Introduction
The FDA's 510(k) Premarket Notification is a critical pathway for getting medical devices to market in the United States. Manufacturers seeking approval must demonstrate that their device is substantially equivalent to a legally available device, known as a predicate device. This process involves a detailed comparison of the new device's intended use and technical characteristics with those of the predicate.
Understanding the FDA classification for the device and conducting a comprehensive review of the competitive landscape are crucial steps in navigating this complex process. Recent trends have seen regulatory agencies and industry stakeholders working towards more streamlined approval pathways, particularly in response to the COVID-19 pandemic. The FDA upholds public health by ensuring the safety and efficacy of medical devices, with a strong emphasis on clear communication about the risks and benefits.
It is essential for manufacturers to have a thorough understanding of the device, its user base, usage instructions, and potential risks to create a successful submission. Additionally, the FDA's determination of a device's market readiness impacts how it may be marketed and introduced to the healthcare system. By following best practices and adhering to FDA guidelines, manufacturers can navigate the 510(k) process effectively and contribute to better healthcare outcomes for the public.
Understanding the FDA 510(k) Medical Device Approval Process
The FDA's 510(k) Premarket Notification is a critical pathway for getting medical devices to market in the United States. Manufacturers seeking approval must show that their product is 'substantially equivalent' to a device already legally available, known as a predicate device. This process involves a detailed comparison of the new device's intended use and technical characteristics with those of the predicate.
The journey begins by determining the correct FDA classification for the device, which depends on the level of risk it poses to patients. With classifications ranging from Class I (low risk) to Class III (high risk), the pathway to market will vary. For lower-risk Class I and II devices, a 510(k) submission may suffice, while Class III devices typically require a more stringent Pre-Market Approval (PMA) or the De Novo classification pathway, reserved for novel, low-to-moderate risk devices that lack a suitable predicate.
To navigate this complex process, it's imperative to gain a thorough understanding of the device in question, including its user base, usage instructions, and any potential risks. Additionally, a comprehensive review of the competitive landscape is necessary, often requiring an analysis of research literature, clinical studies, and existing market devices to establish a suitable predicate. This due diligence culminates in the creation of a comparative table, highlighting the similarities and differences between the new device and its predicate.
