the submission, as it supports the claim of substantial equivalence, which the FDA requires for a 510(k) clearance.
Understanding the extensive nature of regulatory submissions is also vital. They encapsulate detailed information across various facets of the company, including experiment reports and meeting notes. To prevent unnecessary delays in the submission, it is crucial to ensure that this information is well-organized and formatted in accordance with FDA guidelines.
Moreover, it is imperative to be mindful of the confidentiality of the information submitted. Any public comments or documents attached to the submission will be accessible to the public unless specifically submitted as confidential paper submissions, as outlined by the FDA. This includes personal, medical, and sensitive business information which could have implications if disclosed.
With the recent scrutiny of the 510(k) clearance process, as highlighted by the 2018 documentary 'The Bleeding Edge', the onus is on the submitter to provide a robust and comprehensive dossier that meets the FDA's expectations and ensures patient safety. Although clinical trials may not be required for all products under the 510(k) pathway, the submission must still show that the item is as safe and effective as the predicate.
To summarize, preparing a 510(k) submission involves more than just a writing exercise; it's a strategic compilation of pertinent, well-researched information that conforms to regulatory standards and ultimately supports the secure and effective utilization of healthcare instruments.
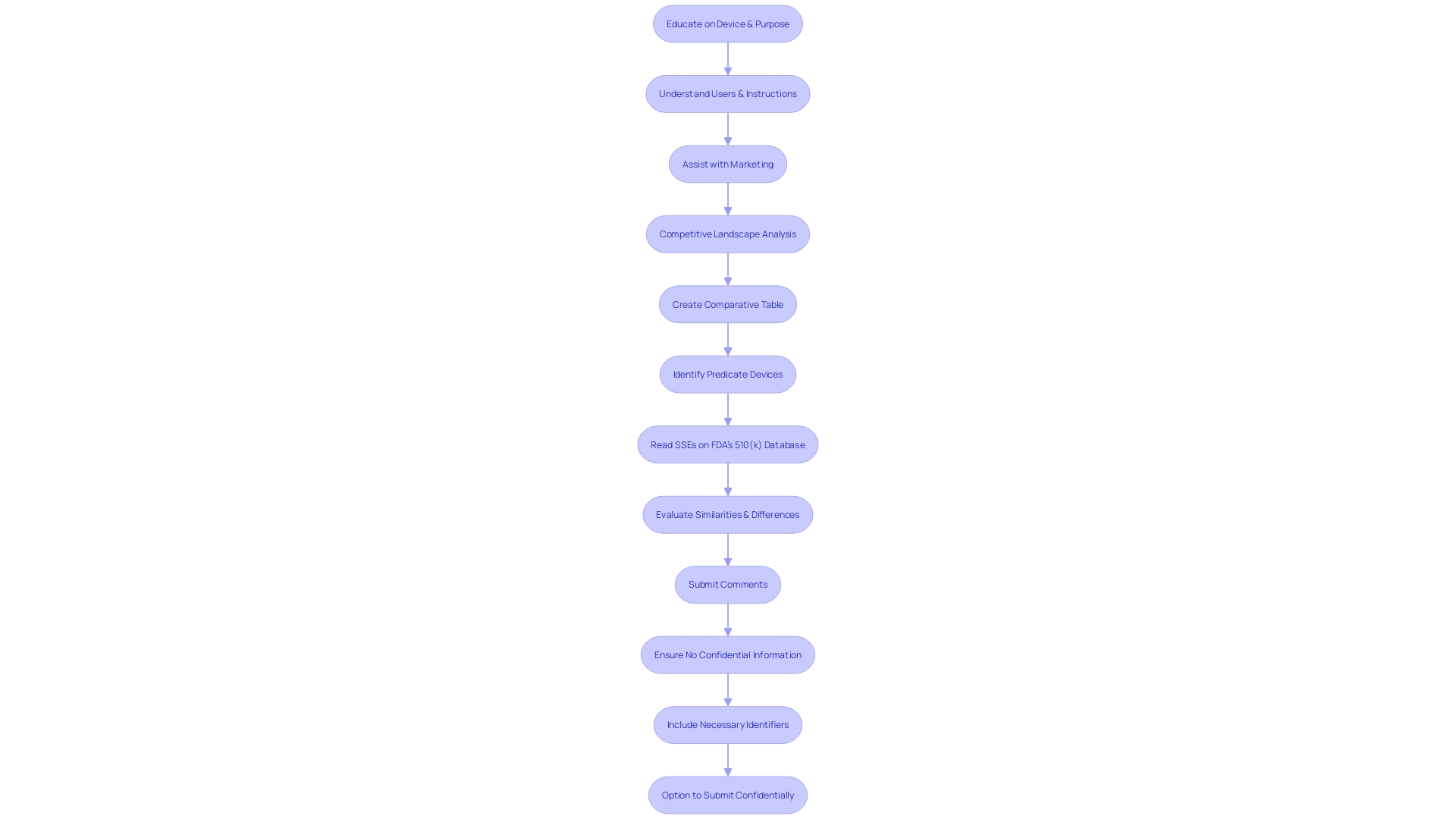
Types of 510(k) Submissions
The 510(k) submission process is a crucial pathway for introducing medical instruments to the U.S. market, particularly for those instruments that do not necessitate the more rigorous Pre-Market Approval (PMA). Understanding the nuances between the different types of 510(k) submissions can be pivotal for manufacturers. The Traditional 510(k) is the standard route, typically utilized when a new apparatus is substantially equivalent to a predicate instrument already on the market. The Abbreviated 510(k) relies on guidance documents or special controls, with the Special 510(k) permitting modifications to an apparatus with an existing clearance without impacting its safety and effectiveness.
