Introduction
The FDA's 510(k) clearance process is a critical pathway for medical device manufacturers to introduce their products to the U.S. market. This regulatory mechanism allows a new device that is demonstrated to be substantially equivalent to a predicate device, which is already legally marketed, to be cleared for sale. The classification of medical devices by the FDA into three categories, based on risk, is the beginning of this comprehensive process.
Class I includes low-risk devices and Class III includes those with the highest risk, often requiring a Pre-Market Approval (PMA) due to their critical role in life-support or sustenance. The 510(k) process often applies to Class II devices, which comprise a vast array of medical tools and apparatuses used in the healthcare industry. Understanding the 510(k) submission, different types of 510(k) submissions, identifying and selecting a predicate device, content requirements for a 510(k) submission, preparing and submitting a 510(k) application, the FDA review process and timeline, substantive review, communications with FDA reviewers, common challenges and best practices, and final clearance and post-clearance requirements are all crucial aspects of navigating the FDA's 510(k) clearance process.
By adhering to the FDA's guidelines and providing comprehensive device information, manufacturers can successfully navigate the 510(k) clearance process while ensuring patient safety and promoting innovation in the medical device industry.
What is FDA 510(k) Clearance?
The FDA's 510(k) clearance process is a critical pathway for medical apparatus manufacturers to introduce their products to the U.S. market. This regulatory mechanism allows a new apparatus that is demonstrated to be substantially equivalent to a predicate instrument, which is already legally marketed, to be cleared for sale. Substantial equivalence means that the new product is as safe and effective as the reference and does not raise new questions regarding safety or effectiveness.
The FDA's categorization of medical equipment into three groups, according to their level of risk, marks the initiation of this extensive procedure. Class I includes low-risk products and Class III includes those with the highest risk, often requiring a Pre-Market Approval (PMA) due to their critical role in life-support or sustenance. The 510(k) process frequently pertains to Class II tools, which encompass a wide range of medical apparatuses and instruments utilized in the healthcare industry.
It is crucial for manufacturers to have a profound understanding of the purpose of the product, its users, and the competitive market landscape, including potential reference devices. A comparative analysis is necessary to align the new product with FDA's standards and to support its claim of substantial equivalence.
Recent examination, like that shown in the 2018 documentary 'The Bleeding Edge,' emphasized the potential risks associated with the 510(k) procedure, including cases where clinical trials were not necessary, resulting in unfavorable patient outcomes. Such revelations have prompted calls for more stringent regulatory oversight to ensure patient safety, reflecting the FDA's ongoing commitment to public health.
Moreover, the FDA continues to enhance transparency and communication standards, as evidenced by its recent final rule on direct-to-consumer prescription drug advertisements, mandating that major side effects and contraindications be presented in a clear, conspicuous, and neutral manner. These evolving regulations and practices underscore the FDA's role in safeguarding public health while facilitating medical innovation.
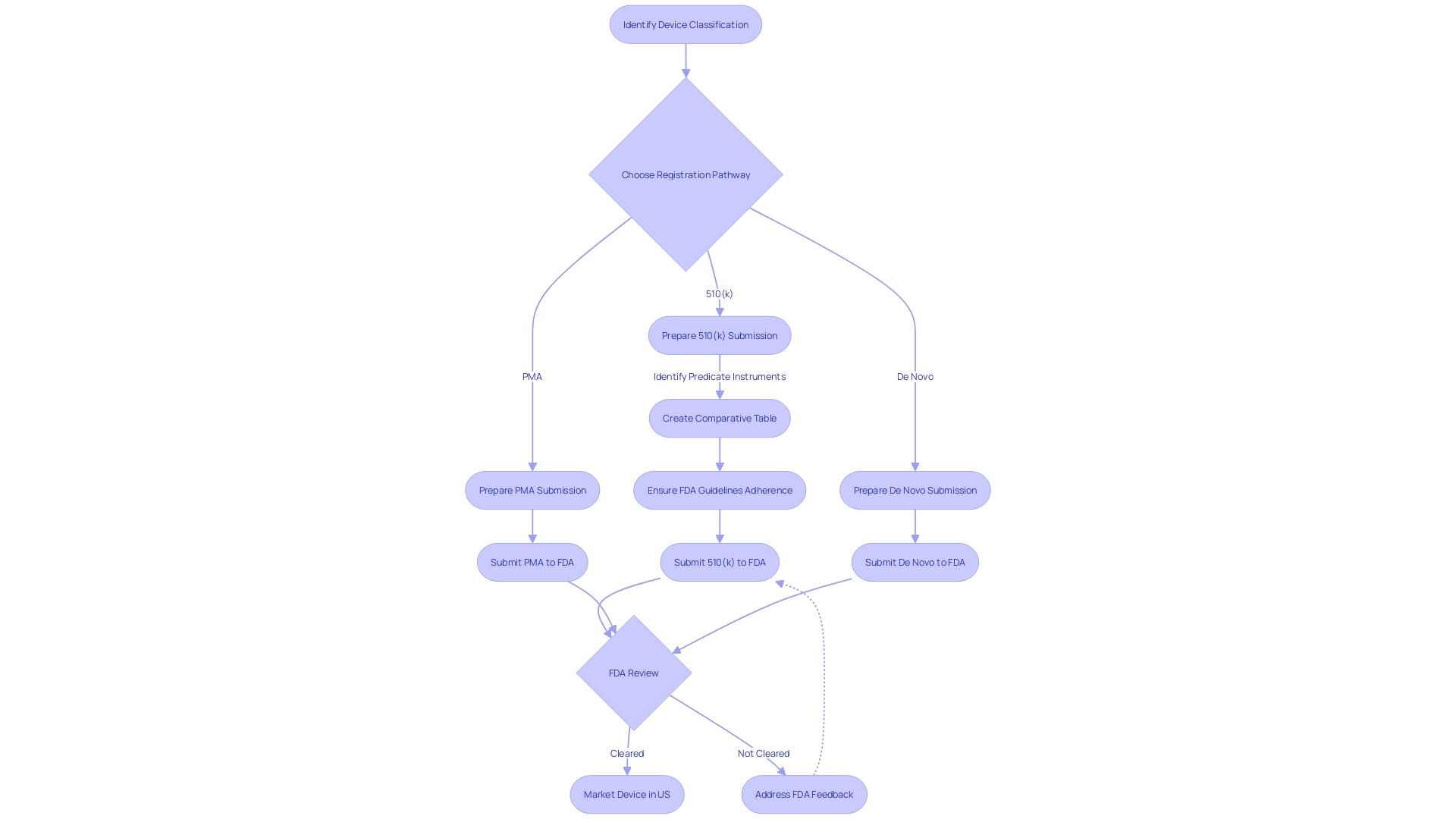
Understanding the 510(k) Submission
Successfully navigating the FDA's 510(k) clearance procedure is a crucial stage for medical instrument manufacturers aiming to showcase the safety and efficacy of their product. The process requires a comprehensive understanding of the object in question. This includes a comprehensive analysis of its intended use, detailed instructions for use, along with any necessary warnings and cautions. It is essential to identify and examine potential predicate devices—those already in the market with similar intended use and technological characteristics—to establish substantial equivalence. A comparative table should be created to concisely demonstrate the similarities and differences between the new equipment and the reference item.