Understanding the nuances between being 'Registered,' 'Cleared,' 'Approved,' or 'Granted' by the FDA is essential for regulatory professionals. These designations reflect the level of review and authorization given to a medical device, impacting how it may be marketed and introduced to the healthcare system.
Recent trends have seen regulatory agencies and industry stakeholders working towards more streamlined approval pathways, particularly in response to the COVID-19 pandemic. This has been especially prominent in rapidly evolving fields like digital health and personalized medicine, where there is a significant need for timely innovations.
The FDA, as part of the U.S. Department of Health and Human Services, upholds public health by ensuring the safety and efficacy of medical devices. It places a high emphasis on clear communication about the risks and benefits of medical products, as evidenced by its recent final rule on the presentation of major statements in direct-to-consumer prescription drug advertisements. Such measures underscore the FDA's commitment to transparency and consumer protection in the medical device approval process.
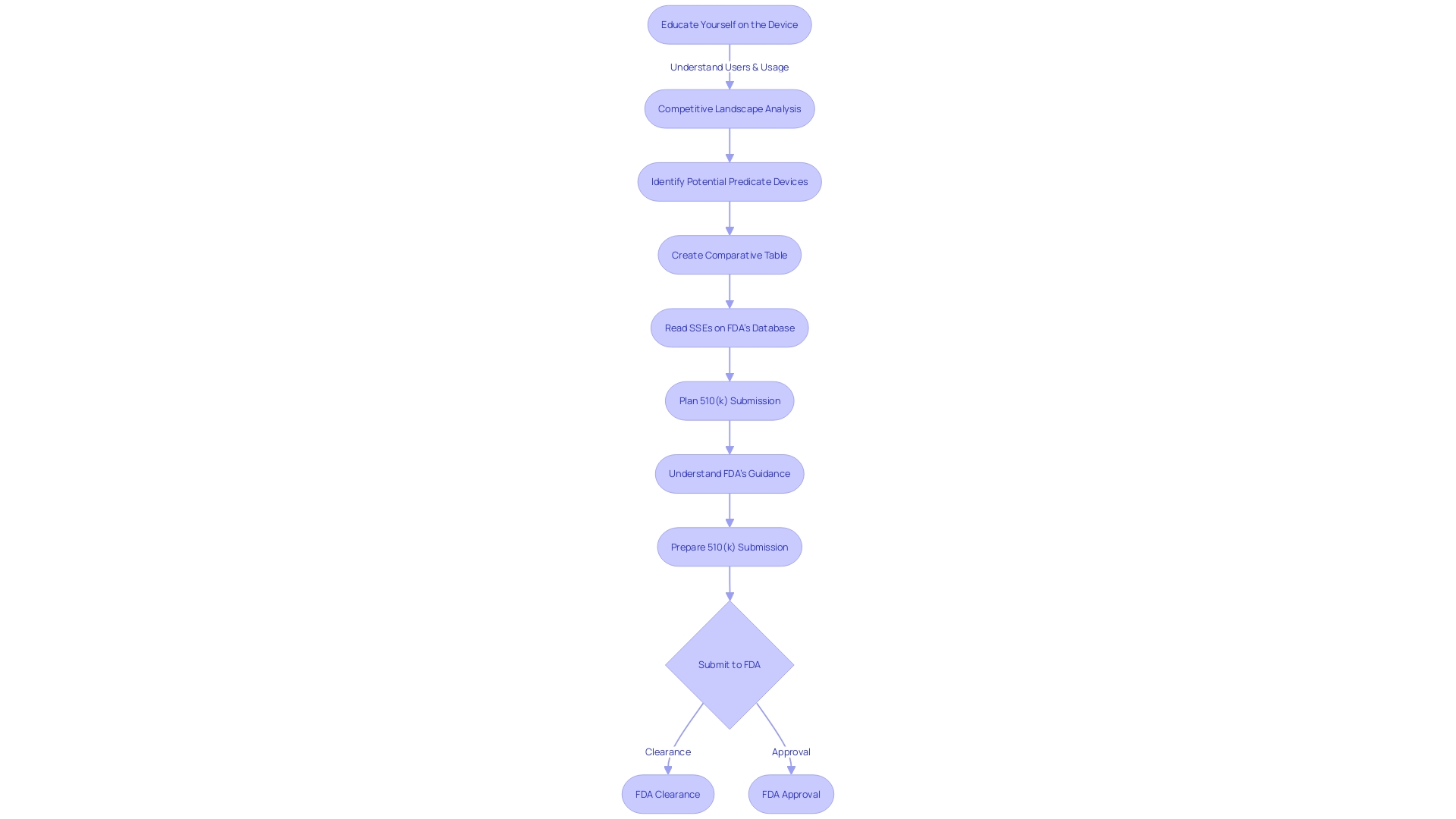
Types of 510(k) Submissions
The 510(k) submission process is a pathway that allows medical device manufacturers to obtain clearance from the FDA for marketing their devices. It is crucial to understand the specific type of 510(k) submission appropriate for a device, which may be traditional, special, or abbreviated. Each category necessitates particular documentation and adherence to distinct requirements.
For a traditional 510(k), one must comprehensively grasp the device in question, including its clinical applications, instructions for use, and any associated warnings. This involves a deep dive into research literature, clinical studies, and competitive analysis to identify predicate devices with analogous intended use and technological characteristics. Creating a comparative table is a fundamental step in this process.
Special 510(k) submissions may be suitable when a manufacturer modifies an existing device for which they have already obtained clearance. It requires the submission of summaries of safety and effectiveness data, and the manufacturer must demonstrate that they can control the device changes in compliance with the quality system regulation.
For an abbreviated 510(k), the emphasis is on utilizing guidance documents, special controls, and recognized standards to demonstrate compliance with regulatory requirements. It is essential to ensure that confidential information is safeguarded during the submission process, which may involve submitting certain documents as written/paper submissions.
The FDA's 510(k) database is an invaluable resource, providing access to safety and effectiveness summaries that can guide manufacturers in evaluating similarities and differences with potential predicate devices. It is important to note that public comments on these submissions are made available in the docket, so one must be cautious not to include confidential or sensitive information in such comments.
