Introduction
The 510(k) premarket notification process is a critical step in ensuring that a new medical device can be legally marketed in the United States. This process requires manufacturers to demonstrate that their device is substantially equivalent to another device that has already been legally marketed, known as the predicate device. By comparing the safety and effectiveness of the new device to its predicate, regulatory professionals ensure the device meets stringent standards while fostering innovation.
In this article, we will explore the different types of 510(k) submissions, how to access the FDA 510(k) database, the searchable information within the database, key components of a 510(k) submission, and tips for a successful submission. We will also discuss common challenges and pitfalls in the submission process and the use of the FDA 510(k) database for research and development in the medical device industry. By understanding these aspects, professionals can navigate the regulatory landscape with precision and ensure the safety and efficacy of their medical devices.
What is a 510(k) Premarket Notification?
The 510(k) premarket notification represents a critical step in ensuring that a new medical device is legally marketable in the United States. Put simply, manufacturers must demonstrate that their device is substantially equivalent to another device that has already been legally marketed, known as the predicate device. This process, which is generally required for devices not classified as Class I exempt, hinges on a meticulous comparison of the new device to its predicate in terms of safety and effectiveness.
A poignant example of the 510(k) process in action involves a recent case where a novel baby monitoring system, Stork, was introduced. Stork capitalizes on Signal Extraction Technology®, or SET®, which has played a pivotal role in reducing neonatal blindness and enhancing screening for congenital heart disease. By providing continuous monitoring of a baby's vital signs and video surveillance at a fraction of traditional costs, Stork exemplifies how advanced technology can be leveraged for patient safety and well-being.
Similarly, the Impella Connect System illustrates the breadth of devices subject to FDA's 510(k) process. This system, comprising both hardware and software, allows remote monitoring of ventricular support devices, delivering crucial patient-specific medical information and generating time-critical alarms. The Impella example underscores the FDA's comprehensive approach to device classification and the rigorous assessment required for premarket notification.
The significance of these processes is underpinned by the overarching definition of a medical device by the WHO, which encompasses a wide range of products from simple tongue depressors to complex diagnostic software. The classification and subsequent pathway to market authorization, whether through 510(k), PMA, or De Novo process, are predicated on the intended use and potential risk to patients.
Regulatory professionals must navigate these pathways with precision, as the terms 'Registered,' 'Cleared,' 'Approved,' and 'Granted' carry distinct meanings and implications for market access. The eCFR provides a structured and user-friendly interface to aid in this process, ensuring regulatory intent is maintained without altering agency guidelines. This meticulous approach to medical device regulation is designed to foster innovation while prioritizing patient safety and efficacy of the devices.
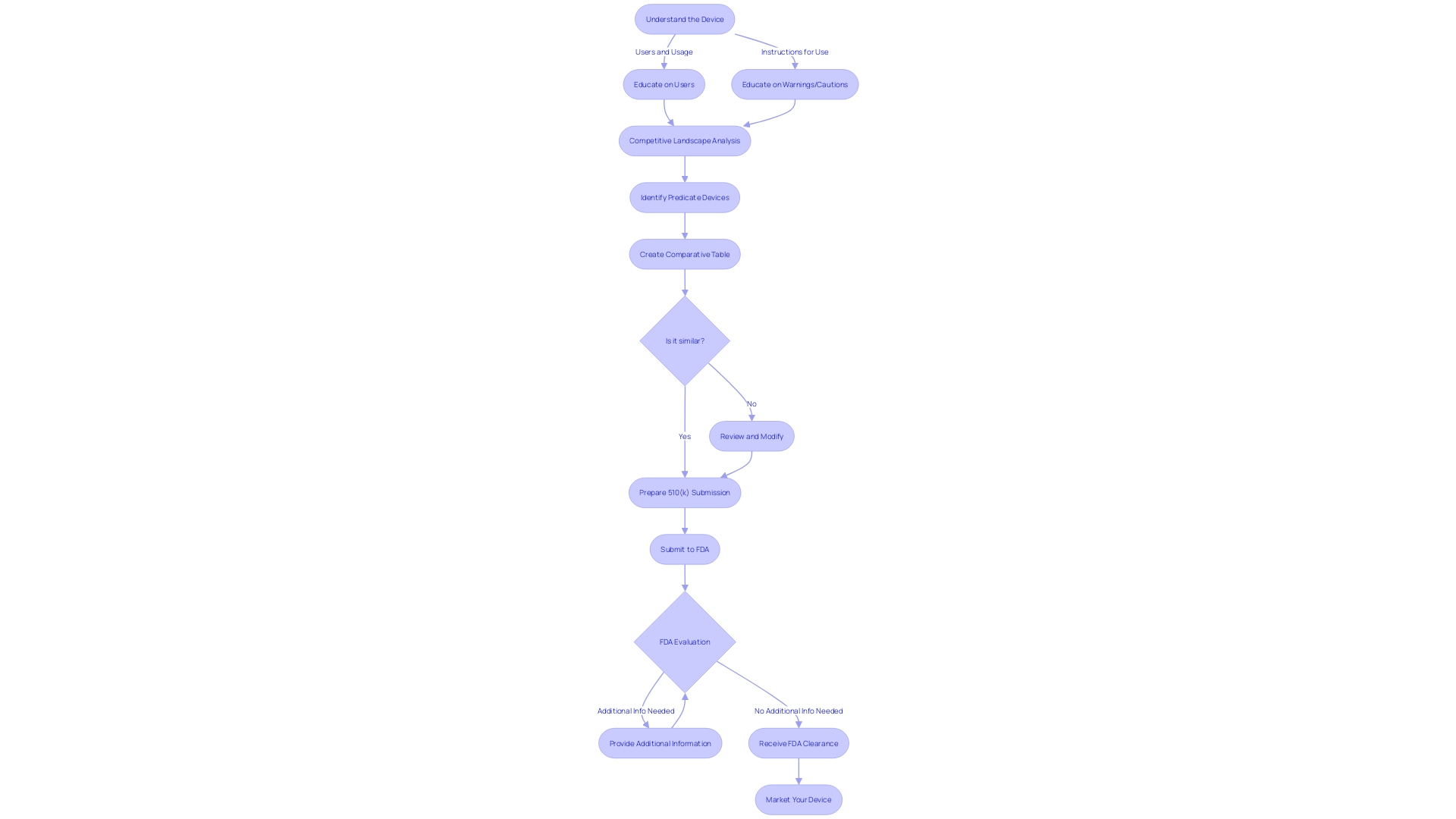
Types of 510(k) Submissions: Traditional, Special, and Abbreviated
Understanding the various types of 510(k) submissions is a critical step in managing the regulatory process for medical devices. The three primary types are traditional, special, and abbreviated submissions. Each has distinct requirements based on the device's intended use, technological characteristics, and the availability of predicate devices.