Determining the appropriate submission type is not just a regulatory formality but a strategic decision. It requires a comprehensive understanding of the equipment, its intended use, and how it compares to existing products. It's not unusual to dive deep into research literature, clinical studies, and competitive analysis to identify appropriate precursor gadgets. Creating a comparative table highlighting similarities and differences with predicate devices—and reviewing the Summaries of Safety and Effectiveness Data (SeEDs) on the FDA's database—can provide the necessary insights for a successful submission.
As an example, the documentary 'The Bleeding Edge' brought attention to possible drawbacks when gadgets are expedited through the 510(k) procedure, occasionally resulting in patient damage because of the absence of mandatory clinical trials. This underscores the importance of rigorous comparative analysis and adherence to FDA regulations to ensure both compliance and patient safety.
In the rapidly evolving healthcare landscape, where technology and private practice management are becoming increasingly intertwined, the ability to efficiently navigate the 510(k) submission types can offer a competitive edge. As independent practices strive to maintain relevance against larger healthcare entities, comprehending regulatory pathways can ensure that innovative healthcare tools reach the market effectively and safely, thereby enhancing patient care.
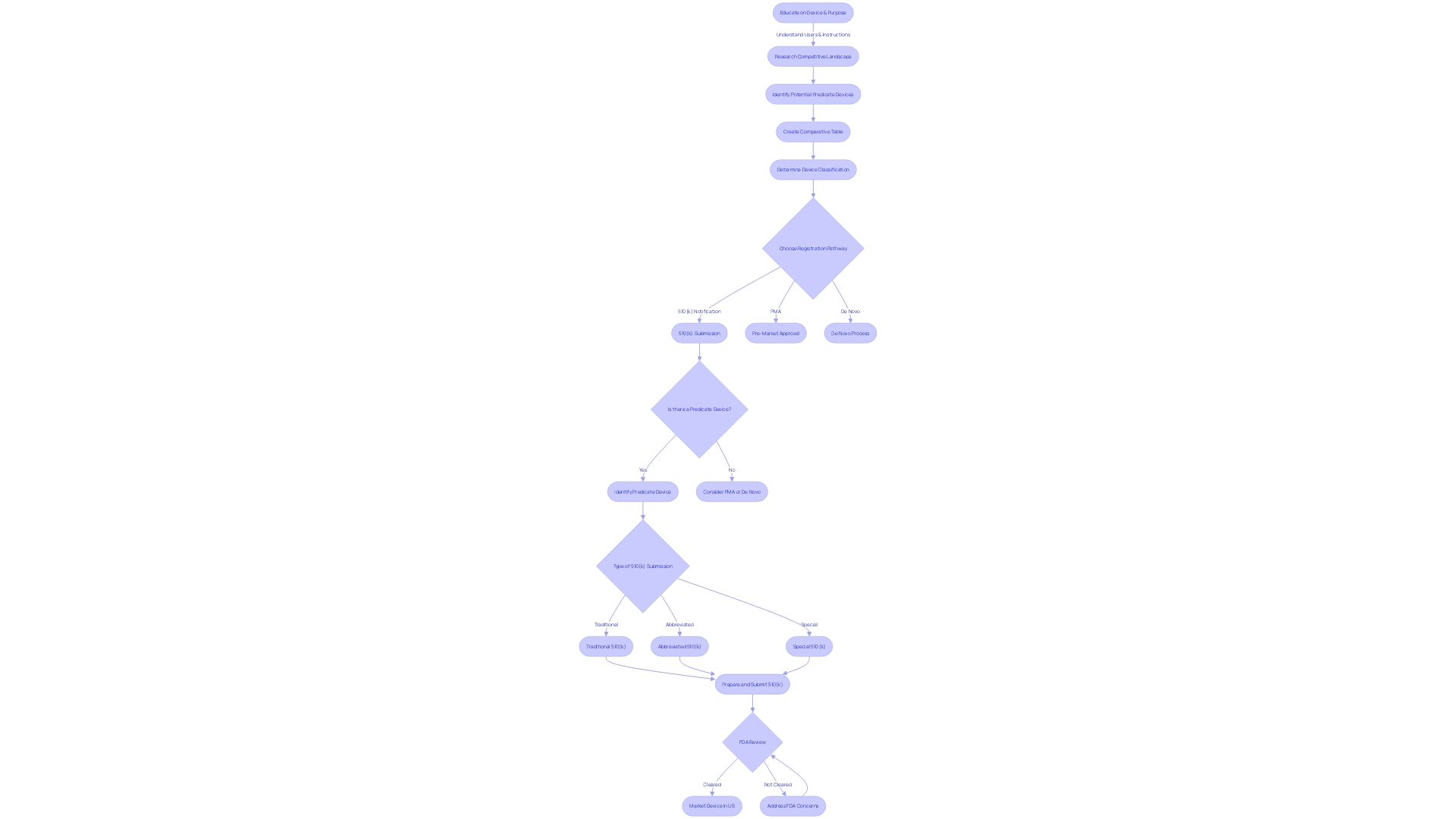
Submission Process and Timeline
The 510(k) submission process is a critical pathway for medical instrument approval in the United States, and understanding the intricacies of this process is paramount for regulatory success. At first, it's important to completely familiarize oneself with the equipment in question, including its intended use, user base, and any associated risks or cautions. This extensive comprehension acts as the basis for identifying appropriate predicate instruments that have comparable traits and intended applications, which is a fundamental aspect of the 510(k) submission.
