Introduction
Navigating the path to drug approval in the United States can be a complex process, especially for pharmaceutical companies aiming to introduce generic drugs to the market. The Abbreviated New Drug Application (ANDA) offers a streamlined route for generic drug approval by the FDA, differentiating from the New Drug Application (NDA) required for brand-name drugs. The ANDA process is critical for generics, as it ensures that these medications are safe and effective without requiring the same level of clinical data needed for an NDA.
In this article, we will explore the key differences between ANDAs and NDAs, the rigorous ANDA review process, the requirements for ANDA submissions, the significance of bioequivalence studies, common challenges and best practices in ANDA submissions, the electronic submission and review process, and the importance of post-approval changes and continuous monitoring. By understanding these aspects, pharmaceutical companies can navigate the ANDA process effectively and contribute to the availability of more affordable generic drugs for patients in need.
Understanding the Abbreviated NDA Process
Finding the way to gain approval for medications in the United States can be a complicated journey, particularly for pharmaceutical organizations aiming to introduce non-branded medications to the market. The Abbreviated New Drug Application (ANDA) provides an efficient pathway for approval by the FDA for non-brand drugs, distinguishing it from the New Drug Application (NDA) necessary for brand-name medications. The ANDA process is crucial for non-brand medications, as it guarantees that these drugs are safe and effective without needing the same amount of clinical data required for an NDA.
The FDA's thorough evaluation involves examining whether a non-branded medication can be deemed comparable to a brand-name medication that has already received approval. This involves verifying bioavailability, ensuring the active ingredients are the same, and that the generic performs in the same manner. For instance, the FDA recently confirmed that the medication products listed in the 'Discontinued Drug Product List' section of the Orange Book, which comprises medications removed from the market for reasons other than safety or effectiveness, can still be referenced in approved ANDAs. This decision highlights that previously approved ANDAs remain valid, and additional applications for these medications can also gain approval if they meet current legal and regulatory criteria.
In one case, the FDA reviewed the withdrawal of DUEXIS (ibuprofen and famotidine) tablets and, after a thorough investigation, found no safety or effectiveness concerns. This has resulted in the continuous listing of DUEXIS in the Orange Book, ensuring that the non-branded version remains available to consumers. The FDA continues to be watchful in safeguarding public health by ensuring the safety, effectiveness, and security of medications while also promoting the accessibility of more cost-effective non-brand pharmaceuticals.
As the industry continues to evolve with new health challenges and scientific advancements, the FDA adapts its regulatory practices. The draft guidance "Requests for Reconsideration at the Division Level Under GDUFA" reflects the latest Generic Drug User Fee Amendments and offers clarity on the reconsideration process for ANDA applicants. It is a demonstration of the FDA's dedication to open and effective regulatory processes, which is crucial for the prompt delivery of non-branded medications to patients in need. Pharmaceutical companies must remain informed and compliant with these evolving guidelines to ensure their products reach the market effectively and responsibly.
Key Differences Between ANDAs and NDAs
Understanding the distinctions between Abbreviated New Drug Applications (ANDAs) and New Drug Applications (NDAs) is crucial for ensuring the availability of effective and safe medications. While AND are used for non-branded medications, which are essentially bioequivalent versions of already approved brand-name medications, NDAs are required for new brand-name medications seeking initial approval. Unlike NDAs, ANDAs are not required to provide new clinical data to demonstrate safety and efficacy, as they can refer to the existing data of the brand-name medication, greatly simplifying the approval process. This dependence on previously established safety and efficacy through bioequivalence studies, which may involve both in vitro and in vivo evaluations, enables a more streamlined entry of non-brand medications into the market. As a result, patients gain access to more affordable medications, and the healthcare system benefits from the increased availability of therapeutic options and potentially improved supply chain stability.
The ANDA Review Process
When evaluating an Abbreviated New Drug Application (ANDA), the FDA rigorously examines the medication without a brand name to ensure it upholds the same standards of quality, safety, and effectiveness as its brand-name counterpart. The examination encompasses an analysis of the active ingredient, dosage form, strength, route of administration, and labeling details. An essential component of the review is the bioequivalence studies, which must show that the non-branded medication performs identically to the brand-name medication in terms of absorption, distribution, metabolism, and excretion. Certainly, the function of the FDA goes beyond mere approval; it actively promotes the progress of non-brand pharmaceutical development and evaluation through its scientific research programs. These efforts include collaborating with international regulatory authorities to develop uniform guidelines that streamline non-branded medication development, such as the recent harmonized draft guidance for bioequivalence studies for immediate-release solid oral dosage forms. The commitment of the FDA to these initiatives is reflected in the Office of Generic Drugs 2023 Annual Report, which provides insight into the agency's achievements and its readiness to continue its vital work into the upcoming year.