For a traditional 510(k) submission, a comprehensive comparison to a predicate device is essential. This involves a thorough examination of research literature, clinical studies, and comparative analysis of the device's specifications and performance data against those of an existing, similar device that has already been cleared by the FDA. Reviewing the Summaries of Safety and Effectiveness Data (Seeds) in the FDA's 510(k) database is an integral part of this process, providing insights into the similarities and differences that could impact the submission's outcome.
Special 510(k) submissions are a pathway for certain modifications to an existing cleared device. This type of submission focuses on the design and labeling changes that do not affect the device's intended use or alter its fundamental scientific technology.
Abbreviated 510(k) submissions are intended for devices that can demonstrate conformance with FDA guidance documents, special controls, or recognized consensus standards. This pathway can streamline the review process by relying on a declaration of conformity to these established benchmarks.
Recent FDA actions, such as issuing warning letters to companies for non-compliance, underscore the importance of meticulous regulatory adherence. The FDA's data-driven approach to oversight and enforcement, using tools such as rapid surveillance and analysis of retail sales and survey data, highlights the need for companies to be diligent in their submissions.
As the medical device industry evolves, staying informed about the latest regulatory requirements and trends is crucial. The Medical Devices: Filings Trends & Signals report offers valuable insights into market dynamics, helping companies navigate the regulatory landscape effectively.
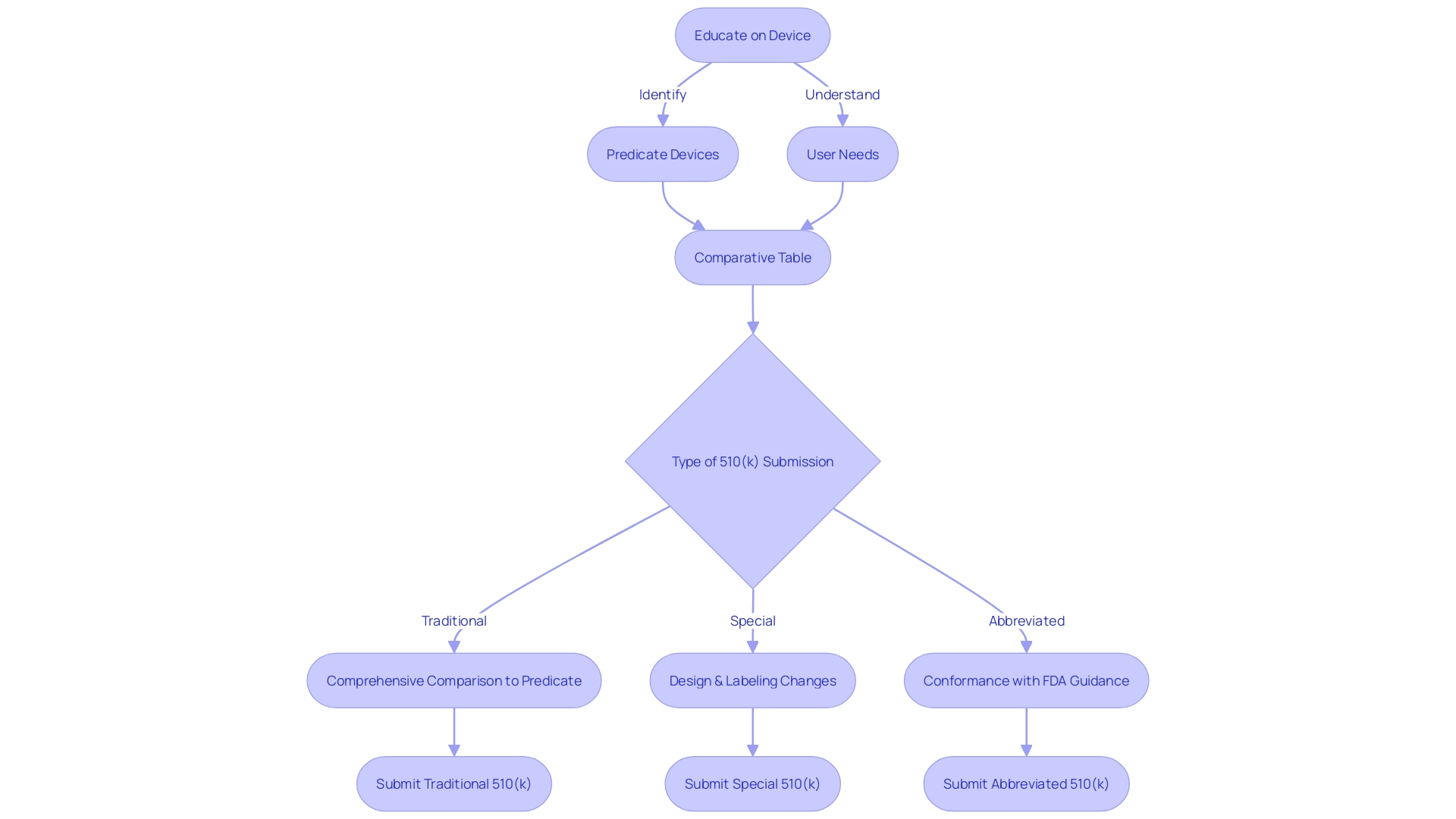
How to Access the FDA 510(k) Database
The U.S. Food and Drug Administration (FDA) maintains an essential online resource, known as the FDA 510(k) database, which houses applications for the 510(k) clearance process—a pathway responsible for the approval of approximately 99% of all human medical devices. As a central tool for professionals in the clinical research field, the 510(k) database enables a comprehensive examination of medical devices, providing detailed information such as Summaries of Safety and Effectiveness and instruction manuals, which are critical for understanding the devices' purposes, users, and usage guidelines. This information is not only pivotal for regulatory compliance but also serves as a foundation for comparative analysis against competitive devices in the market.