In preparation for the 510(k) submission, it is advisable to research various resources such as clinical studies, competitor websites, and marketing materials to fully grasp the competitive landscape. This research informs the submission's content and ensures a well-rounded presentation of the product's position within the market. Additionally, examining the Summaries of Safety and Effectiveness Data (Seeds) from the FDA's 510(k) database offers valuable insights into the performance and safety profiles of previously cleared products.
It is crucial to highlight that all documentation and comments submitted to the FDA during this procedure are subject to public disclosure, so it is vital to omit any confidential information from these submissions unless it is filed as a written/paper submission with special instructions for handling sensitive data. The FDA's responsibility in guaranteeing the safety and efficacy of medical tools is crucial, and the 510(k) clearance procedure demonstrates the agency's dedication to safeguarding public health while encouraging advancement within the medical equipment industry. By carefully following the FDA's guidelines and offering thorough information about the product, manufacturers can successfully navigate the 510(k) clearance process with increased confidence and efficiency.
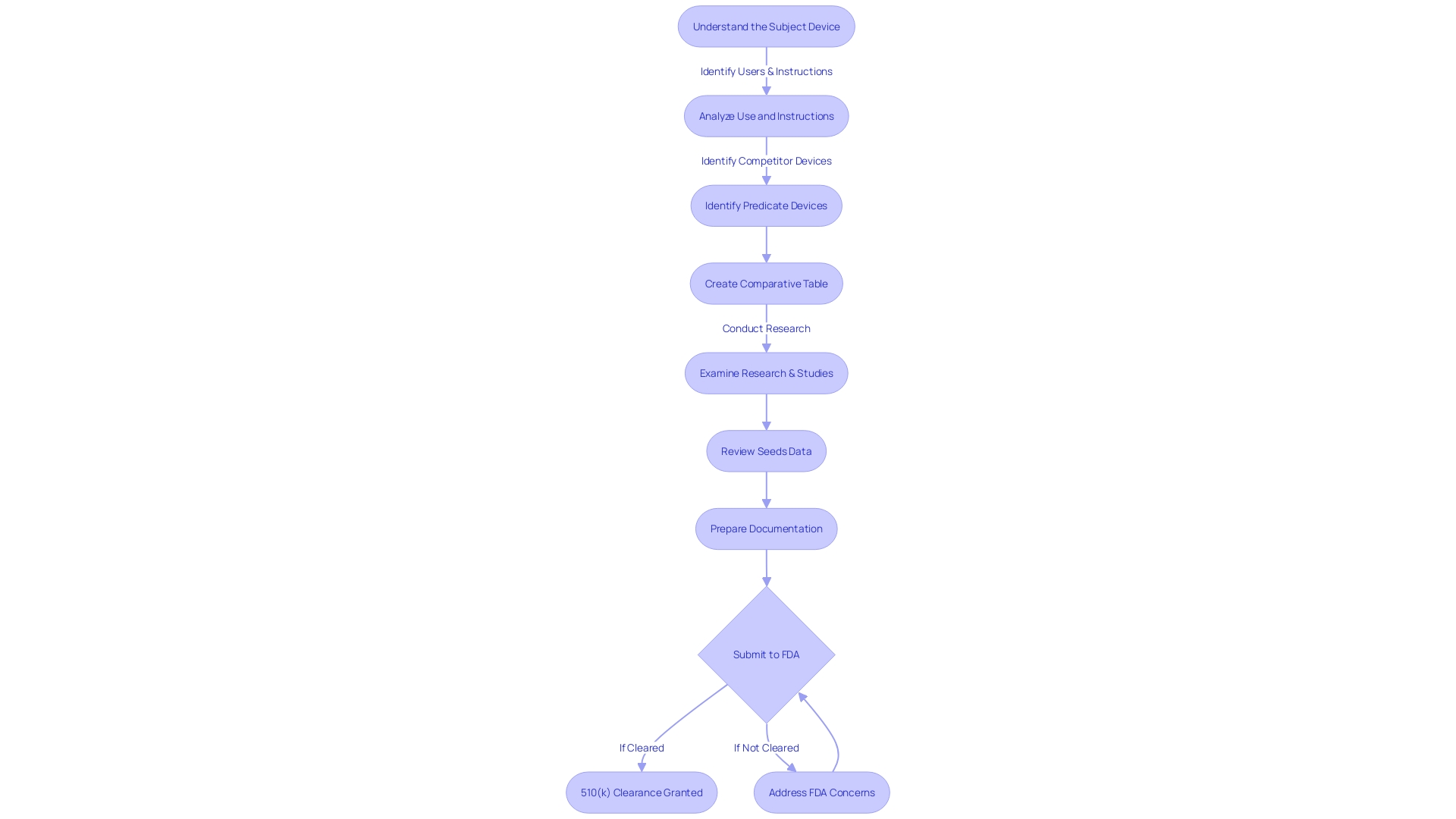
Different Types of 510(k) Submissions
Mastering the intricacies of 510(k) submissions is vital for manufacturers in the medical industry. The FDA delineates three primary pathways: Traditional, Special, and Abbreviated 510(k). A Traditional 510(k) requires a comprehensive comparison with a predicate product, demonstrating that your item is as safe and effective. The Special 510(k) pathway, appropriate for minor modifications to an apparatus that already has 510(k) clearance, highlights design control requirements. Lastly, the Abbreviated 510(k) leverages guidance documents, special controls, and FDA-recognized consensus standards to support compliance.