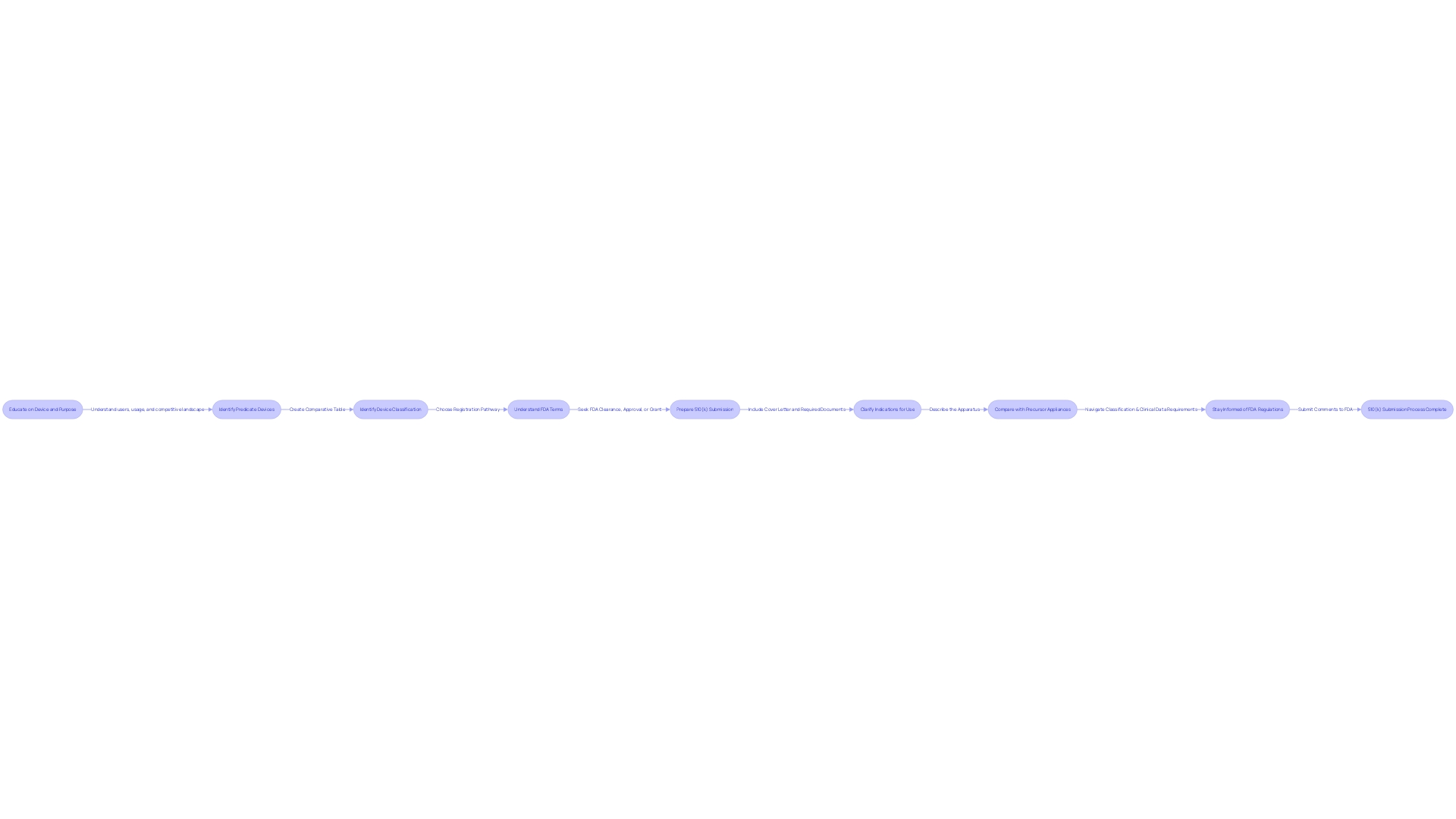
Preparing a 510(k) Submission
Compiling a 510(k) submission is a meticulous process that requires a comprehensive understanding of the medical device in question. It's essential to outline a clear and detailed description of the device, its intended application, the materials and components it comprises, as well as the performance specifications. Additionally, the submission should encompass any relevant clinical study data that corroborates the device's safety and efficacy.
To ensure a robust submission, it's imperative to delve into the device's purpose and the users it's intended for, which include healthcare professionals like clinicians, physicians, and dentists, as well as the patients themselves. The preparation must involve a thorough review of the device's instructions, paying particular attention to any warnings and precautions.
Moreover, partnering with the marketing team can be instrumental in learning about the competitive landscape. This collaboration will aid in identifying potential predicate devices—those with the same intended use and similar technological characteristics. A comparative analysis, supported by a carefully constructed table, should be included in the submission to illustrate the similarities and differences between the new device and the predicate.