To effectively navigate this database, it is imperative to have a systematic approach. By analyzing research literature, clinical studies, and existing marketing materials, one can identify predicate devices—those with the same intended use and similar technological characteristics—as a starting point. The creation of a comparative table can further facilitate the evaluation of similarities and differences between devices, a process that ultimately informs strategic decision-making and ensures alignment with the FDA's commitment to public health and safety.
The FDA 510(k) database has been a cornerstone of medical device regulation since at least October 18, 2000, reflecting the agency's broader role in safeguarding public health through the oversight of a diverse range of products. Given the importance of transparent and accessible regulatory information, it is notable that comments and submissions to the FDA are made public, underscoring the agency's dedication to an open dialogue with stakeholders while emphasizing the responsibility of commenters to omit confidential information from the public submissions.
Understanding the intricacies of the FDA's processes and databases is crucial for professionals overseeing medical device projects. From the initial team meetings to define project priorities, to the alignment of stakeholders with project vision and objectives, the FDA's resources play a pivotal role in guiding the successful navigation of the regulatory landscape.
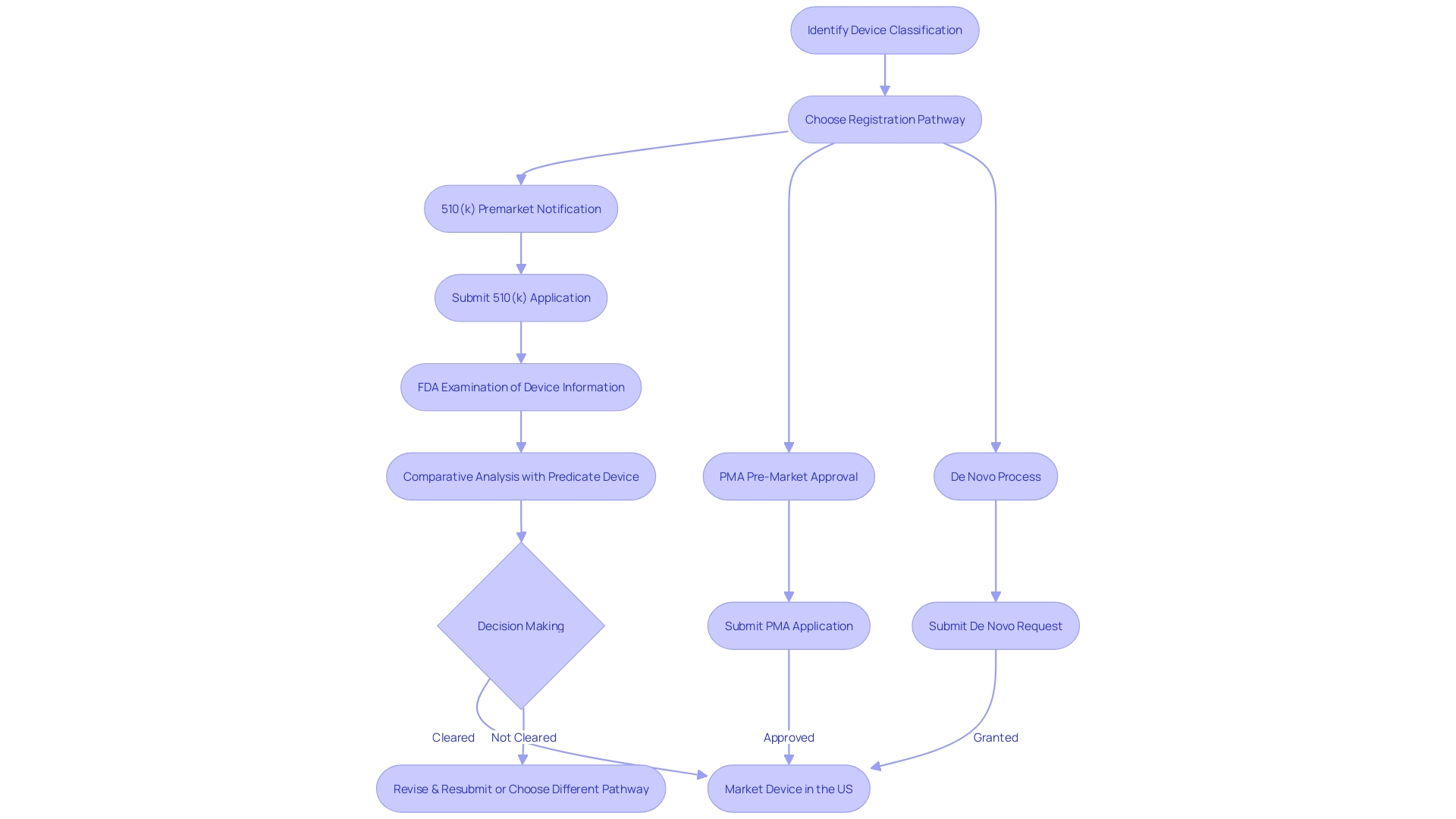
What Information is Searchable in the FDA 510(k) Database?