For a successful submission, it's crucial to thoroughly investigate the subject, its users, and the competitive landscape, as well as comprehend the detailed instructions for use. Engage with Marketing to discern competitor devices and construct a comparative table, underlining the similarities and differences based on research literature, clinical studies, and labeling. This approach, guided by the FDA's recommendations, streamlines the submission procedure, guaranteeing that the required information is carefully compiled and presented to establish substantial equivalence.
As the process is public, it’s imperative to omit any confidential data from your submission, including personal identifiers or sensitive business information. Comments and documents submitted are made available unedited, so one must provide only non-confidential information or opt for written submissions if confidentiality is required.
Recent legislative efforts, such as the Consolidated Appropriations Act, 2023, underscore the importance of clear and concise disclosure, advocating for registration forms that enable informed decisions and utilizing a summary prospectus framework for complex products. This initiative mirrors the FDA's expectations for 510(k) submissions, where clarity and accessibility of information are paramount for both regulatory approval and user comprehension.
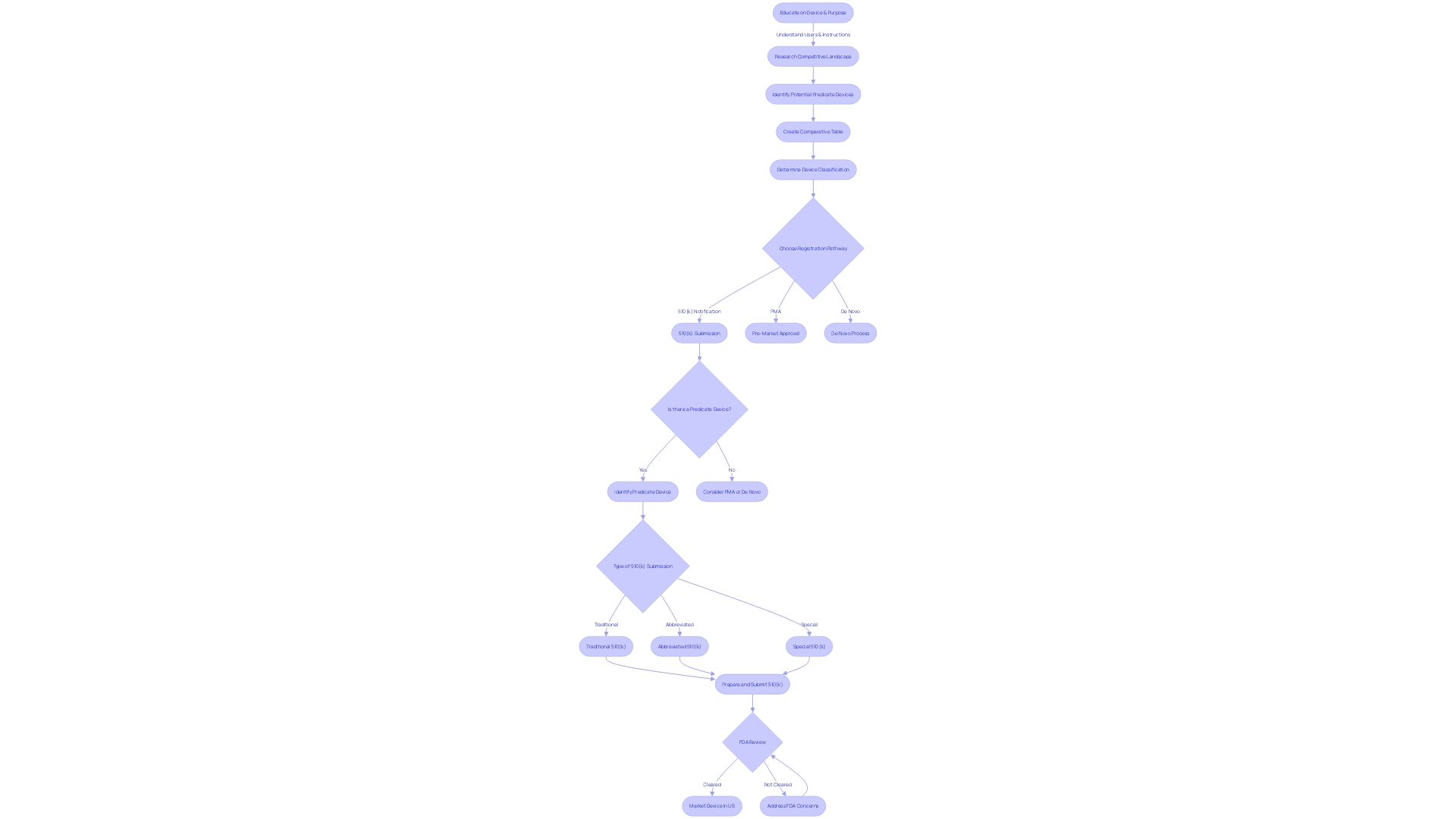
Identifying and Selecting a Predicate Device
Choosing the right predicate tool is a crucial step in the 510(k) clearance pathway for medical instruments. To establish substantial equivalence to a predicate, it is essential to gain a comprehensive understanding of the subject instrument. Examine the user base of the apparatus, which may involve clinicians, physicians, dentists, or patients, and thoroughly assess instructions for use, giving special consideration to warnings and precautions. Work together with marketing teams to analyze the competitive landscape and identify potential predicate objects that share the intended use and possess analogous technological characteristics. Acquire a plethora of information from research literature, clinical studies, and the marketing materials of competitor products to build a comprehensive comparative analysis.
In parallel, ensure that the potential predicate is a legally marketed product currently registered with the FDA. Confirm that the intended use aligns with that of your equipment and that any technological differences do not raise new safety and effectiveness concerns. The FDA's recent modernization efforts, including the draft guidance document released on September 7, 2023, emphasize the importance of selecting a predicate that utilizes well-established methods like FDA-recognized consensus standards, guidance documents, or qualified medical instrument development tools. In cases where more widely accepted scientific methods are used, thorough documentation and vetting, such as public comment or peer review, are advised.