The significance of this comparative evaluation is supported by numerous studies and expert opinions. For instance, the effectiveness of Masimo SET Measure-through Motion and Low Perfusion™ pulse oximetry, a technology introduced in 1995, has been validated by over a hundred independent studies. Such technologies have contributed to reducing severe retinopathy of prematurity in neonates and decreasing rapid response team activations, ICU transfers, and overall costs when used for continuous monitoring in post-surgical wards with Masimo Patient SafetyNet™. These real-world applications underline the critical nature of a detailed 510(k) submission that not only demonstrates safety and effectiveness but also the potential to improve patient outcomes.
In light of the FDA's mandate to protect public health by ensuring the safety and effectiveness of medical devices, it is crucial to recognize the agency's role in assessing these submissions. Devices requiring premarket authorization, such as the Impella Connect System, are scrutinized to ensure their features meet the statutory definitions and standards set by the FDA.
Finally, comments and feedback are a fundamental part of the regulatory process. Submitters should follow the guidelines for providing comments, ensuring that all submissions are devoid of confidential information unless appropriately filed as confidential submissions according to FDA instructions.
Each component of the 510(k) submission plays a vital role in the FDA's determination of a device's market readiness. The submission needs to be as informative as possible, leveraging research studies, the competitive landscape, and the FDA's comprehensive databases, to establish a product's suitability for clinical use.
Content Requirements for a 510(k) Submission
To ensure a successful 510(k) submission, it is imperative to include comprehensive content that enables the FDA to thoroughly evaluate the device's safety and utility. This content should encompass detailed device labeling, precise indications for use, a full device description, results from performance testing, and any pertinent clinical data. For instance, the Impella Connect System, a device comprising both software and hardware components, demonstrates the importance of clear descriptions of device functions that require premarket authorization from the FDA. In this case, the system's capability to provide remote monitoring and critical notifications aligns with the FDA's definition of a device under section 201(h) of the Act.
Moreover, as illustrated by Masimo SET Measure-through Motion and Low Perfusion™ pulse oximetry, it's crucial to provide evidence of the device's performance superiority and its impact on patient outcomes and healthcare costs. Masimo's technology has been validated in over 100 studies, underscoring the significance of robust clinical evidence in the 510(k) process.
The submission must also include a list of all device components, specifications for each, the name and address of each manufacturer, a description of manufacturing and packaging procedures, in-process controls, and necessary specifications to ensure the device's identity, strength, quality, purity, potency, and bioavailability. Additionally, stability data with proposed expiration dating should be presented, with the possibility of including alternatives to meet these requirements.
In-depth knowledge of the device is essential, including its users, instructions for use, and the competitive landscape. This understanding aids in identifying suitable predicate devices with analogous intended use and technological characteristics. As per recent FDA guidance, the selection of a predicate device is a strategic step in the 510(k) submission process. This includes ensuring the predicate is legally marketed and assessing whether any differing technological characteristics raise new questions regarding safety and effectiveness.
The submission should detail the general description of the condition the device intends to diagnose, treat, prevent, cure, or mitigate, including a description of the intended patient population. It's also important to include the generic and proprietary names, device specifications, pictorial representations, and a list of each functional component or ingredient if the device consists of multiple parts.
In essence, a thorough and well-documented 510(k) submission, backed by substantial clinical data and clear device descriptions, is vital for the FDA's assessment of the device, which ultimately has far-reaching implications for public health and safety.
