Introduction
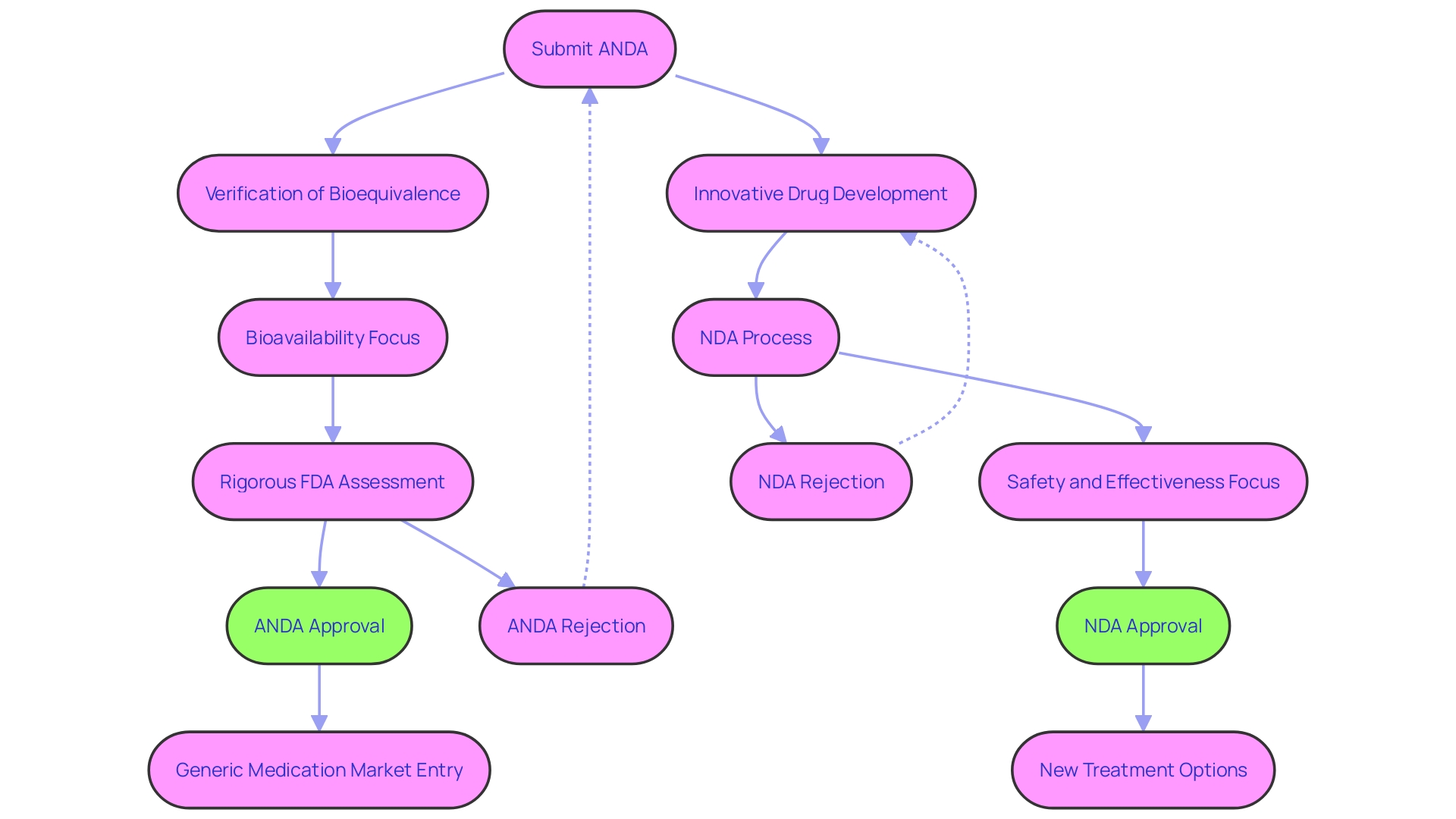
The Abbreviated New Drug Application (ANDA) is a crucial pathway for generic drugs to gain FDA approval. Unlike the New Drug Application (NDA) process for brand-name drugs, which requires extensive clinical trials, the focus of the ANDA process is on demonstrating bioequivalence to a previously approved reference listed drug (RLD). By delivering the same active ingredient at the same rate and extent as the RLD, generic drugs can provide safe and effective alternatives to their brand-name counterparts.
This article explores the key components of an ANDA submission package, the ANDA submission process, regulatory requirements and guidelines, changes to an approved ANDA, postmarketing reporting and compliance, facility fees and self-identification, labeling and advertising requirements, common deficiencies, best practices for avoiding them, and the role of regulatory consulting in ANDA approval. Navigating the complex landscape of FDA regulatory compliance requires a thorough understanding of these processes and adherence to guidelines to ensure the availability of cost-effective and high-quality generic drugs.
What is an Abbreviated New Drug Application (ANDA)?
An Abbreviated New Medication Application (ANDA) is a pathway for generic medications to gain FDA approval. Generic medications, as verified by the FDA's Center for Drug Evaluation and Research (CDER), must exhibit bioequivalence to a previously approved reference listed medication (RLD). This means the generic medication should deliver the same active ingredient at the same rate and extent as the RLD. The ANDA procedure differs from the New Drug Application (NDA) approach for brand-name medications, which necessitates extensive clinical trials to demonstrate safety and effectiveness.
In the ANDA process, the focus is on bioavailability and bioequivalence to the RLD, rather than on the initial proof of safety and effectiveness required for a new molecular entity. The FDA oversees the rigorous assessment of generic medications to ensure they meet these standards and are therapeutically equivalent to their brand-name counterparts. This assessment is based on scientific evaluations and real-time information from various stakeholders.
The approval of an ANDA can be a pivotal moment for patient care, often making treatments more accessible and affordable. However, achieving this approval requires navigating the complex landscape of FDA regulatory compliance, which encompasses a range of standards and regulations to ensure the safety, quality, and efficacy of pharmaceutical products. Pharmaceutical companies must adapt to amendments in FDA regulations, which can significantly impact the development cost and timeline of bringing a generic medication to market. For example, the approval journey of a revolutionary Alzheimer's treatment emphasized the complexities within the FDA's regulatory procedures, emphasizing the significance of adherence and the possible consequences of any errors.
Generic medications are crucial to the healthcare system, providing patients with cost-effective alternatives while upholding the high standards established by the FDA. The ANDA process provides a streamlined route to approval, ensuring that generic medications are a safe and effective option for the public.