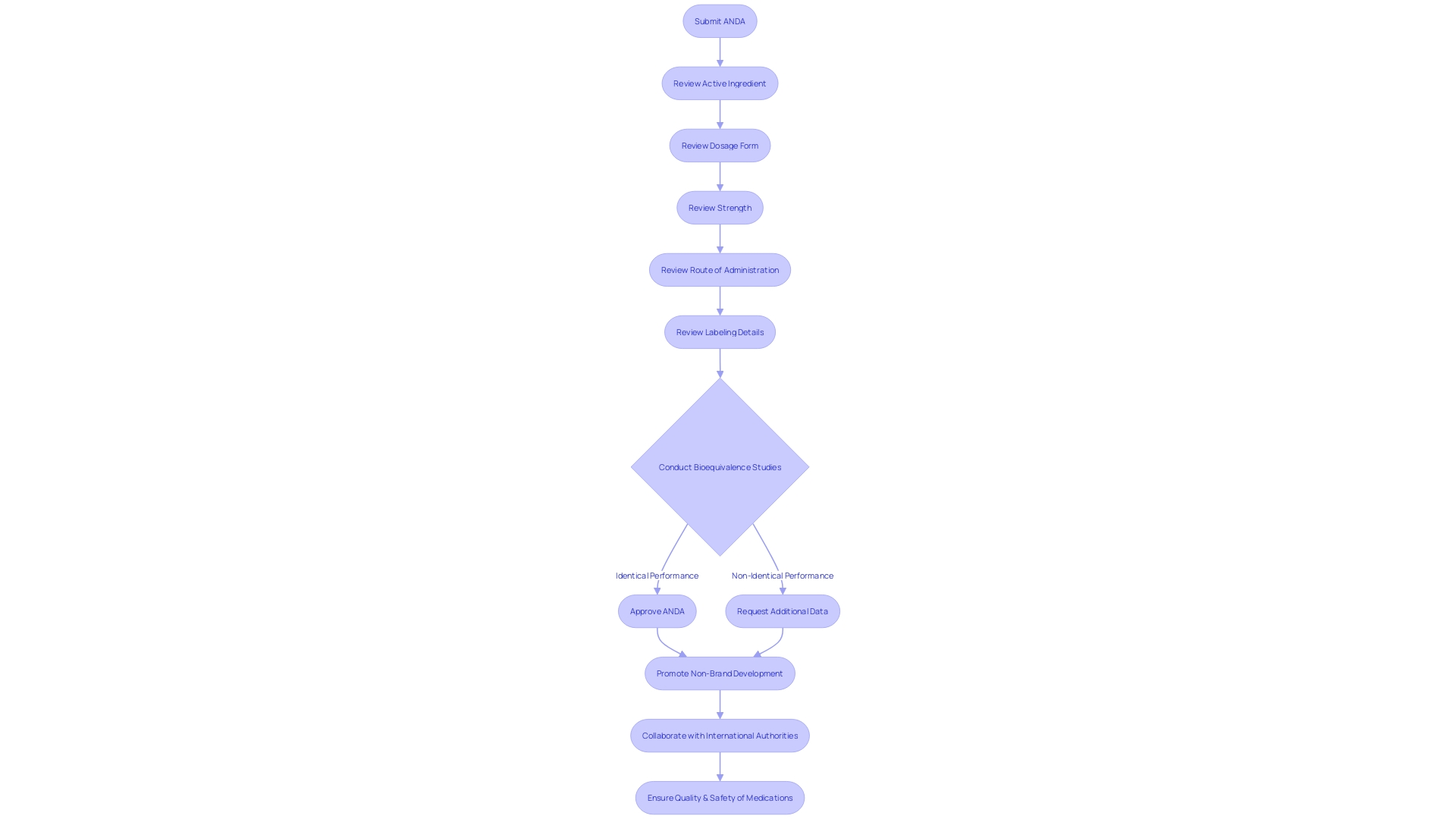
ANDA Submission Requirements
Filing an Abbreviated New Drug Application (ANDA) is a thorough procedure that requires a pharmaceutical company to provide detailed information about the non-branded medicine product. This includes the medication's composition, production procedures, labeling specifics, and intended application. Crucially, the ANDA submission must be supported by bioequivalence studies, which scrutinize the alternative medication against its branded counterpart, evaluating their absorption rates and extents to confirm bioequivalence.