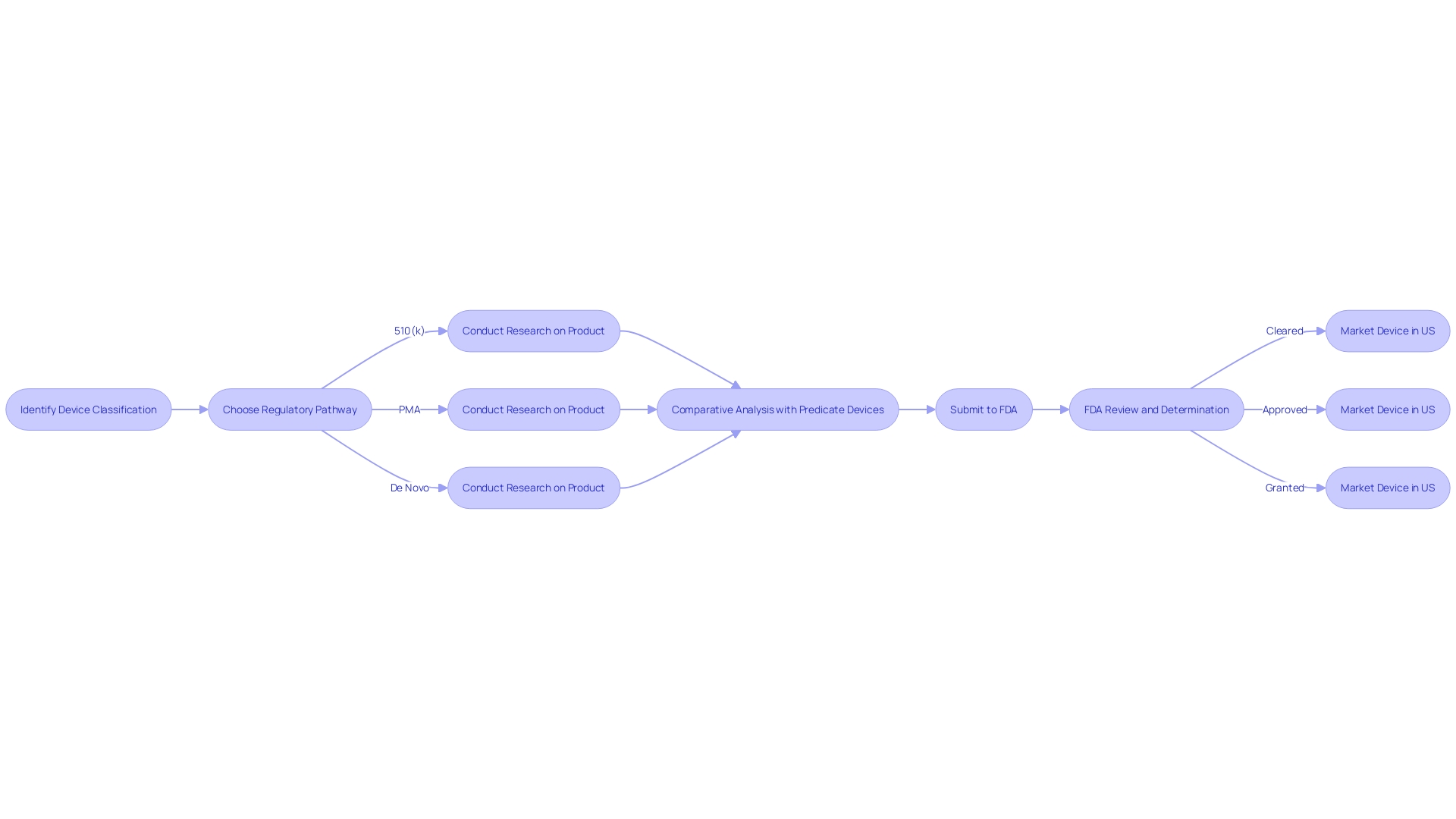
To start, determine the accurate FDA classification for the product, as this will determine the registration pathway - whether it is 510(k), Premarket Approval (PMA), or the De Novo procedure. The distinction among being FDA 'Cleared', 'Approved', or 'Granted' is critical, with each term carrying substantial implications for the marketing and regulatory strategy of the product. Equipped with this understanding and a thoroughly researched comparative table, you can confidently maneuver the 510(k) submission process, substantiating your assertion of substantial equivalence with the most suitable precedent.