Key Components of an ANDA Submission Package
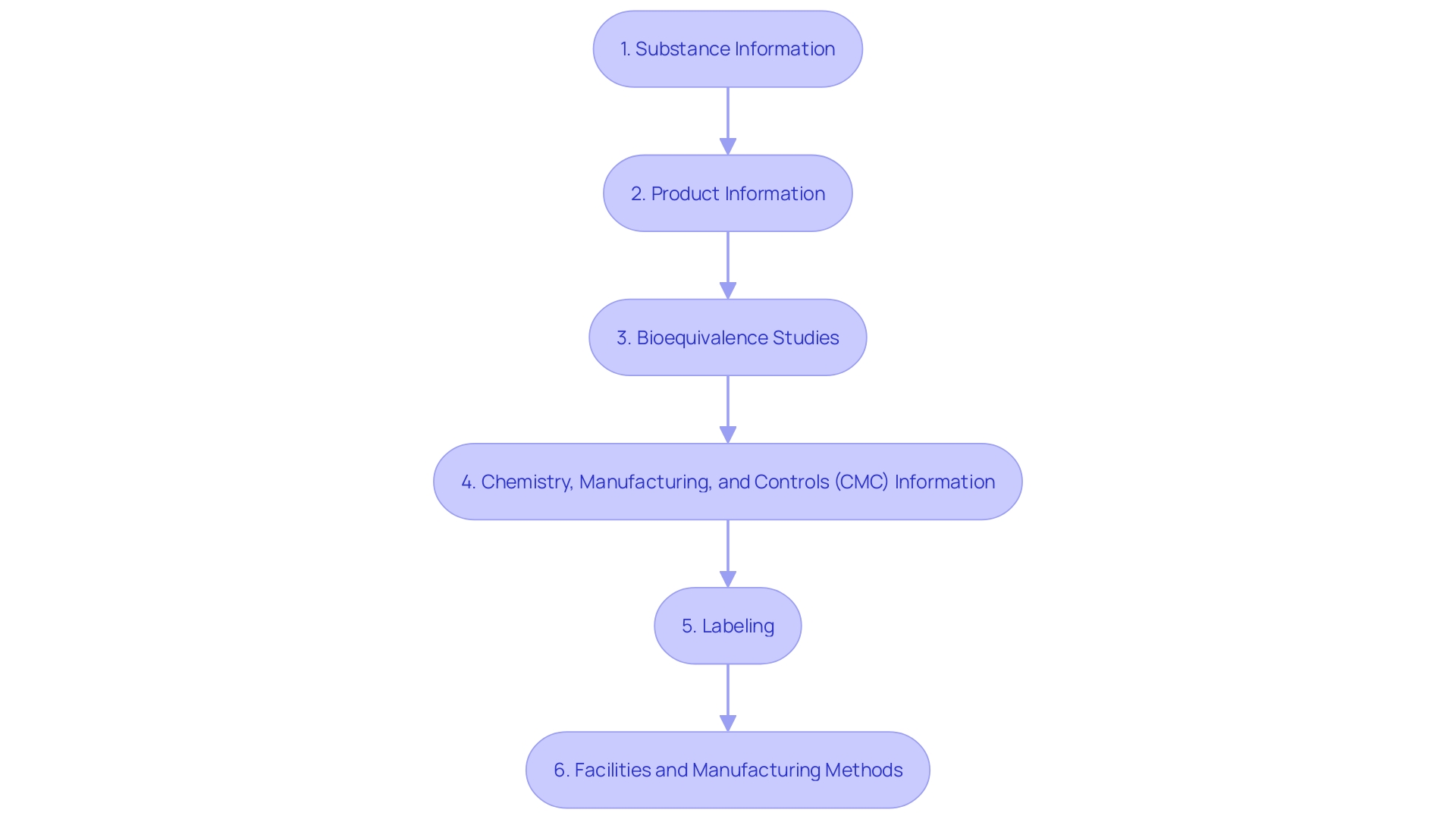
Creating a thorough ANDA filing is crucial for the FDA's assessment of a generic medication's safety, effectiveness, and quality. The submission must encompass:
-
Substance Information: This includes a comprehensive list of all components involved in the manufacture of the product, accompanied by detailed specifications and the name and address of each manufacturer.
-
Product Information: A clear statement of the medication's composition is necessary, along with a description of manufacturing and packaging processes, in-process controls, specifications for ensuring the product's identity, strength, quality, purity, potency, and bioavailability.
-
Bioequivalence Studies: These studies are crucial as they show that the generic medication functions in the same way as the reference listed medication.
-
Chemistry, Manufacturing, and Controls (CMC) Information: The submission should include information on the tests, analytical procedures, and acceptance criteria related to various aspects such as sterility and dissolution rate, which are necessary to determine the quality and effectiveness of the medication.
-
Labeling: Labeling must be scrutinized to ensure it provides accurate information on the usage, warnings, and cautions of the drug.
-
Information about the facilities and the manufacturing methods employed must be disclosed to ensure adherence to FDA standards.
Each element contributes significantly to the FDA's decision-making process. It is also important to consider that any public comments or documentation submitted electronically become part of the public record. As such, submitters must be careful to exclude confidential information such as medical details, Social Security numbers, or confidential business information.
The Center for Medication Assessment and Examination (CMAR) highlights that groundbreaking medications not only provide new treatment alternatives but also signify advancement in healthcare. CDER's annual approvals of new drugs and biological products highlight the significance of thorough applications that adhere to the agency's scientific and regulatory standards. Therefore, the preparation of the documents should be done with a grasp of the science behind new products and the diseases they aim to treat, guaranteeing the inclusion of all essential data for a comprehensive review.