Furthermore, the dossier should confirm the generic medication's pharmaceutical and therapeutic parity to the branded medication. As emphasized in recent FDA guidance, this comprises an extensive list of all components involved in the medication's manufacture, along with a declaration of the medication product's composition, component specifications, and manufacturer details. The guidance also stipulates the necessity of describing the manufacturing and packaging procedures, in-process controls, and the specifications required to guarantee the product's identity, strength, quality, purity, potency, and bioavailability. This is further supported by stability data and proposed expiration dating, with the possibility of using alternative components or manufacturing methods as specified.
The significance of this comprehensive examination is emphasized by the important function non-branded medications play in the U.S. healthcare system, as indicated in the Office of Non-branded Medications' 2023 Annual Report. This report celebrates the endorsement of non-brand medicines, which frequently brings in numerous producers, thus enhancing the distribution network and reducing medication deficiencies. In 2023, generic medications have been crucial in offering patients with more accessible medication choices, often at a lower cost than branded medications, supporting the FDA's mission to provide high-quality, safe, and effective generic medicines to the public.
Feedback from industry stakeholders, such as the International Pharmaceutical Aerosol Consortium on Regulation & Science (IPAC-RS), has further shaped the guidance on ANDA submissions. For instance, their call for clarity regarding the applicability of guidance to orally inhaled and nasal products (OINDPs) and the integration of connected 'smart' devices illuminates the evolving nature of medication delivery technologies and the need for guidance to address these specific considerations.
Applicants are encouraged to follow the instructions for submitting comments to ensure their input is considered without compromising confidential information. The procedure of public commentary and revision reflects the FDA's commitment to collaborative dialogue and continuous improvement of regulatory procedures for the benefit of public health.
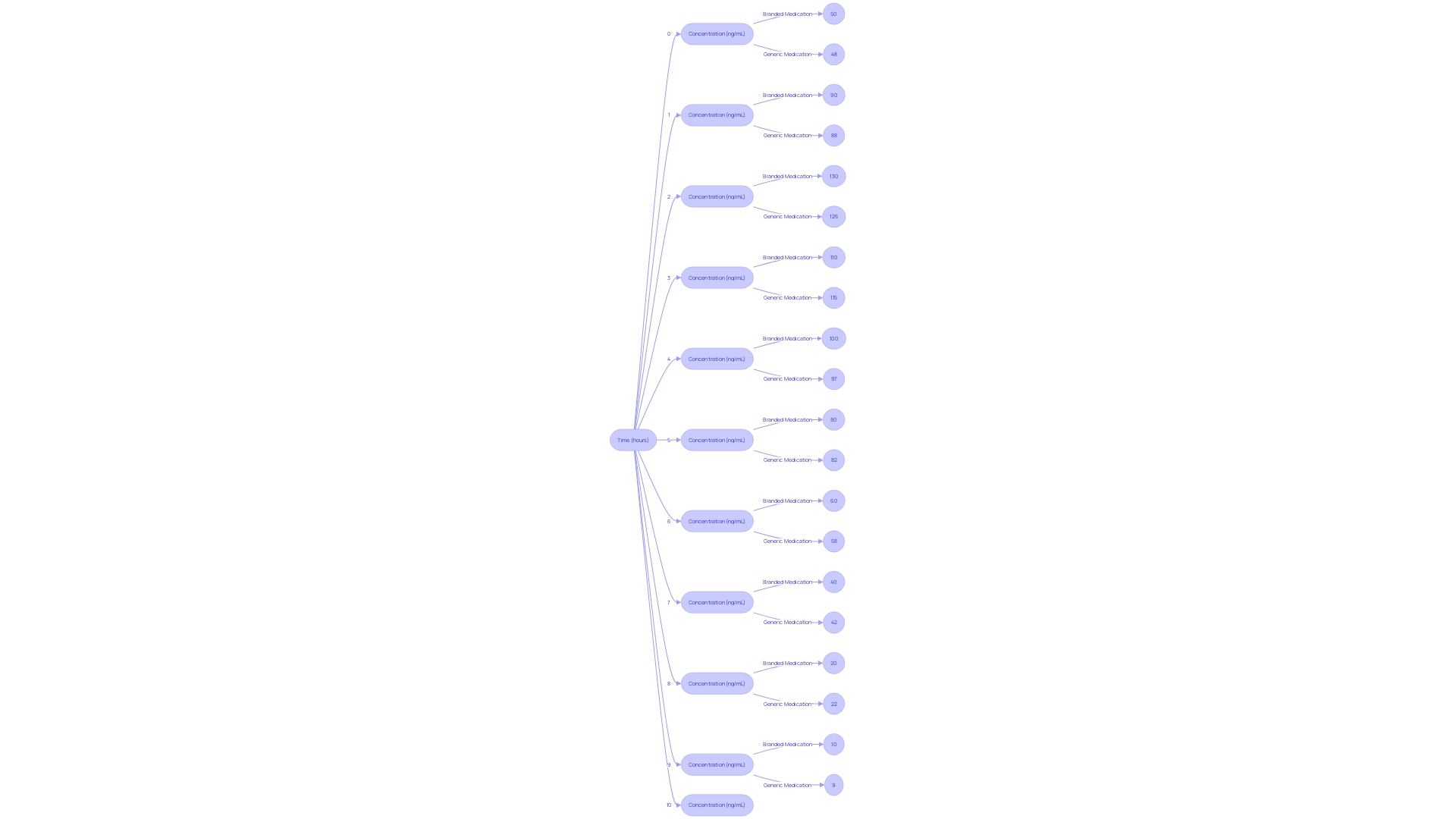
Bioequivalence Studies in ANDA Submissions