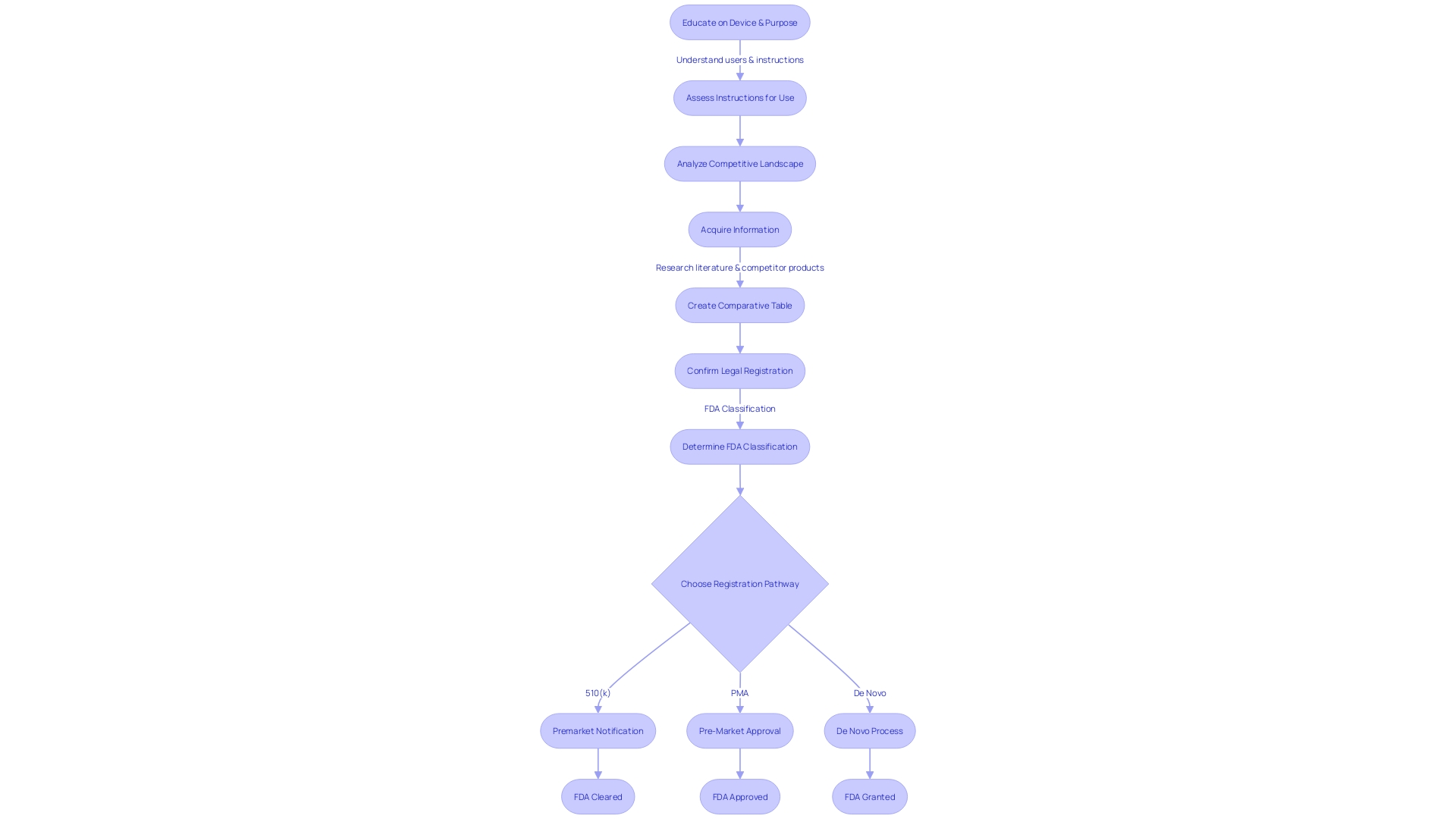
Content Requirements for a 510(k) Submission
A thorough understanding of the equipment you are submitting for FDA 510(k) clearance is crucial. Start by fully engaging with the purpose of the apparatus and its users, who may consist of clinicians, physicians, dentists, and patients. Get acquainted with the instructions for use of the equipment, paying careful attention to any warnings and precautions. Collaborate with your marketing team to gain insight into the competitive landscape. This entails analyzing research literature, clinical studies, and marketing materials from competitor products to identify a precedent item with the same intended purpose and comparable technological features. Creating a detailed comparative table is an invaluable step in this process.
Once a suitable predicate apparatus is identified, it is crucial to review the Summaries of Safety and Effectiveness Data (SSE) available in the FDA's 510(k) database to discern the similarities and differences. This review will inform your submission, ensuring that your documentation is thorough and reflects the necessary performance data, labeling, and any relevant clinical data.
It is also important to remember that any comments or information submitted to the FDA, including attachments, become part of the public record. Confidential information should not be included unless submitted according to the FDA's guidelines for written/paper submissions.
Preparing and Submitting a 510(k) Application
When compiling your 510(k) submission for FDA review, it's critical to begin with a thorough understanding of the medical equipment in question. This entails exploring the particular applications of the apparatus, the intended users comprising clinicians, physicians, dentists, and patients, and the comprehensive guidelines for usage, while carefully considering any alerts and safety measures. Working together with your marketing team can provide insights into the competitive landscape, highlighting competitor products and helping to identify potential precursor items that have similar intended uses and technological characteristics.
Create a comprehensive comparative table by examining various sources such as research literature, clinical studies, and marketing materials like websites, brochures, and sell-sheets. This table will serve as a vital component of your submission, illustrating how your apparatus compares with an existing predicate. Furthermore, examine the Summaries of Safety and Effectiveness Data (Seeds) in the FDA's 510(k) database, which can offer a clearer understanding of the similarities and differences between your product and the predicate.
When submitting comments to the FDA, especially electronically, it's paramount to ensure that no confidential information is inadvertently made public. This includes sensitive medical data, personal identifiers like Social Security numbers, or proprietary business information. If your submission contains such confidential information, opting for a written/paper submission following the detailed instructions provided by the FDA is advisable.
The FDA's goal to safeguard public health by ensuring the safety and effectiveness of medical equipment highlights the significance of a thorough 510(k) submission. The agency's recent final rule on direct-to-consumer prescription drug advertisements emphasizes the necessity for clear and understandable presentation of information, mirroring the level of clarity that should be aimed for in your 510(k) submissions.
Considering the documentary 'The Bleeding Edge', which brought attention to worries regarding the 510(k) clearance process, it's clear that manufacturers bear the duty to guarantee that their products are not only in compliance but also safe for users. By following these guidelines, your submission will not only meet regulatory requirements but also contribute to the FDA's overarching goal of safeguarding public health.