Navigating the FDA 510(k) database is a crucial step for any entity involved in the production and distribution of medical devices intended for the U.S. market. With a comprehensive repository of information on cleared devices, this database offers insights into product codes, device names, and detailed indications for use. However, effective utilization of the database requires an understanding of the FDA's classification system, which stratifies medical devices into three distinct categories based on patient risk.
Only after identifying the appropriate device classification can one determine the most suitable registration pathway—be it Premarket Notification (510(k)), Pre-Market Approval (PMA), or the De Novo process. Each pathway has its own set of requirements and nuances, which are critical to comprehend for successful navigation.
Moreover, regulatory professionals must be adept at distinguishing between the terms Registered, Cleared, Approved, and Granted—terms frequently used within the medical device industry that carry significant implications for market entry. The FDA's approval process is designed to ensure that new medical devices meet stringent safety and effectiveness standards, and the 510(k) database is a valuable tool for researchers and developers to assess potential predicate devices, examine their safety and effectiveness summaries, and understand the competitive landscape.
The importance of the FDA's role in safeguarding public health cannot be overstated. The agency's oversight extends beyond medical devices to drugs, biological products, food supply, cosmetics, and more, with the Center for Drug Evaluation and Research (CDER) annually approving a broad spectrum of new drugs and biological products, excluding certain categories managed by the Center for Biologics Evaluation and Research. The FDA continues to modernize its processes, as exemplified by the 510(k) modernization initiative, which aims to enhance the efficiency and effectiveness of the review process.
In light of this, it is imperative for anyone involved in device development or Regulatory Affairs to stay informed about the latest FDA guidance documents and updates, such as the draft guidance on best practices for selecting a predicate device released on September 7, 2023. This guidance is part of the FDA's ongoing efforts to refine the 510(k) submission process, providing clarity and support to industry professionals navigating the regulatory landscape.
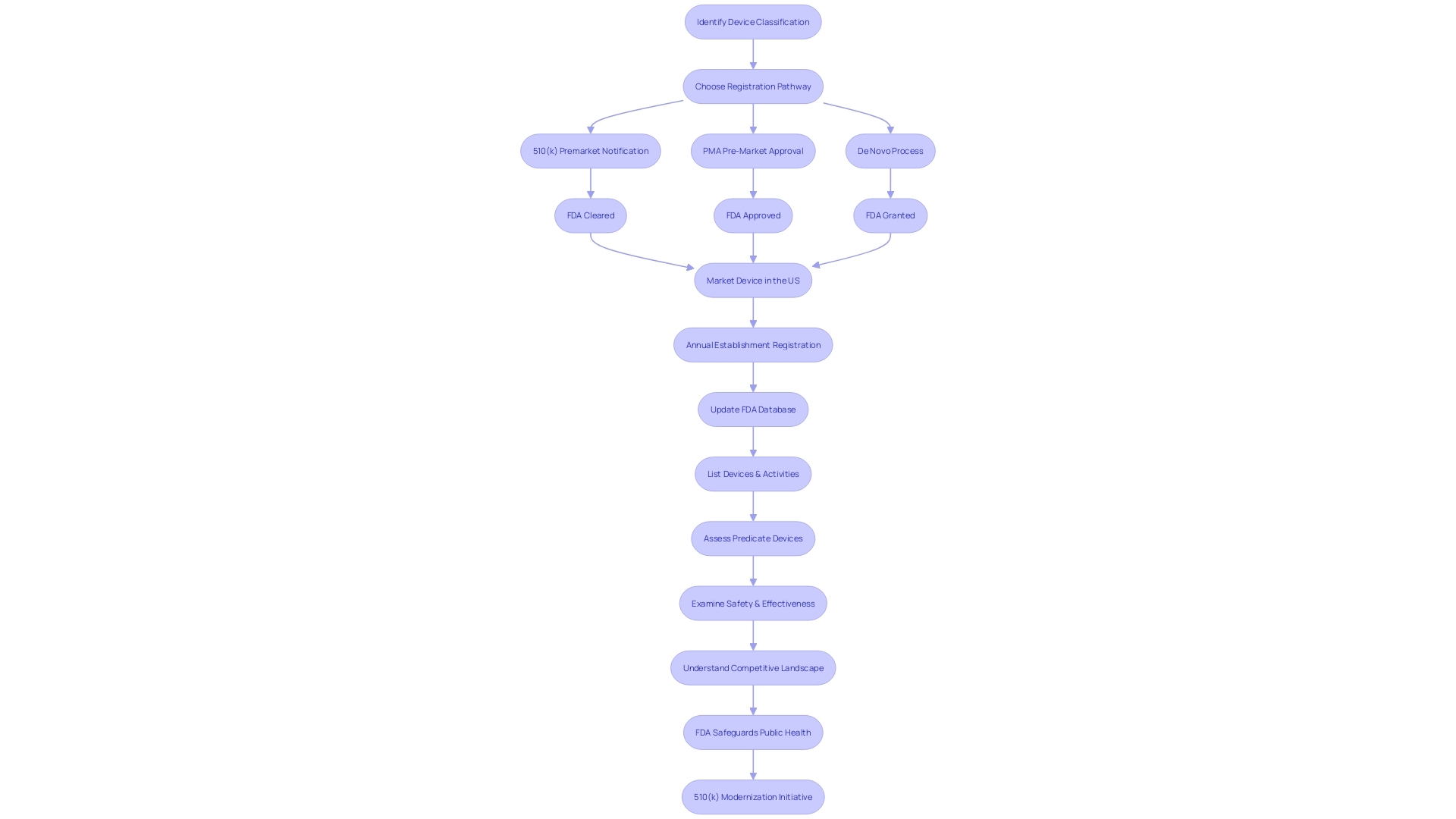
Key Components of a 510(k) Submission
Crafting a successful 510(k) submission is crucial for gaining FDA clearance for medical devices. This involves a thorough explanation of the device's characteristics, including a detailed description, performance data, and labeling. It's vital to understand the intended users, such as clinicians and patients, and the device's use instructions, including any warnings and precautions.
Additionally, it's important to identify potential predicate devices with similar intended uses and technological characteristics to create a comparative analysis.