Ensuring the similarity of unbranded medications to their name-brand counterparts is a crucial procedure in the ANDA submissions. This entails thorough pharmacokinetic studies that examine how the non-brand medication is absorbed, distributed, metabolized, and ultimately excreted from the body, aligning these processes with those of the reference medication. The crucial measure in these bioequivalence studies is the concentration of the medication in the bloodstream over time. By analyzing this data, it's determined whether the product performs in the same manner as its branded equivalent, adhering to the stringent criteria set forth by the FDA. These standards are designed to guarantee that patients receive the same therapeutic benefits from generic drugs as they would from the original patented medications.
Common Challenges and Best Practices in ANDA Submissions
Pharmaceutical companies face several challenges when navigating the submission of ANDA, including strict regulatory requirements and the crucial need for data integrity and quality. The submission procedure demands careful attention to detail, particularly when addressing deficiencies highlighted by the FDA. Companies must adopt a strategic approach that encompasses maintaining precise documentation, conducting comprehensive quality control measures, and responding proactively to the FDA's queries and concerns. By adhering to these best practices, pharmaceutical entities can enhance the likelihood of a successful review and approval for their generic drug applications.
In the context of regulatory compliance, comments made public during the submission require careful consideration to avoid the disclosure of confidential information, such as proprietary manufacturing processes or personal data, to protect both the company and individuals involved. The FDA's initial guidance for 'Requests for Reconsideration at the Division Level Under GDUFA' provides a pathway for addressing concerns during the review. It's essential for industry professionals to be well-versed in these procedures and to submit comments in accordance with established guidelines to ensure that their feedback is considered effectively.
The use of artificial intelligence (AI) has been transforming various medical fields, including cardiology, by enhancing diagnostic accuracy and reducing the pressure on medical professionals. Likewise, the incorporation of AI and advanced analytics in the ANDA submission procedure could transform the manner in which pharmaceutical companies approach data analysis and regulatory submissions, potentially resulting in more efficient and accurate outcomes.
It is also crucial for pharmaceutical companies to remain informed about the evolving landscape of clinical trials, as these can influence the FDA's expectations for safety and efficacy data. Participation and innovation in clinical trials are vital for the continuous advancement of healthcare and the introduction of new treatments. As the industry advances, companies that stay ahead of these trends and utilize state-of-the-art technologies and methodologies are more likely to succeed in the competitive ANDA submission.