Once you have identified a predicate product, the next step involves determining the correct classification of your medical equipment, as outlined by the FDA. Devices are classified into three categories based on the risk they pose to patients, with Class I representing the lowest risk and Class III the highest. This classification determines the regulatory pathway to be followed, with Class I and II products typically going through the 510(k) procedure, which involves a comparison to an already cleared predicate item. On the other hand, Class III devices, like life-sustaining implantables, go through a more rigorous evaluation procedure because of their elevated risk profile.
Moreover, the regulatory landscape is not static. Recent initiatives have aimed to simplify the approval procedure, especially in light of the COVID-19 pandemic, which has emphasized the necessity for swift implementation of healthcare advancements. These efforts are geared towards enhancing the collaboration between the industry and regulatory agencies, thereby expediting the delivery of medical devices that cater to unmet medical needs, especially in burgeoning fields like digital health and personalized medicine.
The timeline for the FDA's decision-making can be influenced by different factors, including the complexity of the apparatus, the nature of the health condition it targets, and the effectiveness of the regulatory agency. It is crucial for industry stakeholders to stay aware of these factors and the changing regulatory landscape, as they navigate the approval routes for their healthcare equipment.
Given these factors, companies must also stay informed about potential risks and uncertainties that may affect the approval procedure, as mentioned in forward-looking statements from industry entities. These statements highlight the inherent difficulties and unpredictability of introducing a healthcare instrument to the market, stressing the importance of comprehensive preparation and comprehension of the regulatory structure.