The FDA categorizes medical devices into three classes based on risk, with class one and two devices typically undergoing a less stringent regulatory process, like 510(k) clearance, which involves comparing the new device to an existing, legally marketed predicate device. Understanding the nuances of the FDA's classifications and the differences between terms like 'Registered,' 'Cleared,' 'Approved,' and 'Granted' is essential for navigating the regulatory landscape.
To ensure safety and effectiveness, the FDA requires accurate documentation of the manufacturing process, which includes validation of aseptic processes and environmental monitoring, especially for products claiming sterility. Recent case studies highlight instances where firms neglected these critical aspects, leading to FDA enforcement actions.
The 510(k) process has been scrutinized for its approach to device clearance, as seen in the 2018 documentary 'The Bleeding Edge,' which revealed that clinical trials are not always mandatory for device clearance, raising concerns over patient safety. Despite this, the process allows for medical advancements, such as the introduction of 'twiist,' a new product aimed at improving the lives of those with type 1 diabetes.
In conclusion, a successful 510(k) submission requires meticulous attention to the FDA's regulatory requirements, a comprehensive understanding of the device and its competitive landscape, and a commitment to ensuring the safety and effectiveness of the product.
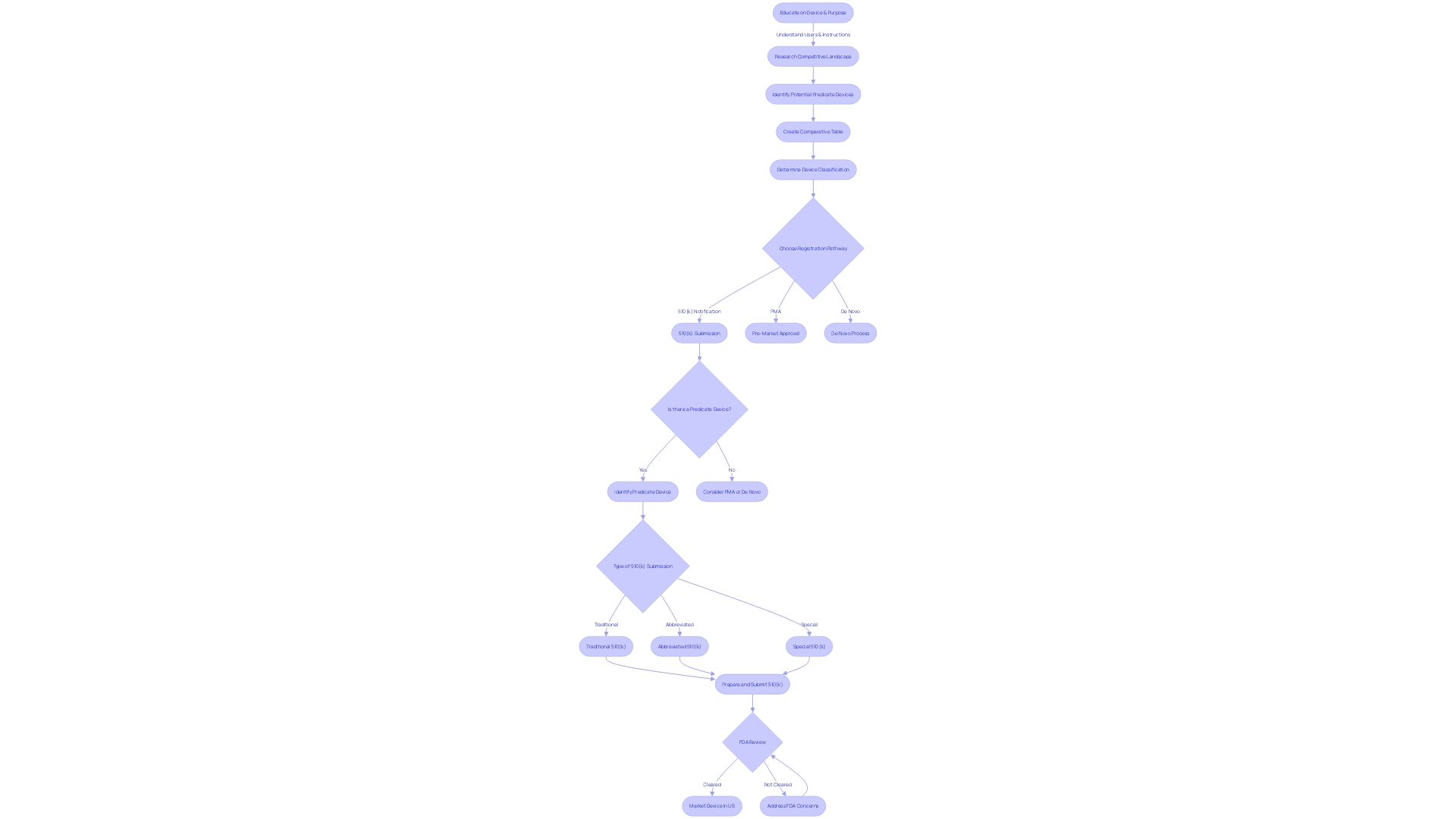
Finding a Predicate Device
Choosing the correct predicate device is a vital component of the FDA 510(k) submission process, serving as a benchmark to demonstrate substantial equivalence to your new medical device. To ensure a robust foundation for your submission, it is essential to conduct a comprehensive review of the FDA 510(k) database. This involves a meticulous understanding of the device in question, including its intended users—clinicians, physicians, dentists, patients—and its proper use, taking into account all warnings and precautions.
In collaboration with Marketing teams, one should explore the competitive landscape, examining research literature, clinical studies, and commercial materials to pinpoint potential predicate devices that share the same intended use and technological characteristics. Creating a comparative table becomes a strategic step in this process. Following the identification of such devices, it is advised to delve into the Summaries of Safety and Effectiveness Data (Seeds) available in the FDA's database, to assess the nuances between your device and the chosen predicate.
Recent efforts by the FDA to modernize the 510(k) process have spotlighted the advantages of selecting appropriate predicates, such as the availability of long-term safety data. These insights, derived from public feedback, have been encapsulated in a draft guidance document issued on September 7, 2023, outlining best practices for predicate selection. The preliminary steps include confirming that the potential predicate is currently registered and legally marketed, followed by a thorough comparison of intended uses and technological attributes to ensure no new safety or effectiveness concerns are introduced.