The ANDA Submission Process
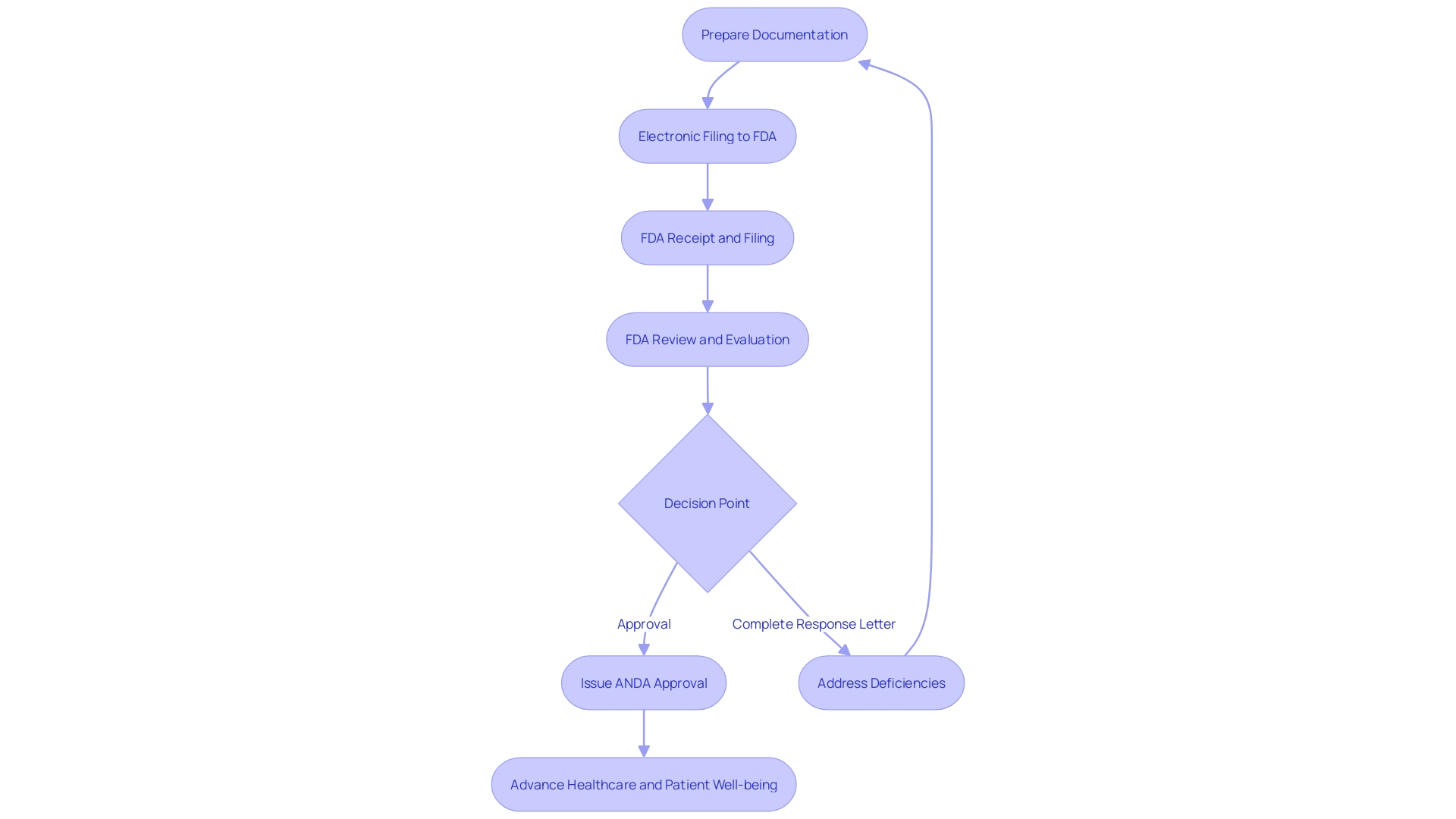
The journey toward submitting an Abbreviated New Drug Application (ANDA) is a comprehensive process that demands a thorough understanding and strict adherence to the FDA's guidelines. The process begins with a comprehensive preparation of the document, where one must acquire a deep understanding of the subject device, including its users, usage instructions, and the competitive environment, which may require extensive research literature and comparative analysis of similar devices.
After this, an electronic filing is necessary, which involves thorough documentation to guarantee that all comments and attachments are properly entered into the FDA's docket, with careful attention to protecting confidential information.
Once the ANDA is received, the FDA's receipt and filing phase begins, where the docket number FDA-2017-D-5868 plays a crucial role in tracking the document through its journey. The recent draft guidance from the FDA, which incorporates updates from the latest Generic Drug User Fee Amendments (GDUFA), offers a clearer comprehension of the reconsideration procedure within the review discipline.
The review and evaluation phase is where the FDA rigorously examines the application against safety standards and regulatory requirements. This phase can be seen as analogous to qualitative assessments in other research fields, such as the peer evaluations at INRAE guided by French law, focusing on the research methodology rather than just the outcomes.
Finally, the outcome of the ANDA process will culminate in either an approval or the issuance of a Complete Response Letter. This stage is crucial, as it can have a substantial effect on healthcare providers and patients alike, similar to the encounter shared by Florence Luna, where administrative procedures in healthcare can result in significant setbacks and unfavorable results.
Every one of these steps is crucial for guaranteeing a seamless transition through the ANDA submission, reflecting the level of attention to detail needed in other domains, like the safety protocols followed by electrical companies or the assessment of scientific research. It is a procedure that not only requires attention to detail but also a dedication to the broader objective of advancing healthcare and patient well-being.
Regulatory Requirements and Guidelines
Navigating the complex landscape of regulatory compliance is a critical aspect of the ANDA and FDA approval processes. Ensuring all documents are thorough, precise, and meet the FDA's expectations requires a profound comprehension of key regulatory requirements and guidelines. These consist of the Hatch-Waxman Act, which promotes the market entry of generic medications while incentivizing innovation; the FDA's Bioequivalence Guidance, which establishes the criteria for proving that a generic product is comparable to its brand-name equivalent; the Current Good Manufacturing Practices (CGMP), guaranteeing the excellence and uniformity of pharmaceutical manufacturing; and the compliance with the Electronic Common Technical Document (eCTD) format for electronic filings.