Common Challenges and Tips for Success
Navigating the 510(k) process for approval involves a thorough understanding of the intended use and technological characteristics. A crucial step is to identify an appropriate predicate instrument with the same intended use and similar technological features. This involves analyzing research literature, clinical studies, and competitor information to create a comparative table which can be a linchpin for a 510(k) submission. The Food and Drug Administration (FDA) categorizes healthcare instruments into three risk categories, with Class III instruments being the highest risk and subject to the most stringent regulatory controls. Although comprising only approximately 10% of FDA-regulated equipment, these items, like life-preserving implants, go through a more demanding authorization procedure. Recent initiatives to streamline regulatory pathways, especially during the COVID-19 pandemic, emphasize the significance of cooperation between regulatory agencies and industry stakeholders to accelerate the approval of products that fulfill essential healthcare requirements. The time to regulatory approval varies greatly depending on the complexity of the apparatus, the health condition addressed, and the efficiency of the regulatory process. In the US, the FDA oversees regulation of healthcare equipment, while in Europe, devices are regulated at the Member State level with involvement from the European Medicines Agency (EMA). Understanding these nuances and working closely with contract manufacturers to bridge the gap between design and mass production are essential for a successful 510(k) application and eventual market entry.
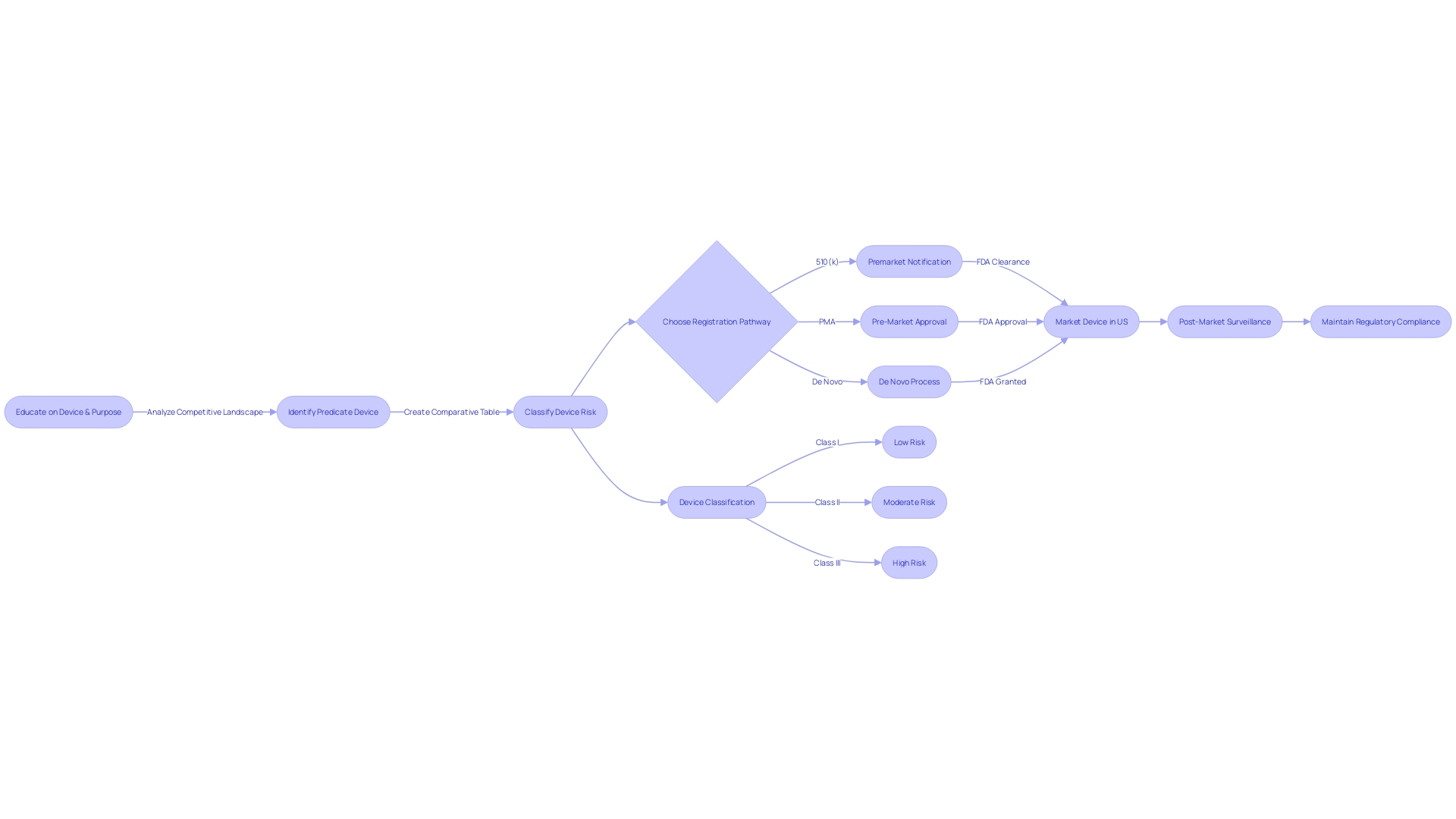
Post-Submission: Regulatory Compliance and Device Listing
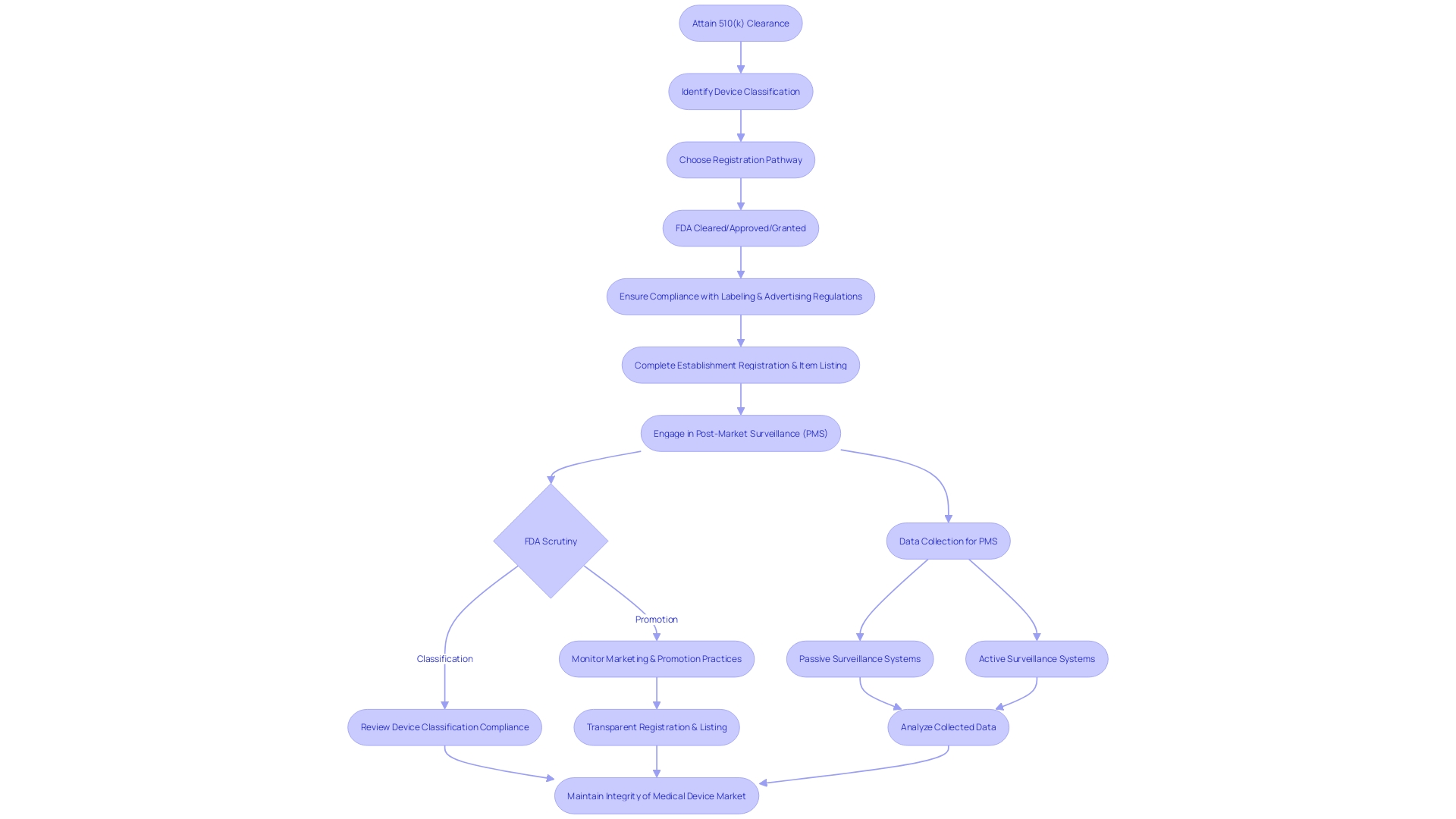
Navigating the post-clearance landscape following the attainment of 510(k) clearance involves several crucial steps to ensure adherence to the Food and Drug Administration's (FDA) regulatory framework. This includes ensuring compliance with specific labeling and advertising regulations, completing establishment registration and item listing, as well as engaging in diligent post-market surveillance (PMS) of the healthcare apparatus.