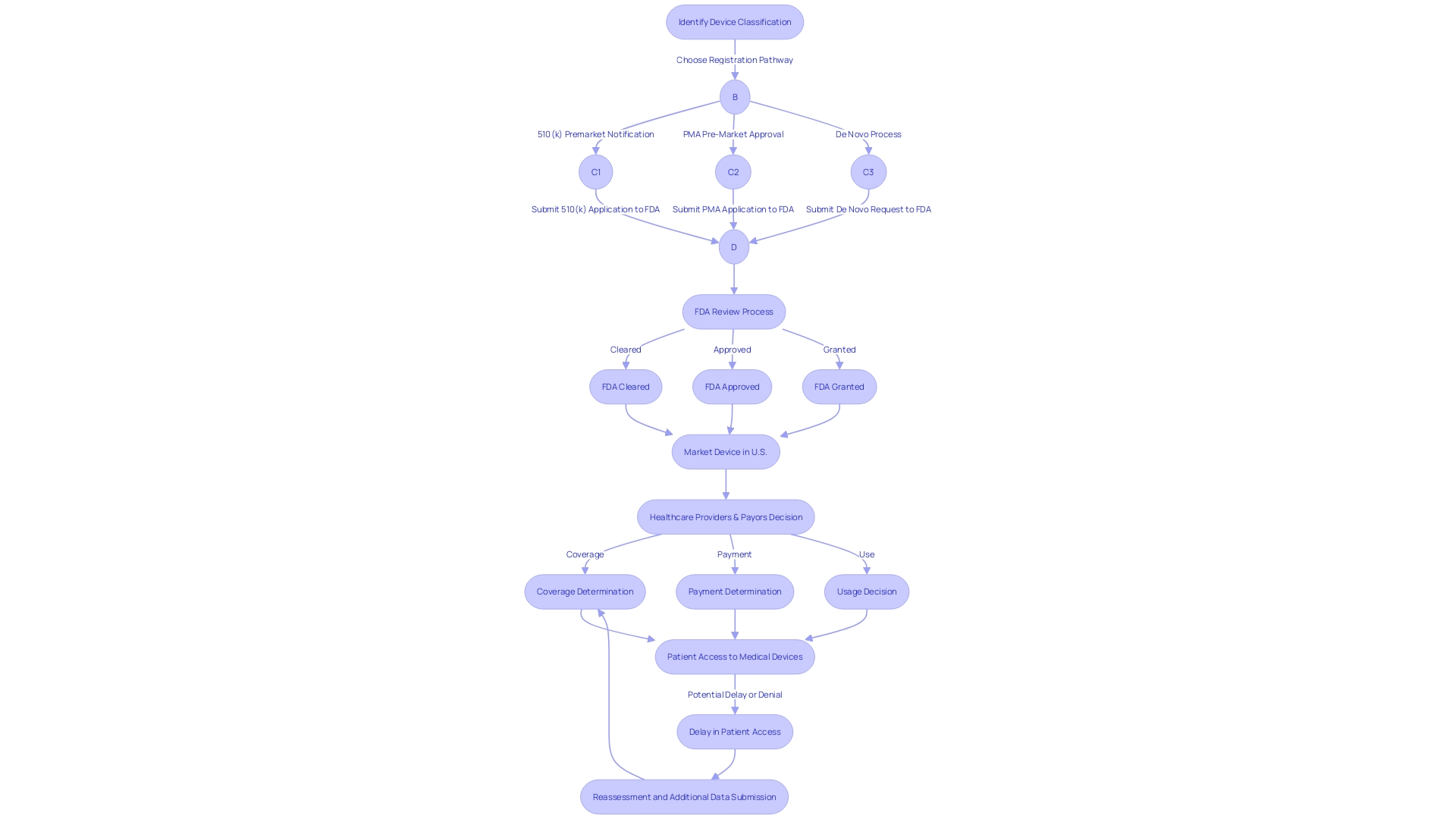
FDA Review Process and Timeline
The FDA plays a crucial role in ensuring the safety and effectiveness of medical instruments in the U.S. When a 510(k) application is submitted, it undergoes a comprehensive review where the FDA assesses whether the new apparatus is substantially equivalent to an existing, legally marketed prototype. Understanding the purpose of the subject object, including its users, usage instructions, warnings, and cautions, is crucial. Equally important is understanding the competitive landscape, which involves analyzing research literature, clinical studies, competitor websites, brochures, and product labeling.
Medical apparatus are classified into three categories depending on their level of risk and necessary regulatory control, with class three apparatus subject to the most rigorous examination process due to their high-risk nature and potential to sustain or support life. Only approximately 10% of medical products regulated by the FDA belong to this classification, which encompasses essential life-saving implants such as pacemakers.
Once the FDA approves a product, healthcare providers and payors, such as CMS and private health insurers, play a significant role in deciding its coverage and reimbursement. However, the data required by the FDA for clearance may differ from the data payors need for coverage decisions, potentially leading to delays or denials in equipment coverage even after FDA clearance.
The eCFR, an electronic representation of the Code of Federal Regulations, offers detailed information on regulatory procedures with well-structured formatting for the convenience of users. To fully understand the regulatory landscape and the steps involved in obtaining FDA clearance for medical devices, it is crucial to refer to this and other trustworthy sources.
Submission Acceptance and Substantive Review
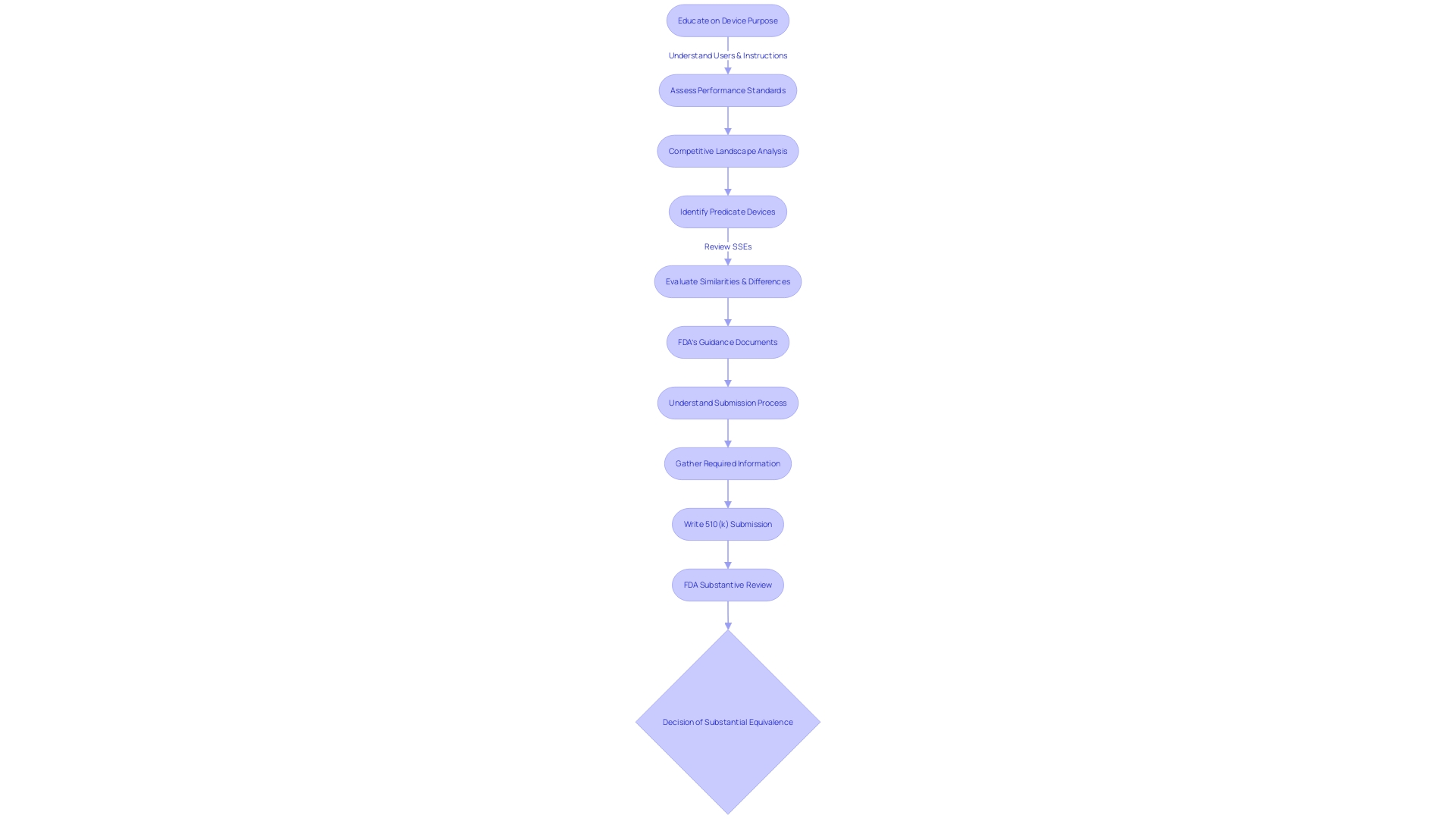