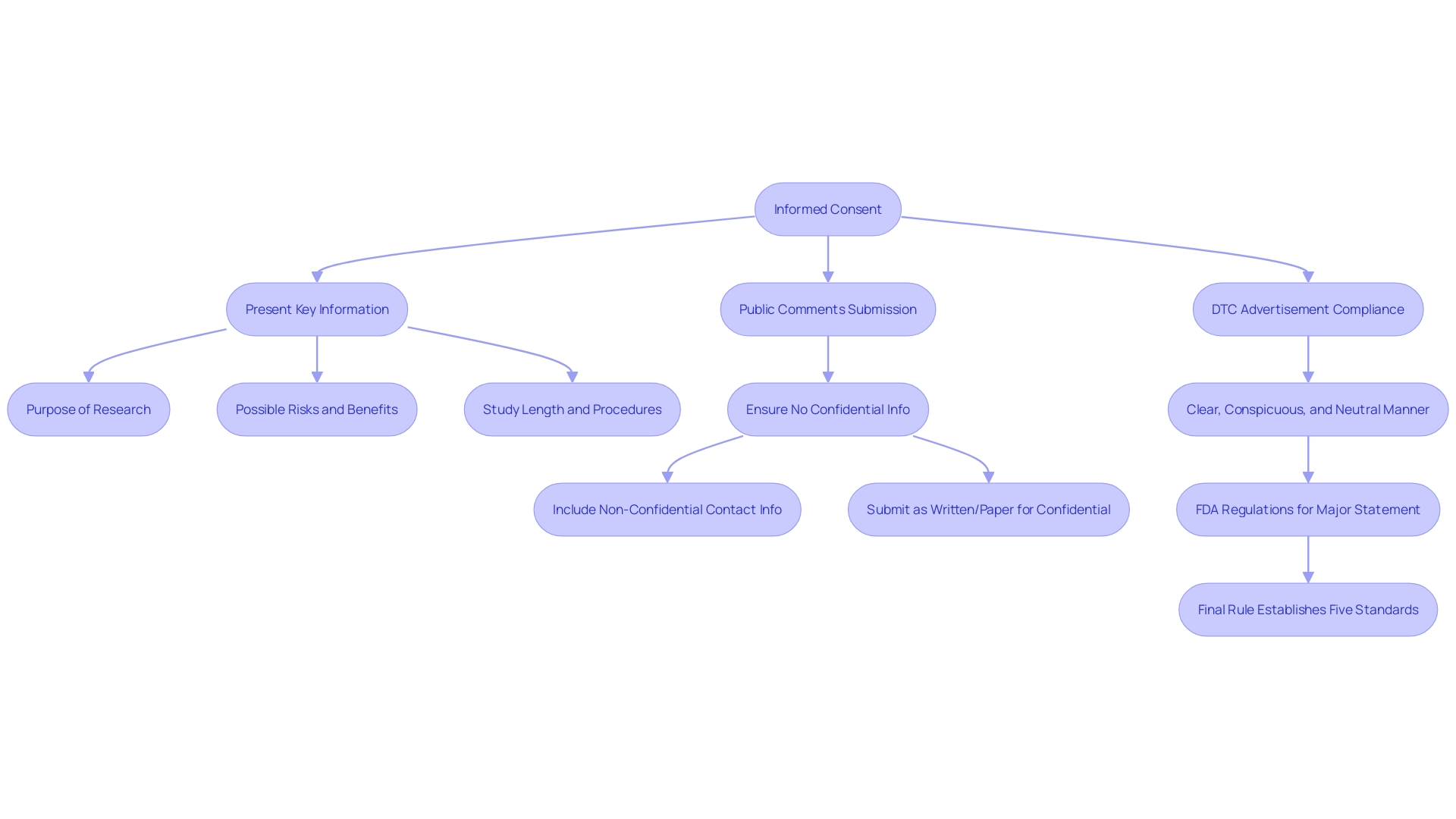
Keeping abreast of regulatory changes is crucial. For example, the FDA's recent final rule on direct-to-consumer prescription medication advertisements mandates clear and neutral presentation of a medication's major side effects, using consumer-friendly language and ensuring both audio and text are easily understandable. This standard reflects the FDA's commitment to transparency and public comprehension.
Additionally, the handling of documents must be done with caution to safeguard sensitive information. Any public comments or data shared with the FDA becomes part of the public record, so confidentiality must be maintained for personal and proprietary information.
Professionals in the field, such as Chris, a biomedical engineer with experience in clinical studies for Class III devices, recognize the critical nature of compliance. Likewise, Cardinal Health's efforts on regulatory starting material strategy for an investigational medication application emphasizes the significance of a holistic approach for timely IND filings.
Statistically speaking, the FDA's Center for Drug Evaluation and Research (CDER) emphasizes the importance of rigorous research development, with the drug approval process lasting an average of 12 to 15 years. This is highlighted by the alarming increase in untrustworthy data reports from third-party labs, especially from China and India, which obstructs device authorization and ultimately impacts patient care and device supply chains.
In essence, a successful ANDA submission hinges on meticulous attention to regulatory requirements, proactive adaptation to new rules, and a vigilant approach to data integrity and confidentiality.
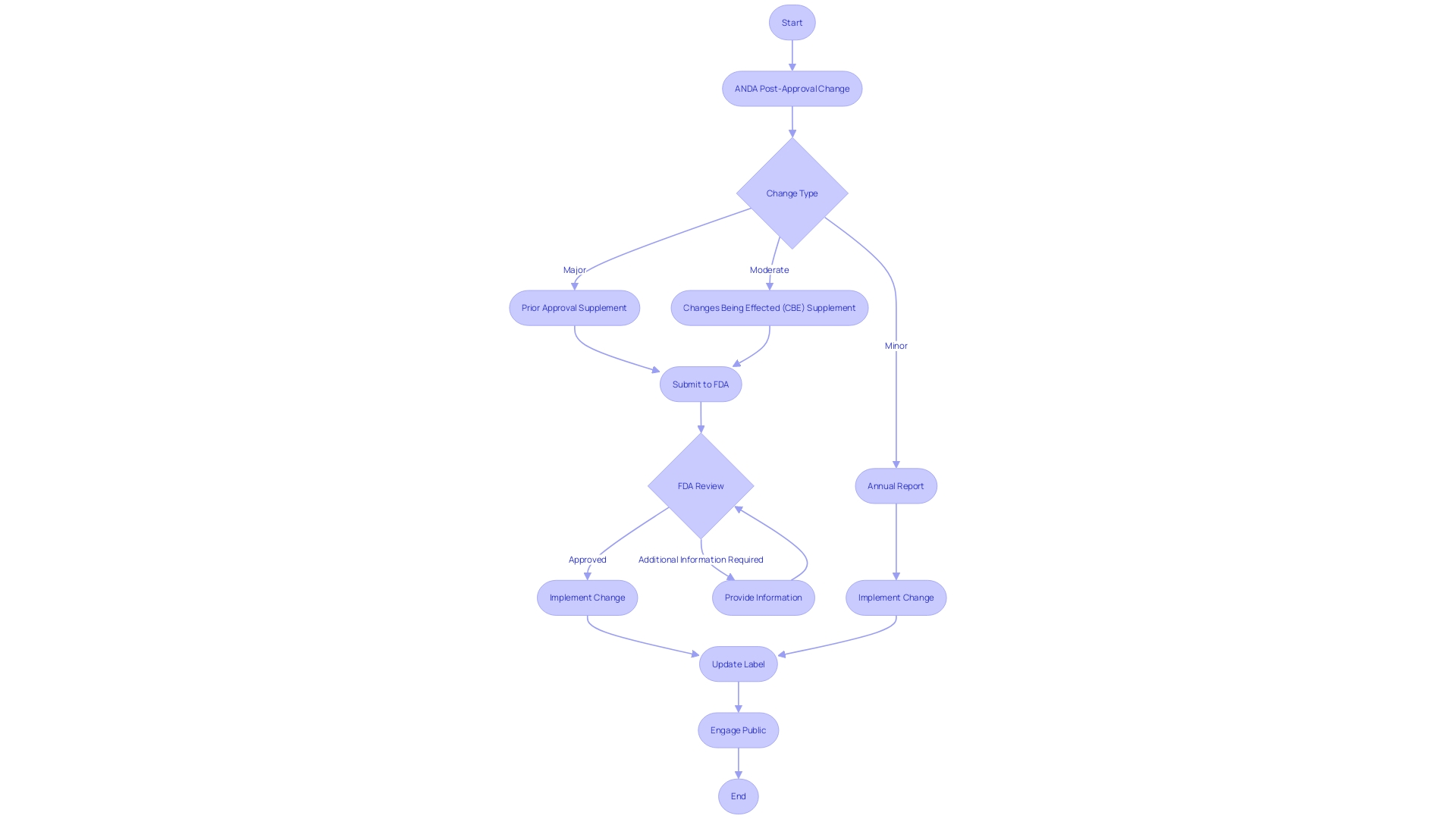
Changes to an Approved ANDA
Navigating the intricacies of post-approval changes to an Abbreviated New Drug Application (ANDA) is critical for maintaining compliance with FDA regulations. Such alterations may come from innovative scientific developments, enhancements in manufacturing techniques, or other significant factors. The FDA categorizes these changes and stipulates clear protocols for their implementation, which include:
-
Types of Changes: Recognizing the nature and scope of the change is the first step. Whether the change is major, moderate, or minor will determine the subsequent process and documentation required.