Firstly, the classification and promotion of medical equipment are subject to FDA scrutiny. Manufacturers are obligated to supply a representative sampling of advertisements and any other labeling materials, which must accurately reflect the promotional claims made for the product. This is to prevent misleading information that could compromise patient safety. Furthermore, any substantial alteration in labeling or advertisements that could impact the identity, safety, or effectiveness of the product must adhere to established FDA guidelines.
Registration and listing of establishment is another crucial post-clearance necessity. As a component of this procedure, the producer or first importer must yearly enter information into the FDA database, outlining the apparatuses and the actions conducted on those apparatuses at their establishment. This ensures a transparent chain of production and distribution, which is essential for maintaining public safety and trust.
Moreover, post-market surveillance is a pivotal aspect of post-clearance activities. The objective of PMS is to consistently oversee the safety and efficiency of healthcare instruments once they are available for use. This phase involves various data collection methods, including passive surveillance systems and active surveillance through registries or studies. Such vigilance is vital for identifying potential safety issues that may not have been apparent during pre-market testing.
The management of healthcare technology, including post-clearance activities, has developed over time, presenting challenges and emphasizing the significance of ethical, legal, and social considerations. As shown in case studies and the documentary 'The Bleeding Edge,' certain products have been expedited without the requirement for clinical trials, resulting in serious patient injuries. These incidents underscore the significance of rigorous post-market surveillance (PMS) and the FDA's commitment to protecting public health, as emphasized by FDA officials who assert that the distribution of unapproved healthcare equipment is a risk to patient safety.
In summary, the post-510(k) clearance phase is not merely a procedural formality but a comprehensive set of activities aimed at safeguarding the wellbeing of device users and maintaining the integrity of the medical device market.
Conclusion
In conclusion, successfully navigating the 510(k) approval process for medical devices in the United States requires a thorough understanding of the FDA's classification system, the device's level of risk, and the regulatory landscape. It is crucial to educate oneself about the device, its users, and the competitive landscape to accurately determine the device's classification and select the appropriate regulatory pathway.
Creating a comprehensive comparative table by comparing the device to predicate devices is a critical step in the 510(k) submission process. Understanding industry terminology, such as 'Registered,' 'Cleared,' 'Approved,' and 'Granted,' is essential for navigating the FDA review and approval process.
Building a robust Quality Management System (QMS) is vital for meeting the highest standards of safety and effectiveness. The integration of a digital QMS can streamline processes and elevate product quality. Device testing and validation are essential to establish the device's safety and performance, ensuring compliance with regulatory requirements.
Preparing a 510(k) submission involves strategically compiling relevant information that aligns with regulatory standards and supports the safe and effective use of medical devices. Choosing the appropriate submission type, understanding the submission process and timeline, and addressing common challenges are crucial for regulatory success.
Compliance with labeling and advertising regulations, establishment registration and device listing, and diligent post-market surveillance are vital in the post-clearance phase to ensure adherence to FDA regulations and maintain patient safety.
By following the appropriate steps and meeting the necessary requirements, medical device manufacturers can bring their products to market while ensuring safety and effectiveness for patient care.