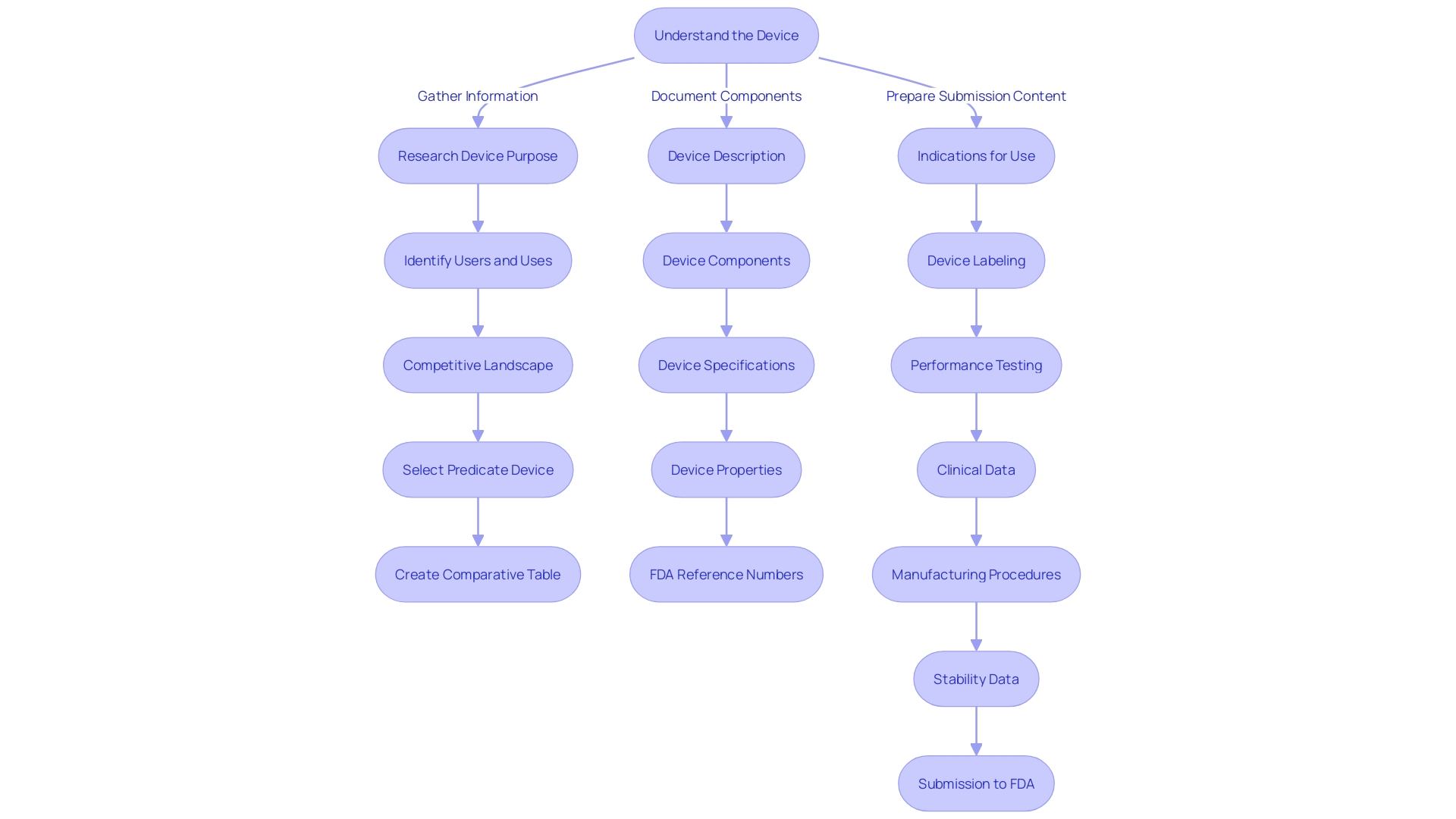
Finding and Using Predicate Devices
The backbone of the 510(k) submission process lies in the strategic selection of a predicate device—a legally marketed device used as a benchmark to demonstrate substantial equivalence to a new device. This is critical for medical device manufacturers as it may circumvent the need for costly clinical trials, as highlighted by the 2018 documentary 'The Bleeding Edge', which revealed that certain devices have been fast-tracked without such trials, sometimes resulting in patient harm.
To identify an appropriate predicate, it is imperative to gain a comprehensive understanding of the subject device's intended users, such as clinicians and patients, and to thoroughly review its instructions for use, alongside warnings and cautions. Collaboration with Marketing teams is also essential to assess the competitive landscape. Delving into a wide array of resources, including research literature, clinical studies, and competitor marketing materials, facilitates the identification of devices with comparable intended uses and technological characteristics.
A critical tool provided by the FDA is the 510(k) Premarket Notification database. This resource allows for meticulous research into potential predicates, enabling manufacturers to construct comparative tables and pore over Summaries of Safety and Effectiveness to discern critical similarities and differences.
FDA's primary mandate is to ensure the safety and effectiveness of medical devices in the U.S., a role that extends beyond the approval or clearance phase. It is noteworthy that data submitted for FDA clearance might not align with the evidence required by payors for coverage decisions, which can lead to delays in device availability to patients after FDA clearance.
Voluntary consensus standards, developed by Standards Development Organizations (SDOs), play a significant role in the regulatory process. These standards, which must adhere to principles of transparency, openness, balance, and due process, underpin the rigorous conformity assessments—activities crucial for a robust regulatory framework. The FDA's use of these standards ensures that medical devices meet high safety and effectiveness criteria before reaching the public domain. By leveraging these databases and standards, manufacturers can navigate the 510(k) process with greater precision and confidence.