It's important to remember that despite the streamlined nature of the 510(k) process, it has faced scrutiny, as highlighted by the 2018 documentary 'The Bleeding Edge,' which revealed instances where the expedited clearance of devices led to adverse patient outcomes. Such revelations have reinforced the necessity of careful predicate device selection to safeguard patient health and uphold the integrity of the medical device industry.
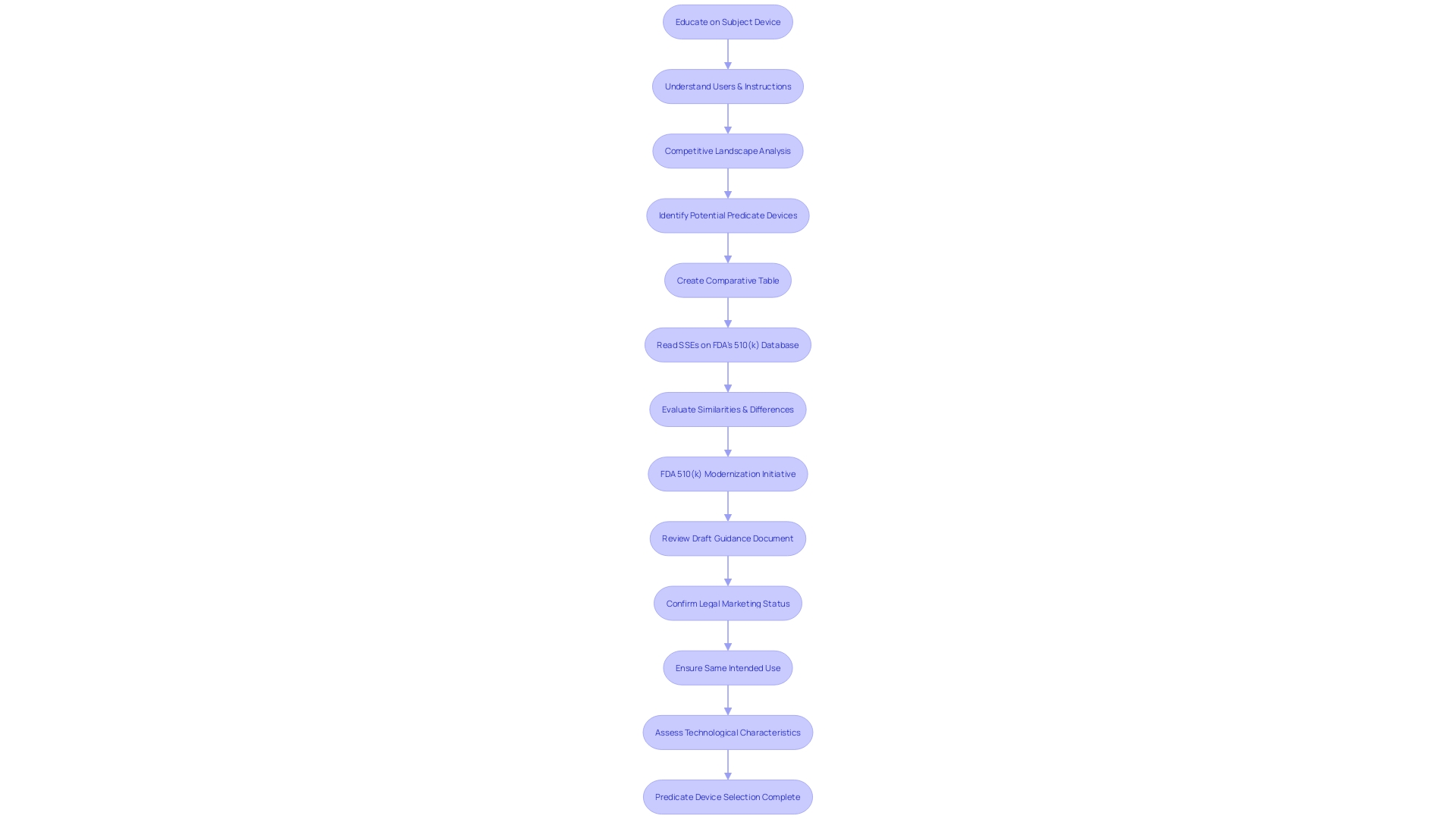
Content and Format Requirements for a 510(k) Submission
For a successful 510(k) submission, it's essential to meticulously organize the required documentation in line with FDA guidelines. A comprehensive submission should include a detailed list of all components used in the manufacture of the medical device, along with specifications and manufacturer details. Additionally, thorough descriptions of the manufacturing and packaging procedures, in-process controls, and tests to assure sterility, potency, and quality are vital.
Stability data and proposed expiration dating must also be included, with reference to the current U.S. Pharmacopeia standards.
A deep understanding of the device's purpose and its competitive landscape is equally crucial. This involves researching literature, clinical studies, and competitor products to identify predicate devices with similar intended uses and technological characteristics.
Recent FDA initiatives, such as setting clear standards for direct-to-consumer prescription drug advertisements, illustrate the agency's commitment to clarity and consumer safety, which aligns with the precise documentation expected in 510(k) submissions. Moreover, awareness of the evolving regulatory landscape is fundamental, as it shapes industry preparedness and the development of medical devices.
As the medical device industry grows, particularly with the rise of digital health solutions, companies must adapt to ensure expedient and compliant market entries. A successful track record in product launches can be indicative of a company's ability to navigate the intricate process from concept to commercialization, which is a valuable asset in the competitive medical device market.
Submission Process and Timeline
To orchestrate a successful 510(k) submission, one must delve into a detailed understanding of the subject device, its users—ranging from clinicians and dentists to patients—and the specific instructions for its use, including any warnings and precautions. This foundational knowledge is crucial as it guides the entire submission process. Furthermore, collaboration with Marketing teams to comprehend the competitive landscape is pivotal.
Researching and analyzing materials such as research literature, clinical studies, and competitor marketing materials can illuminate potential predicate devices that share similar intended uses and technological characteristics.