Navigating the FDA's substantive review process is crucial for medical equipment manufacturers seeking 510(k) clearance. This thorough analysis involves a thorough assessment of different aspects of the product, including its performance standards, labeling, and any clinical data submitted. To get ready for this phase, it is crucial to educate oneself thoroughly on the apparatus's intended use and operation, along with the users it's designed for, such as clinicians, physicians, dentists, and patients. Furthermore, understanding the competitive scenery through research literature, clinical studies, and marketing materials can offer insights into potential models that have similar intended uses and technological characteristics.
Furthermore, the FDA is dedicated to safeguarding public health by guaranteeing the safety and effectiveness of medical instruments. It is vital to follow FDA guidelines when submitting any comments or documentation, ensuring that no confidential information is inadvertently made public. As part of the agency's efforts to maintain transparent and clear communication, standards have been established, such as using consumer-friendly language and ensuring that information is presented in an easily understandable manner, both audibly and visually.
It is also important to be aware of the FDA's continuous updates and publications, such as the recent final rule on direct-to-consumer prescription drug advertisements, which underscores the agency's commitment to clarity and neutrality in public communications. In this context, creating a thorough comparative table that emphasizes the similarities and distinctions between the subject gadget and current ones is an essential stride in the evaluation. By doing so, manufacturers can anticipate and address any potential issues that the FDA may raise during the substantive review, thereby facilitating a smoother clearance procedure.
Communications with FDA Reviewers
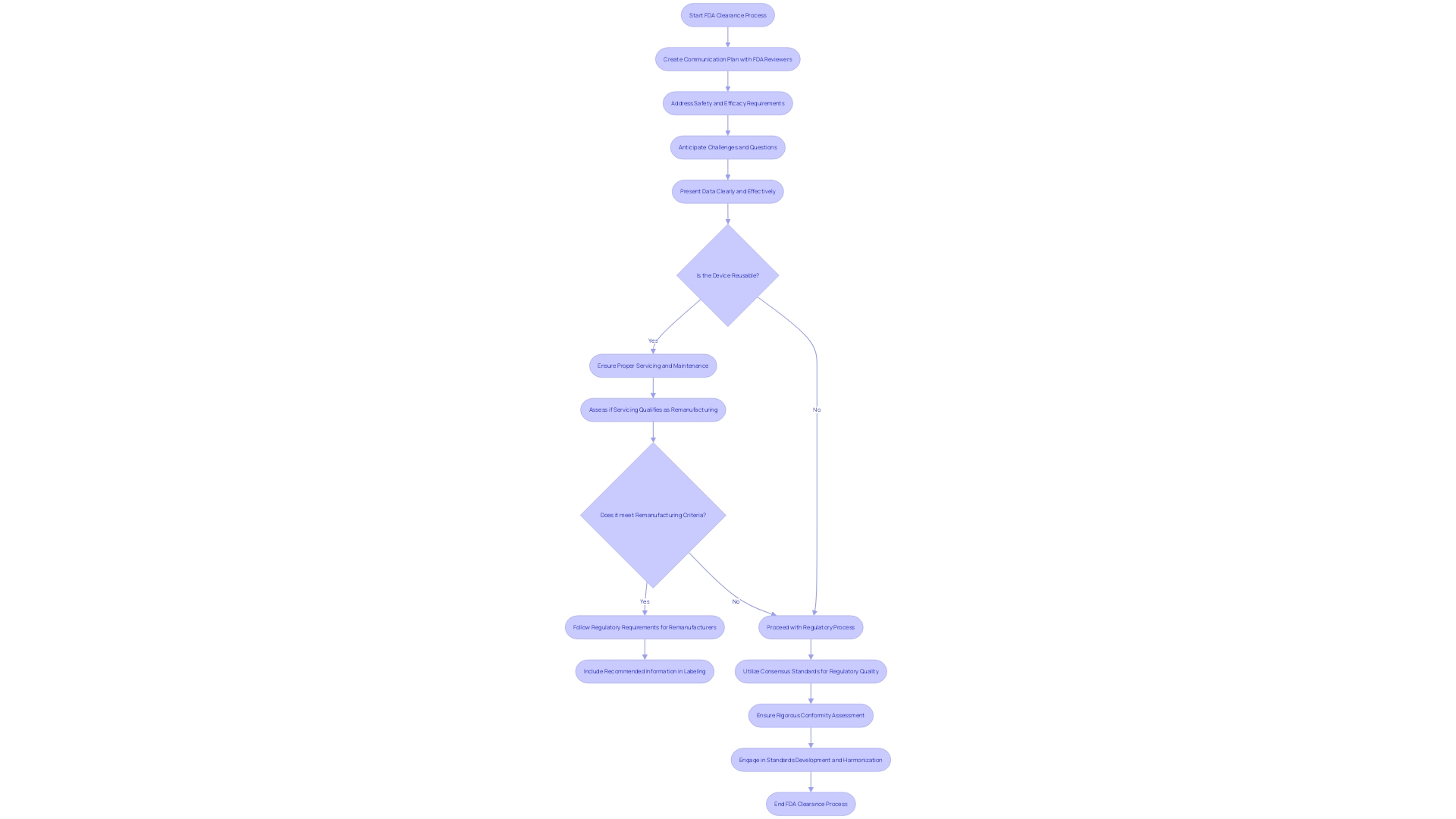
Navigating the complexities of FDA clearance, including the 510(k) pathway, is a critical step for medical equipment manufacturers. An essential component of this process is fostering effective communication with FDA reviewers. To accomplish this, it is recommended to create a plan that includes transparent and succinct delivery of information, addressing safety and efficacy, which is in line with the FDA's function in the assessment of medical equipment.