-
For significant modifications that might impact the medication's safety, quality, or effectiveness, a Prior Approval Supplement is required. This involves a detailed review by the FDA before any implementation.
-
Changes Being Effected (CBE) Supplement: Modifications that do not significantly affect the medication's safety and effectiveness, but still necessitate FDA notification, are classified as the CBE supplement. These can be implemented before FDA approval, but with specific conditions and timelines.
-
Annual Reports: Certain minor changes can be documented and reported to the FDA in the form of Annual Reports. This enables a streamlined procedure that guarantees the FDA is kept informed without requiring a formal supplement.
It is important to mention that the FDA maintains a 'Discontinued Medication Product List' in the Orange Book, identifying medication products that have been discontinued for reasons not related to safety or effectiveness. Approved ANDAs linked to these listed products remain valid, and additional ANDAs for these products may receive approval if they meet legal and regulatory criteria. Moreover, if label revisions are needed to meet current standards, the FDA will guide ANDA applicants accordingly.
The FDA also encourages public engagement and transparency, allowing industry professionals to submit comments on draft guidance and procedures. Such comments are made public, underscoring the importance of excluding confidential information. This open dialogue ensures that stakeholders have a voice in shaping regulatory processes that are both robust and adaptable to emerging industry and health contexts.
The FDA's commitment to evolving guidance reflects the dynamic nature of pharmaceutical development and the challenges faced by industry stakeholders. For instance, as part of the recent reauthorization of the Generic Drug User Fee Amendments (GDUFA), the FDA has updated its guidance on requests for reconsideration at the division level, clarifying appropriate procedures and matters for applicants of ANDAs.
In summary, following the FDA's established framework for post-approval changes to an ANDA is crucial for ensuring continued compliance, avoiding potential delays, and contributing to the advancement of healthcare through the availability of effective and safe generic medication alternatives.
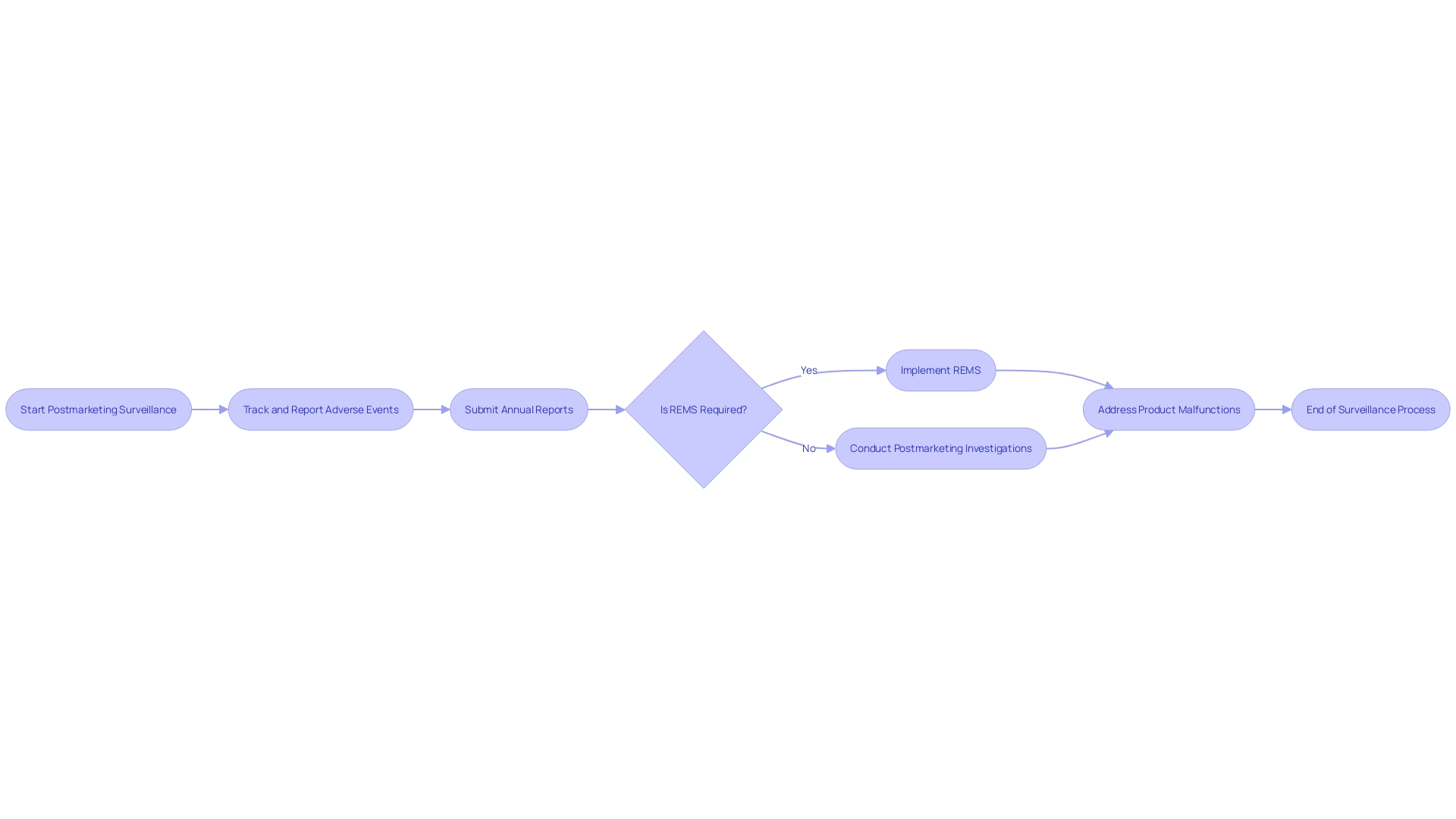
Postmarketing Reporting and Compliance
The FDA's focus on postmarketing surveillance is a crucial aspect of ensuring the safety and efficacy of generic medications once they reach the marketplace. Postmarketing reporting and compliance serve as a safeguard against potential adverse events that could arise from the widespread use of these medications. The cornerstone of this vigilance includes the tracking and reporting of adverse events, the submission of annual reports, and the implementation of Risk Evaluation and Mitigation Strategies (REMS). Furthermore, performing postmarketing investigations, such as pregnancy registries and prospective outcomes studies, is obligatory for specific medications to monitor their impact on maternal and fetal health.