With these predicate devices in mind, it is beneficial to construct a comparative table, which serves as a tangible reference for evaluating similarities and differences. Delving into the FDA's 510(k) database to review the Summaries of Safety and Effectiveness (SSEs) is an essential step in this comparative analysis. This exercise not only provides a clearer picture of the regulatory landscape but also assists in aligning your device with the FDA's expectations for demonstrating substantial equivalence.
It is also important to be aware of the public nature of comments submitted to the FDA, taking care to withhold confidential information to avoid inadvertent disclosure. As for the review process, the timeline can vary based on the complexity of the device and the regulatory pathway it falls under. Devices categorized as class one or two generally undergo a more straightforward process, while class three devices, which include life-sustaining or supporting technologies like pacemakers, face more stringent regulatory scrutiny.
In recent times, there has been a concerted effort to streamline regulatory pathways, particularly for medical devices that meet urgent medical needs, as seen during the COVID-19 pandemic. This has led to a more collaborative approach between regulatory bodies and industry stakeholders, aiming to accelerate approval processes, especially in innovative fields such as digital health and personalized medicine.
Understanding these nuances and preparing thoroughly can significantly impact the efficiency and success of your 510(k) submission. By following these guidelines and staying informed on regulatory trends and updates, you can devise a strategy that navigates the complexities of the FDA's review process and moves your medical device closer to market authorization.

Tips for Successful 510(k) Submission
Adept preparation of a 510(k) submission is a pivotal factor in achieving a favorable review from the FDA. To enhance the likelihood of a smooth 510(k) process, it is critical to immerse oneself in the details of the subject device. This includes understanding its users—ranging from clinicians and patients to dentists—and familiarizing oneself with the device's instructions for use, while paying particular attention to warnings and precautions.
Moreover, it is beneficial to gain insight into the competitive landscape of the device. This can be achieved by assessing a variety of sources such as research literature, clinical studies, and competitor marketing materials to identify a predicate device with analogous intended use and technological features. Creating a comparative table early on can facilitate this process.
Leveraging the FDA's 510(k) database to review Summaries of Safety and Effectiveness Data (Seeds) can further aid in discerning the nuances between your device and the identified predicate.
The FDA's paramount concern is patient welfare, so it's essential to frame submission arguments with patient health and safety at the forefront. Any positions put forth should be substantiated with robust data, as the FDA increasingly relies on empirical evidence. As such, ensuring that every claim is backed by data will greatly enhance the credibility of the submission.