It is not uncommon for there to be a misalignment between the data submitted to the FDA and the information required by payors, such as CMS or private health plans. This difference can result in delays or denials in coverage and reimbursement post-clearance, affecting patient access to new medical equipment. Therefore, it is beneficial to anticipate these challenges and prepare to address any questions or concerns from the FDA with comprehensive data that may also satisfy payor requirements.
Recent standards set by the FDA underscore the importance of presenting information in a consumer-friendly manner. This principle can extend to communications with FDA reviewers, where clarity and understandability are paramount. By presenting data clearly and addressing the FDA's inquiries promptly and thoroughly, manufacturers can facilitate a smoother clearance procedure.
The classification and selected regulatory pathway, whether it is 510(k), PMA, or De Novo, influence the communication strategy. Understanding that science is dynamic and regulatory advice may evolve, it is important to keep the dialogue with the FDA adaptive and informed by current best practices.
Furthermore, real-life examples of professionals in the field, such as Chris, a biomedical engineer with 13 years of expertise, demonstrate the significance of efficiently overseeing clinical studies and the contribution of solutions engineers in simplifying regulatory procedures. Utilizing the knowledge of experienced professionals can help manufacturers in maintaining open lines of communication with the FDA, ultimately improving the likelihood of successful clearance.
Common Challenges and Best Practices
The 510(k) clearance process is a pivotal step for medical instrument manufacturers aiming to enter the U.S. market. It necessitates a comprehensive comprehension of the apparatus, its intended users, and the competitive landscape. A comprehensive approach involves examining research literature, clinical studies, and existing tools with similar functions. This comparative analysis is necessary to identify a suitable predicate instrument that shows similar intended use and technological characteristics, which is the foundation of the 510(k) process.
Manufacturers must navigate the FDA's classification system, which assigns products to one of three regulatory classes based on the level of control necessary to ensure safety and effectiveness. The classification determines the regulatory pathway, be it 510(k) Premarket Notification, Pre-Market Approval (PMA), or De Novo classification. It's crucial to understand these distinctions to avoid regulatory missteps.
In the ever-changing realm of medical equipment, keeping updated is crucial. For instance, the evolving surgical sutures market, projected to grow annually by 3.4% and reach $4.5 billion by 2033, underscores the importance of continued learning and adaptation to market trends. Furthermore, real-world case studies, such as the NIH's handling of plagiarism in the grant application process, highlight the critical nature of maintaining integrity throughout every stage of development and review.
Optimal methods for attaining clearance involve constructing a comprehensive comparative table to establish similarities with a chosen reference item and examining the Summaries of Safety and Effectiveness accessible on the FDA's database. Such due diligence is vital in ensuring a medical instrument's path to market is both compliant and efficient.
The consequences of distributing a medical product without FDA clearance are severe, as emphasized by the FDA's Office of Criminal Investigations. Ensuring adherence to regulatory requirements not only protects patient safety but also shields manufacturers from legal and reputational harm. Therefore, a knowledgeable and careful approach to the 510(k) clearance process is essential for success in the medical instrument industry.
Final Clearance and Post-Clearance Requirements
Obtaining 510(k) clearance from the FDA indicates that your medical equipment is essentially the same as a legally marketed product and is prepared for commercial distribution. It is important to acknowledge that while some equipment may not require clinical trials for clearance, as exposed in the 2018 documentary 'The Bleeding Edge', this absence of necessity has been examined due to linked patient risks in certain instances. The FDA's responsibility, as a component of the U.S. Department of Health and Human Services, is to guarantee the safety and effectiveness of medical equipment. The classification of a device, based on associated patient risk, determines the regulatory pathway—be it 510(k) Premarket Notification, Pre-Market Approval (PMA), or the De Novo process. Regulatory professionals should clearly understand the nuances between terms like Registered, Cleared, Approved, and Granted to navigate the compliance landscape effectively. Following clearance, manufacturers must adhere to post-market surveillance and reporting obligations to maintain compliance with FDA regulations, thus safeguarding public health.
Conclusion
The FDA's 510(k) clearance process is crucial for medical device manufacturers to introduce their products to the U.S. market. Understanding the different types of 510(k) submissions, selecting an appropriate predicate device, and meeting content requirements are essential. Manufacturers must thoroughly understand their device's purpose, intended users, and the competitive landscape.
A comparative analysis using research literature, clinical studies, and marketing materials helps establish substantial equivalence with a predicate device. The FDA's review process categorizes devices into three classes based on risk, with Class III devices undergoing the most stringent review. Clear and concise communication with FDA reviewers is important, addressing safety and effectiveness.
Manufacturers should anticipate challenges and provide comprehensive data that satisfies both FDA requirements and payor needs. Achieving final clearance from the FDA signifies readiness for commercial distribution. Post-clearance, manufacturers must fulfill post-market surveillance and reporting obligations to ensure ongoing compliance with FDA regulations.
By following these guidelines and providing accurate information, manufacturers can successfully navigate the FDA's 510(k) clearance process while ensuring patient safety and promoting innovation in the medical device industry.
Learn more about the FDA's 510(k) clearance process and how to navigate it successfully.