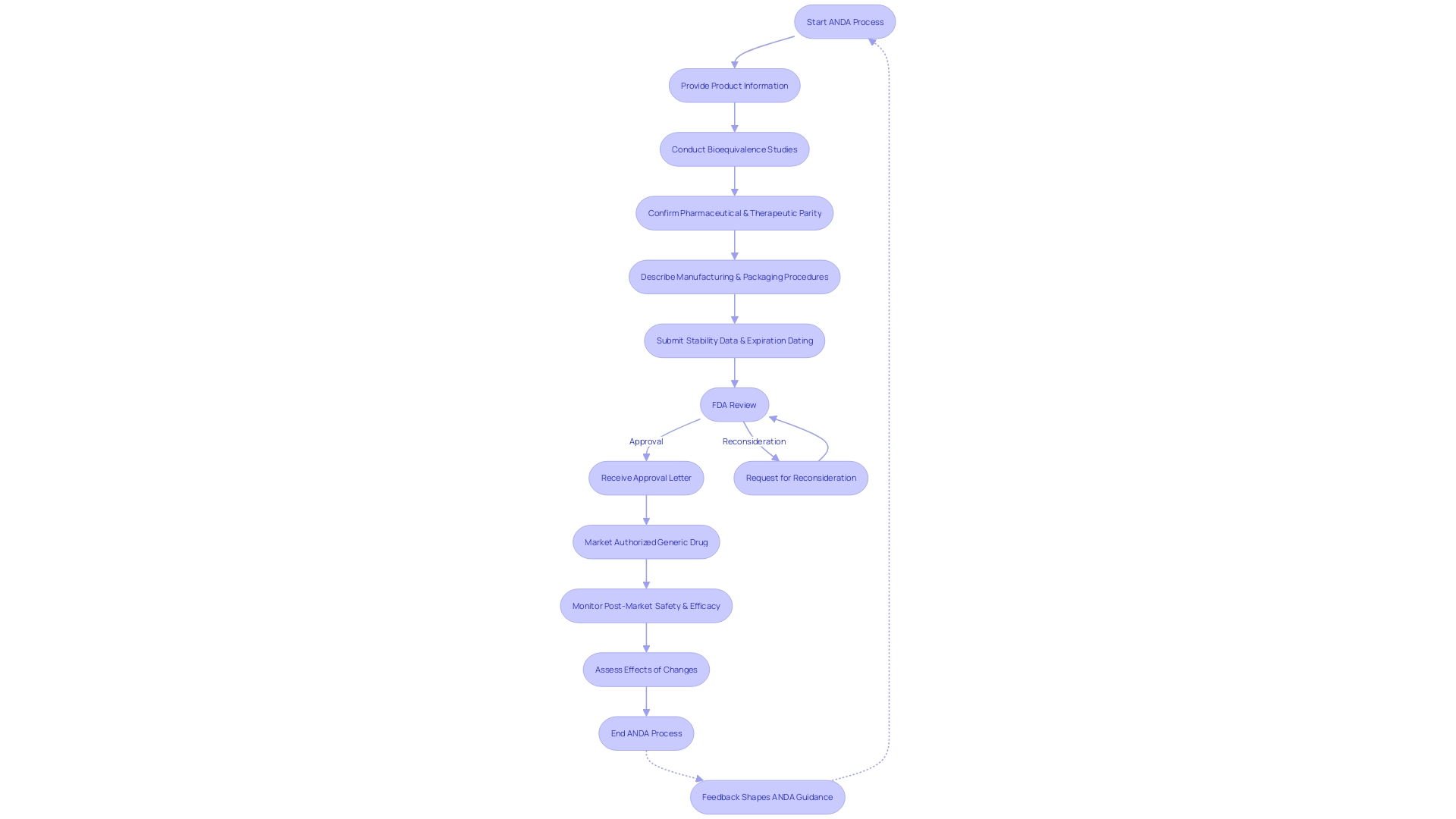
Electronic Submission and Review Process
To expedite the approval process for Abbreviated New Drug Applications (ANDAs), the FDA has championed the use of electronic submissions. This forward-thinking approach facilitates swifter processing of documents and enhances the sharing and analysis of data, fostering a more collaborative environment between regulatory authorities and pharmaceutical entities. The electronic management of ANDA reviews not only expedites the overall assessment period but also allows for more thorough evaluations. With such enhancements in place, the FDA is now better prepared to manage the complexities of ANDA evaluations, thereby expediting the time to market for non-branded medications. It is essential for applicants to adhere strictly to the submission guidelines, which include the careful handling of confidential information. Any public comments and electronic attachments become part of the public record, necessitating a high level of scrutiny to avoid inadvertent disclosures of sensitive details such as proprietary business information or personal identifiers. For those seeking to maintain the confidentiality of certain information, the FDA provides clear instructions for written or paper submissions. The urgency for efficient ANDA reviews is further underscored by the backlog of inspections, especially in overseas manufacturing facilities, a challenge that has been amplified by the COVID-19 pandemic. A recent analysis revealed that approximately 2,000 pharmaceutical manufacturers have not undergone FDA inspection since before the pandemic began, as reported by the Associated Press. This situation heightens the risks within the pharmaceutical industry and places increased emphasis on the quality and integrity of the electronic data submitted with ANDAs. Furthermore, the FDA consistently enhances medication labeling based on up-to-date information from diverse stakeholders, showcasing the dynamic character of medication oversight and emphasizing the significance of top-notch, prompt data submissions.