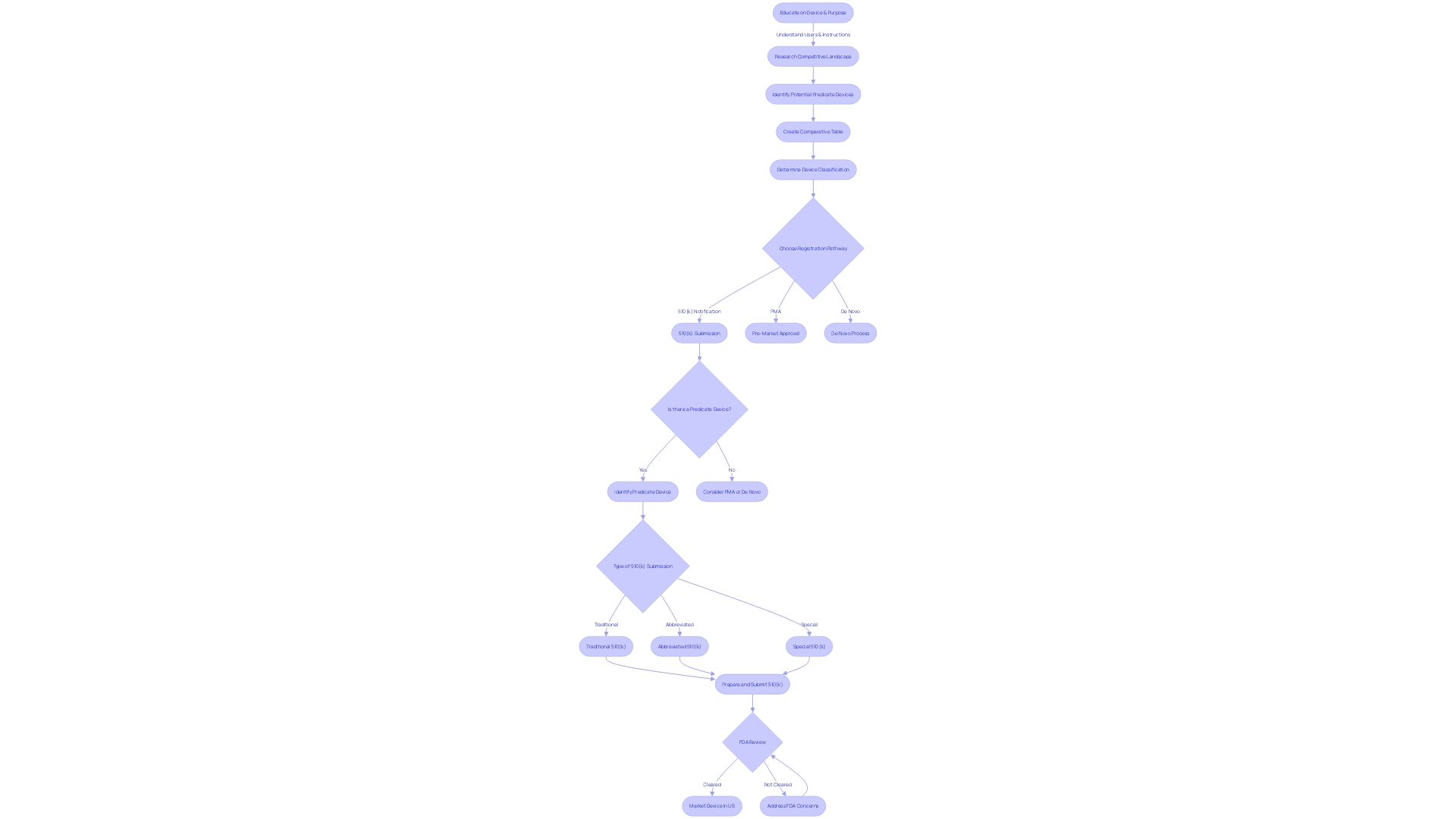
Submission Formats: eSTAR and eCopy
For medical device manufacturers seeking FDA clearance through the 510(k) pathway, understanding the available electronic submission formats is critical. The FDA offers two methods for this process: Estar and eCopy. Estar, short for electronic Submission Template And Resource, is an interactive PDF that guides users through the submission process, ensuring all necessary information is included and properly formatted. It streamlines the review process by allowing for a more consistent and complete submission. On the other hand, an eCopy is a PDF version of the 510(k) submission that must be accompanied by a physical copy on a USB drive or CD-ROM, mailed to the FDA's Document Control Center. Both formats require meticulous attention to detail, as any confidential information, such as proprietary data or personal identifiers, must be carefully excluded to avoid public disclosure upon submission to the docket. The choice between Estar and eCopy will depend on the manufacturer's preference and preparedness to adhere to the FDA's stringent guidelines for electronic submissions.