In the rapidly evolving medical device sector, companies like PharmaSens have made significant strides, as seen with their recent ISO 13485 certification for their insulin pump technology. This reinforces the importance of comprehensive design, development, manufacturing, and distribution processes in successful regulatory submissions.
With only 10% of medical devices falling under the FDA's stringent class three category, the majority are subject to less rigorous regulatory pathways, such as 510(k) clearance. However, despite the streamlined process, thoroughness remains key. As underscored by the 2018 documentary 'The Bleeding Edge,' the 510(k) route does not universally require clinical trials, which places an even greater onus on the accuracy and comprehensiveness of the submission documentation.
By taking these steps and approaching the 510(k) submission process with diligence and strategic forethought, the path towards clearance can be navigated more effectively, ultimately leading to successful outcomes and advancements in medical device innovation.

Common Challenges and Pitfalls in 510(k) Submissions
Navigating the 510(k) submission process involves a strategic understanding of both the medical device in question and the regulatory landscape. Initially, it is crucial to gain a comprehensive understanding of the device's users, ranging from clinicians and physicians to dentists and patients, and thoroughly comprehend the device's instructions for use, including any warnings and cautions. Collaborating with the Marketing team provides insights into the competitive landscape, allowing for the identification of potential predicate devices that share the intended use and technological characteristics with the subject device.
The creation of a comparative table becomes an instrumental part of this process.
Further, it is imperative to delve into the Summaries of Safety and Effectiveness (SSEs) available in the FDA's 510(k) database. This research will illuminate the similarities and differences between your device and the selected predicate, forming the basis of demonstrating substantial equivalence—a cornerstone of the 510(k) process.
The FDA's guidance documents offer a roadmap for submission, outlining clear expectations and the process details necessary for acceptance. The real challenge lies in compiling the requisite information within a given timeframe, ensuring the data aligns with the FDA's criteria for substantial equivalence.
In the initial stages of a medical device project, it is beneficial to convene all stakeholders, including key external parties, to prioritize the project's objectives and align on the project charter. Understanding who is responsible for each aspect of the project through a RACI matrix—identifying who is Responsible, Accountable, Consulted, and Informed—is also crucial for smooth project navigation. An initial team meeting serves as a platform to align the team, providing an opportunity to discuss project vision, objectives, and expectations, thereby setting the stage for successful collaboration.
To ensure a robust submission, it is advised to follow the FDA's recommendations and utilize their comprehensive resources to understand the nuances of the 510(k) submission pathways. This preparation is instrumental in overcoming the complexities inherent in the 510(k) process, enhancing the likelihood of a favorable review and approval by the FDA.
Using the FDA 510(k) Database for Research and Development
Harnessing the FDA 510(k) database is a crucial step in medical device research and development, enabling professionals to delve into the particulars of existing devices, spot market opportunities, and shape product development tactics. To fully utilize the FDA 510(k) database, one must acquire a thorough comprehension of the device in question, including its users, instructions, warnings, and cautions. An exhaustive understanding of the competitive landscape is equally important, mandating a review of research literature, clinical studies, and competitor marketing materials to identify potential predicate devices with analogous intended uses and technological characteristics.
It's essential to correctly classify the device, as the FDA sorts medical devices into three risk-based categories. This classification determines the pathway for registration—be it the 510(k) Premarket Notification, Pre-Market Approval (PMA), or the De Novo process. Each term—Registered, Cleared, Approved, and Granted—carries specific connotations in the industry, and it's crucial to discern and apply them correctly.
Recent FDA initiatives aim to refine the 510(k) submission process. Draft guidelines released on September 7, 2023, encapsulate best practices for selecting predicates, emphasizing the use of legally marketed devices with shared intended purposes and comparable technological features that do not introduce new safety and efficacy concerns.
Traditional methods for predicate selection include FDA-recognized consensus standards and guidance documents, as well as qualified medical device development tools. However, the FDA also acknowledges widely accepted scientific methods that have undergone public scrutiny or peer review. Access to comprehensive information on predicates enhances the selection process, although it may not always be feasible.
Medical devices range from the simple, like tongue depressors, to the complex, such as diagnostic computers and prostheses. Their design represents a significant aspect of mechanical engineering. Medical devices serve to diagnose, prevent, monitor, treat, or alleviate diseases, improving patient quality of life—a mission underscored by the World Health Organization's definition.
The FDA 510(k) database, when navigated with expertise, can be a potent tool in advancing this mission and fostering innovation within the medical device industry.
Conclusion
In conclusion, the 510(k) premarket notification process is crucial for ensuring the legal marketing of medical devices in the US. Understanding the different types of 510(k) submissions and accessing the FDA 510(k) database are key to navigating the regulatory landscape effectively. Crafting a successful submission involves thorough attention to device descriptions and understanding FDA classifications.
Challenges can be overcome by following FDA recommendations and utilizing their resources.
The FDA 510(k) database is a valuable tool for medical device research and development, providing detailed information on cleared devices. Staying informed about FDA guidance documents and updates is vital for successful navigation. Thoroughness and accuracy in documentation are essential for a smooth 510(k) process.
In summary, a successful 510(k) submission requires meticulous attention to regulatory requirements, a comprehensive understanding of the device and its landscape, and a commitment to safety and effectiveness. By following guidelines and utilizing FDA resources, professionals can navigate the complexities of the review process and contribute to medical device innovation.