Post-Approval Changes and Continuous Monitoring
Upon approval of an Abbreviated New Drug Application (ANDA), pharmaceutical companies are tasked with a critical role in safeguarding public health: the continuous post-marketing vigilance for the generic medication they produce. This responsibility extends to the meticulous monitoring of the medication's performance in real-life situations, mirroring the post-market surveillance (PMS) of medical devices that is essential for identifying and addressing potential safety concerns. Companies must systematically collect and analyze data using methods such as spontaneous reporting systems and active surveillance studies, thereby ensuring that any variations in safety and quality are swiftly addressed.
To comply with regulatory standards, manufacturers must promptly report any alterations in their product's manufacturing process or labeling to the FDA. This could range from minor labeling changes, which can be documented in an annual report as advised in the FDA's draft guidance, to more substantial modifications that may require supplemental approval. The FDA underscores the importance of these updates, emphasizing that they are pivotal for consumers to have timely access to the most current information, thereby ensuring the safe and effective use of the drug.
Regular FDA inspections serve as a cornerstone of this compliance framework, affirming that pharmaceutical companies adhere to the stipulated guidelines. The FDA's overarching role is to maintain the safety, effectiveness, and security of medications. The agency's diligent oversight is a testament to its commitment to public health, as it also monitors a broad spectrum of products ranging from food and cosmetics to medical devices. By fulfilling these post-approval obligations, pharmaceutical companies play a crucial part in the FDA's lifecycle approach to product safety that begins with pre-market testing and extends to continuous post-market assessment.
Conclusion
In conclusion, navigating the Abbreviated New Drug Application (ANDA) process is crucial for pharmaceutical companies introducing generic drugs to the US market. ANDAs offer a streamlined route for FDA approval, ensuring the safety and effectiveness of these medications without requiring extensive clinical data. Understanding the key differences between ANDAs and NDAs is essential, as ANDAs are used for generics and can reference existing data.
The ANDA review process is rigorous, with the FDA examining the generic drug's quality, safety, and effectiveness. Bioequivalence studies play a pivotal role, demonstrating that the generic drug performs identically to the brand-name drug. The FDA fosters generic drug development through research programs and collaborations.
ANDA submissions require comprehensive information, including formulation, manufacturing protocols, labeling details, and intended usage. Bioequivalence studies confirm bioequivalence by comparing the generic drug to the branded counterpart. Thorough examination is important due to the significant role generic drugs play in the US healthcare system.
Navigating the ANDA submission process presents challenges, such as regulatory requirements and data integrity. Meticulous attention to detail, precise documentation, and proactive responses to FDA queries are best practices for success. The integration of AI and advanced analytics could revolutionize data analysis.
Electronic submission expedites the ANDA approval process, enhancing collaboration. Adhering to submission guidelines and handling confidential information carefully is essential. The backlog of inspections due to the pandemic underscores the importance of high-quality, timely data submissions.
Post-approval, continuous monitoring and prompt reporting of changes are critical. Pharmaceutical companies must monitor the drug's performance, collect and analyze data, and report any alterations to the FDA. Regular inspections ensure compliance with guidelines and maintain medication safety.
By understanding these aspects and following best practices, pharmaceutical companies can navigate the ANDA process effectively, contribute to the availability of affordable generic drugs, and ensure the safety and efficacy of medications for patients in need.