Acceptance Review Process
When a 510(k) submission arrives at the FDA, it is critical to the review process that the submission is meticulously complete, as it then undergoes a thorough acceptance review. The FDA's mandate is to ensure the safety, efficacy, and security of medical devices, which necessitates a careful examination of each 510(k) submission to confirm that it meets all the necessary administrative criteria. If any deficiencies are found, the submitting manufacturer is promptly informed, and a request for supplementary information may be issued. Manufacturers should be well-prepared for this process by having a robust understanding of the device in question, its users, and all associated instructions, including any warnings. It is equally important to comprehend the competitive landscape, identifying potential predicate devices with analogous intended uses and technological characteristics. This comprehensive approach, including a comparative analysis, is vital for a successful submission. Furthermore, the FDA's recent final rule on direct-to-consumer prescription drug advertisements underscores the importance of clarity and transparency, which are also essential in 510(k) submissions. The FDA's guidance documents offer invaluable insights into the submission process, presenting a clear roadmap for manufacturers to ensure their submissions are accepted without unnecessary delays.

Substantive Review and Decision-Making
The substantive review stage of the 510(k) medical device approval process is a critical juncture where the FDA rigorously evaluates the device to ensure it meets safety and effectiveness standards. This stage goes beyond a cursory comparison to predicate devices; it often necessitates a thorough examination of clinical data and device performance. Although clinical trials may not always be mandatory for 510(k) clearance, as highlighted by concerns raised in 'The Bleeding Edge' documentary, the FDA's emphasis on patient safety remains paramount, particularly for implant devices that present unique risks due to their continuous use within the body.
During this review, the FDA assesses all evidence provided, which may include the results of performance testing and analysis of patient experience data. This is in line with the FDA's draft guidance for implant devices, which underscores the importance of understanding the patient experience to enhance safety. Moreover, recent FDA observations have identified an alarming trend of unreliable data submissions, especially from third-party test labs, emphasizing the importance of integrity in the data provided for 510(k) submissions.
Manufacturers must be proactive and meticulous in their submission process, addressing any issues identified during FDA inspections, such as those noted on a Form 483, and providing clear plans for corrective actions within stringent timelines. A comprehensive understanding of the subject device, its competitive landscape, and potential predicate devices is essential for a successful 510(k) submission. The FDA continuously works to ensure that the approval process for medical devices, including those cleared through the 510(k) pathway, aligns with the agency's mission to protect public health by assuring safety, efficacy, and security.