Case studies illustrate the importance of these requirements. For instance, the reporting of adverse events assists the FDA in identifying trends that may indicate a broader issue with a medication's performance. An example of this is the monitoring of patient complaints related to medical devices through the Manufacturer and User Facility Device Experience (MAUDE) database, which has been instrumental in identifying and addressing product malfunctions.
Moreover, the FDA's recent final rule on presenting major side effects and contraindications in direct-to-consumer prescription medication advertisements underscores the agency's commitment to transparent communication about the risks associated with medications. This rule mandates that such information is presented in a clear, conspicuous, and neutral manner to the public, enhancing patient awareness and comprehension.
Compliance with these postmarketing obligations is not merely a regulatory requirement; it is a fundamental component of patient safety and the trust in pharmaceutical products. According to industry professionals, adherence to these protocols is also a reflection of the pharmaceutical manufacturer's commitment to upholding the highest standards of quality and integrity in clinical research. As reported by Gamertsfelder and Osipenko, the personal toll on patients participating in clinical trials is significant, and the data collected from these trials contributes to the broader understanding of a medication's safety profile.
In terms of financial impact, data analytics have revealed that non-compliance with contractual terms regarding commercial rebates and the 340B Drug Pricing Program can result in substantial revenue losses. It is estimated that at least 5% of commercial rebates are duplicate discounts with 340B, amounting to over $6 billion in potential losses.
In summary, careful attention to postmarketing reporting and compliance is not only a legal obligation for pharmaceutical manufacturers but also a critical factor in safeguarding public health and maintaining the integrity of the pharmaceutical industry.
Facility Fees and Self-Identification
Ensuring compliance with the FDA's regulations is a critical step for generic medication manufacturers. Not only must they adhere to high quality standards, but they also need to navigate the intricacies of the Generic Drug User Fee Amendments (GDUFA). This includes paying facility fees and fulfilling self-identification requirements for their establishments. The GDUFA aims to accelerate the availability of more affordable generic medications by funding the FDA's generic medications program, which includes the assessment of generic medication applications and inspections of facilities.
The arrangement of the facility fees is planned to cover expenses connected with the FDA's generic medication program, which guarantees that the public has access to safe, effective, and high-quality generic medications. These fees are a necessary part of maintaining the integrity of the drug supply chain and ensuring that manufacturers meet the necessary regulatory guidelines.
Self-identification, on the other hand, is a procedure through which manufacturers provide the FDA with certain information about their facilities, sites, and organizations. This transparency allows the FDA to better manage inspections and resources, thus facilitating a more efficient review process. It is crucial for manufacturers to provide accurate and timely information, as failure to comply with these requirements can result in delays or even refusal of product approvals.
The FDA's dedication to the safety, effectiveness, and security of human and veterinary medications is unwavering, as demonstrated by their comprehensive guidelines and the recent final rule to ensure clear communication in direct-to-consumer prescription medication advertisements. Manufacturers can contribute to this mission by diligently adhering to the FDA's facility fees and self-identification guidelines, which are essential for maintaining approval and a presence in the market.
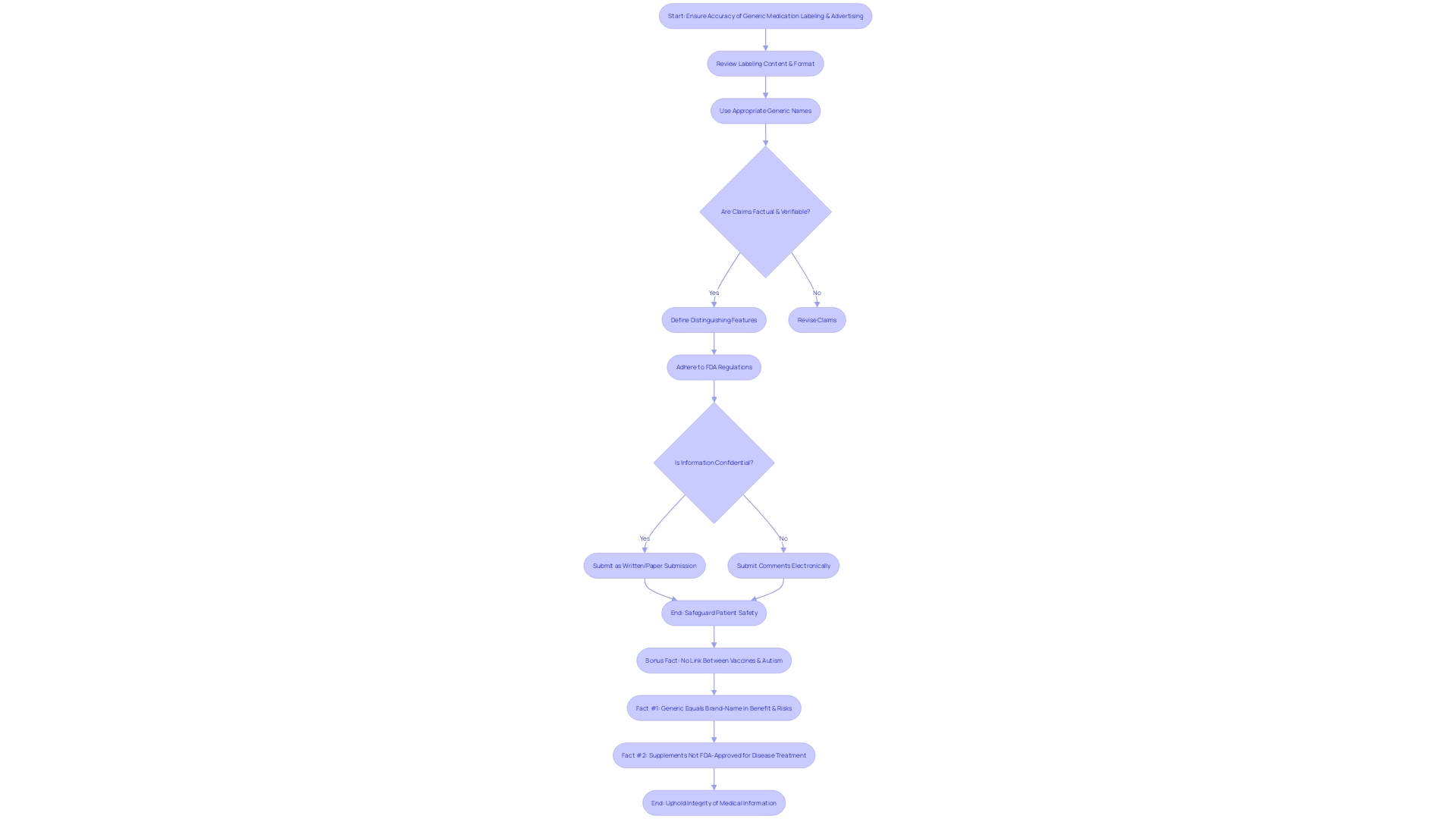
Labeling and Advertising Requirements
The accuracy with which generic medications are labeled and advertised is a cornerstone of patient safety and the integrity of medical information. The FDA enforces strict guidelines to ensure that every aspect of a generic medication's presentation is accurate, preventing any misleading claims that could compromise patient care. Key areas of focus include:
-
Labeling Content and Format: Clarity and neutrality are paramount. The FDA mandates that information be presented in a way that is both conspicuous and neutral to avoid any ambiguity. This includes a meticulously crafted major statement that abides by five distinct standards to ensure its clear and unbiased delivery to the consumer.
-
Generic Name Usage: It is imperative that the generic name is used appropriately. This is not just a legal necessity but also a measure to uphold uniformity and dependability in the identification of substances.
-
Comparative Claims: When making comparisons, it's crucial that they are factual and verifiable. The FDA's lifecycle assessment of vaccines, for example, from pre-human testing studies to post-market surveillance, underscores the agency's commitment to continuous and thorough evaluation.
-
Distinguishing Features: A medication's distinct qualities should be clearly defined to set it apart from others. This helps in establishing a unique identity for the medication, which is crucial in a marketplace where advertising plays a vital role in brand recognition.
FDA-approved generic medicines are confirmed to provide the same clinical benefits and carry the same risks as their brand-name counterparts. However, not all products receive this level of scrutiny and approval—vitamins and dietary supplements do not undergo the same rigorous FDA evaluation. It is critical for healthcare professionals and consumers alike to distinguish between FDA-approved medications and supplements that make unverified health claims.
Medication errors remain a significant concern, with nearly 50% of preventable patient harm globally attributed to the inappropriate use of medicines. Compliance with FDA regulation is not just about following rules; it's about safeguarding lives. The FDA, an integral part of the U.S. Department of Health and Human Services, upholds the safety and efficacy of drugs through comprehensive regulations that protect public health. Adherence to these standards is not only a legal obligation but a moral one, fostering confidence and trust in the healthcare system.
Common Deficiencies and Best Practices for Avoiding Them
Navigating the ANDA approval process requires meticulous attention to detail and adherence to FDA guidelines. A common challenge in this journey is the presenting of applications with deficiencies that can lead to significant delays or outright rejections. Among the typical issues encountered are inadequate bioequivalence studies, which are critical for demonstrating that the generic product performs in the same manner as its branded counterpart. Furthermore, applications often fall short in providing comprehensive Chemistry, Manufacturing, and Controls (CMC) information, which forms the backbone of the product's quality assurance. Additionally, labeling errors can cause significant setbacks, as accurate labeling is not only a regulatory requirement but also ensures patient safety.
To fortify the application against such challenges, it is essential to engage in thorough quality control and quality assurance practices. This involves a proactive approach to identifying and rectifying potential errors throughout the product development lifecycle. For example, as directed by recent FDA initiatives such as Project Orbis and the Real-Time Oncology Review (RTOR) program, applicants are encouraged to streamline data filings and utilize tools like the Assessment Aid to facilitate the FDA's evaluation. These programs have demonstrated success, with some applications receiving approval up to 5 weeks ahead of the FDA goal date.
Moreover, continuous monitoring of the FDA's communications, such as updates to the "Discontinued Drug Product List" in the Orange Book, ensures that applicants remain informed about products that are no longer marketed for reasons unrelated to safety or effectiveness, providing a clearer landscape for the approval of additional ANDAs.
The FDA's dedication to openness is also apparent in their guidelines for public comment postings, advising applicants to refrain from including private information in comments shared to the docket, thereby preserving the integrity of sensitive data.
Essentially, a triumphant ANDA filing is based on a strategic and systematic approach to complying with FDA criteria, emphasized by a strong comprehension of agency procedures and the proactive handling of application materials. By avoiding common deficiencies and leveraging best practices, applicants can significantly enhance the likelihood of a favorable outcome.
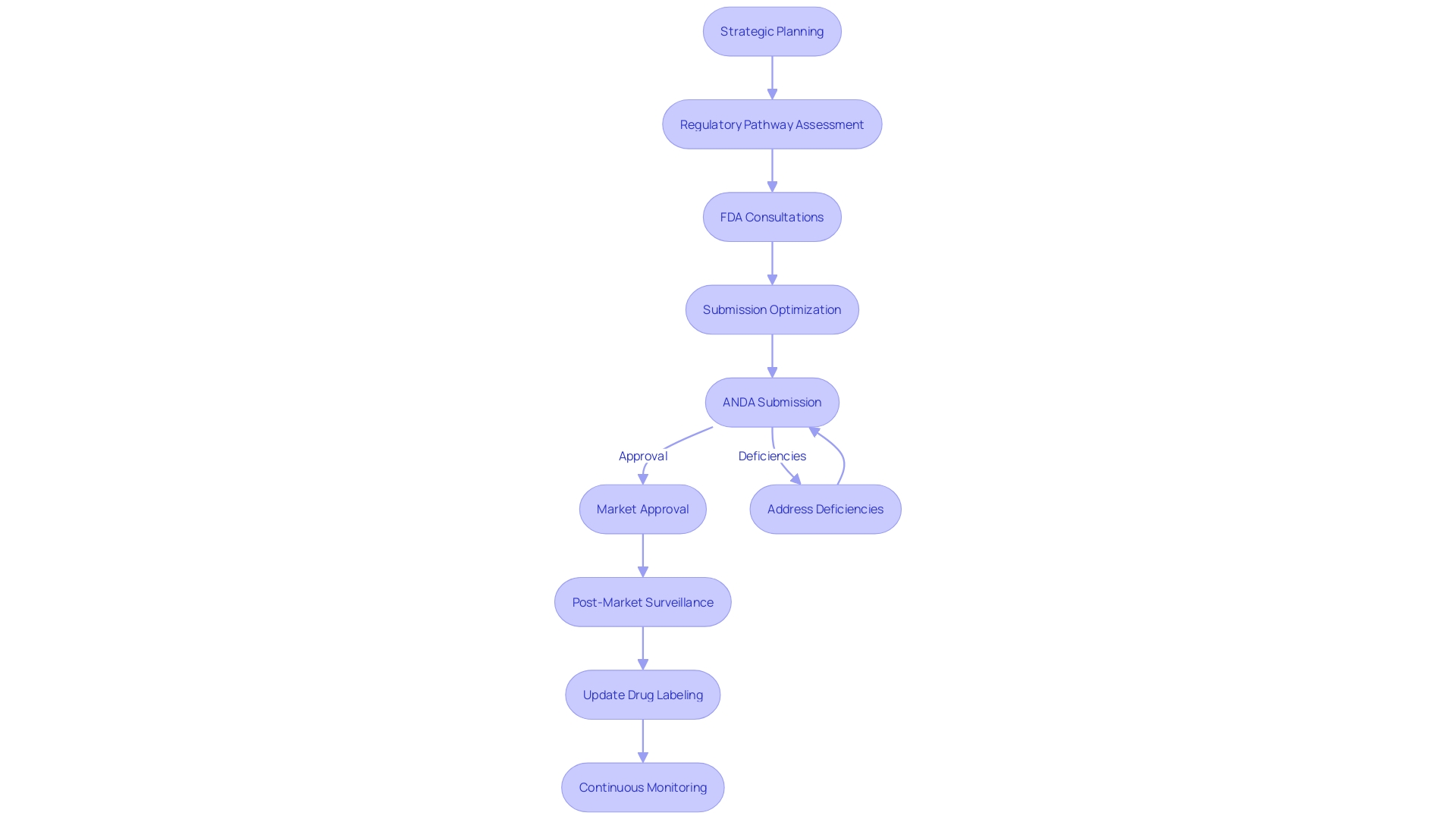
Role of Regulatory Consulting in ANDA Approval
The Abbreviated New Drug Application (ANDA) and FDA approval procedures are meticulous and necessitate a deep comprehension of regulatory requirements. Drawing on approximately 65 hours of research and expert interviews, the significance of regulatory consulting emerges as a crucial factor in navigating these complex procedures. Applying the knowledge from a Senior Research Scholar at Stanford Law School and other experts in the field, it becomes evident that the ANDA process is not solely focused on the act of submitting, but also on strategic planning and anticipation of regulatory pathways.
Regulatory consultants specialize in conducting thorough regulatory pathway assessments, a foundational element in crafting a regulatory strategy. Their role involves exploring FDA databases to discover crucial details about a product, like its code and pathways, and determining the necessity of Q-dialogues with the FDA. This expertise is crucial for addressing potential issues preemptively and ensuring that the ANDA is comprehensive and compliant with FDA expectations.
The FDA's draft guidance document, identified by docket number FDA-2017-D-5868, delineates the procedures for ANDA applicants seeking reconsideration at the division level. It underscores the importance of understanding the agency's processes and the value of regulatory consultants who can guide applicants through these intricate steps.
Furthermore, comments submitted to the FDA docket are made public, emphasizing the need for careful consideration of confidentiality in communications with the agency. Regulatory consultants can assist in managing such submissions, ensuring that sensitive information is protected while meeting the requirements for public commentary.
In the context of medication regulation, the FDA oversees a spectrum of products, from prescription medications to generics and over-the-counter medications. Real-time data from various stakeholders informs the FDA's decisions, highlighting the dynamic nature of drug approval and the ongoing need for strategic regulatory oversight.
In essence, engaging with regulatory consulting services equips companies with the knowledge and foresight necessary to maximize the likelihood of a successful ANDA approval. These professionals offer invaluable support, from initial consultations to addressing deficiencies and optimizing submissions, thereby aligning with the FDA's goal of ensuring safety and efficacy before products enter the market.
Conclusion
The Abbreviated New Drug Application (ANDA) is a crucial pathway for generic drugs to gain FDA approval. By demonstrating bioequivalence to a previously approved reference listed drug (RLD), generic drugs offer safe and effective alternatives to brand-name counterparts. Compliance with FDA guidelines and regulatory requirements is essential throughout the ANDA submission process.
Crafting a meticulous ANDA submission package is pivotal, encompassing comprehensive drug information, bioequivalence studies, CMC information, labeling details, and facility information. Attention to detail and adherence to guidelines are necessary to avoid common deficiencies in submissions.
Post-approval changes to an approved ANDA require careful navigation to maintain compliance. Manufacturers must categorize changes, follow specific protocols, and submit annual reports. Compliance with postmarketing reporting and compliance obligations is crucial for ensuring the safety and efficacy of generic drugs in the marketplace.
Compliance with FDA regulations, including facility fees and self-identification requirements, is critical for generic drug manufacturers. Facility fees cover costs associated with the FDA's generic drugs program, ensuring the availability of safe and high-quality drugs. Adhering to these guidelines is essential for maintaining approval and a presence in the market.
The precision of labeling and advertising is fundamental for patient safety and the integrity of medical information. Compliance with FDA guidelines ensures accurate and unbiased information. Attention to detail in labeling and advertising is necessary to prevent misleading claims and protect patient care.
Regulatory consulting plays a crucial role in navigating the ANDA approval process. Their expertise ensures comprehensive and compliant submissions, addressing potential issues and maximizing the likelihood of a successful approval. Engaging with regulatory consulting services aligns with the FDA's goal of ensuring safety and efficacy.
In conclusion, the ANDA approval process requires a thorough understanding of regulatory requirements, adherence to FDA guidelines, and the utilization of regulatory consulting services. Compliance with FDA regulations contributes to the availability of safe and high-quality generic drugs, advancing healthcare and patient well-being.
Get expert regulatory consulting services to navigate the ANDA approval process successfully.