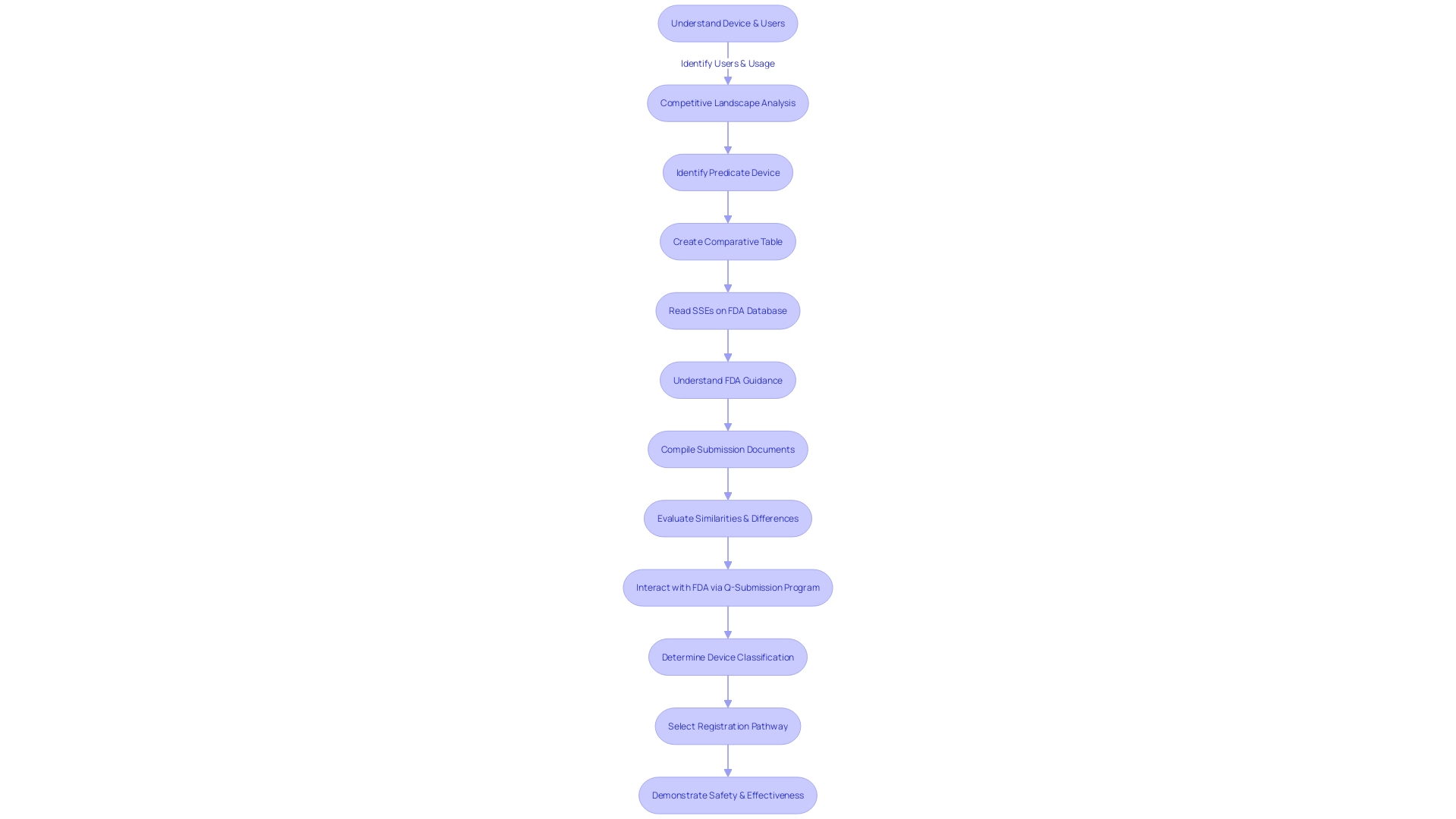
Timeline and Communication with FDA
Navigating the FDA 510(k) review process for medical devices is a nuanced endeavor that can vary in duration based on the device's complexity and the thoroughness of the submitted data. Manufacturers must ensure they have a profound understanding of the device, including its intended use, the users, and any warnings or cautions associated with the instructions for use. This knowledge, coupled with a competitive analysis facilitated by Marketing teams, will allow manufacturers to identify predicate devices that share similar intended uses and technological characteristics. By creating detailed comparative tables, manufacturers can facilitate a more efficient review process.
It is also crucial to determine the correct FDA classification for the device, as it dictates the risk level to patients and the appropriate registration pathway—be it 510(k), Premarket Approval (PMA), or the De Novo process. For example, the documentary 'The Bleeding Edge' highlighted that not all devices require clinical trials, as some can be fast-tracked if they're substantially equivalent to already approved devices, although this may lead to patient safety concerns.
Understanding the FDA's role is key; as they evaluate the safety and effectiveness of medical devices, their approval does not necessarily translate to immediate coverage or payment by other entities like CMS or private health plans. Manufacturers should be prepared for potential delays or denials in device coverage and patient access even after FDA clearance, as noted by the American Medical Association.
Manufacturers are encouraged to maintain proactive and transparent communication with the FDA during the review process. Submitting comments and feedback is part of the process, and it's critical to do so responsibly, ensuring confidentiality and adherence to submission guidelines. The FDA's mission to ensure public health safety extends to a wide array of products, and the 510(k) process is a vital component of their regulatory responsibilities.
Ultimately, the goal is to bring safe and effective medical devices to the market. With a clear understanding of the regulatory landscape and a strategic approach to the FDA's requirements, manufacturers can navigate the 510(k) process more effectively, leading to better health care outcomes for the public.
Common Challenges and Best Practices
The 510(k) approval process through the FDA is a critical pathway for medical device manufacturers seeking to bring their devices to market. Achieving a successful submission often hinges on the comprehensive understanding of the subject device, its intended use, and the context within which it operates. It is imperative for manufacturers to delve into a deep analysis of the device's users, which may include clinicians, physicians, dentists, and patients, and to scrutinize the instructions for use, paying close attention to any warnings and cautions associated with the device.
Furthermore, by collaborating with marketing teams, manufacturers can gain insights into the competitive landscape, identifying competitor devices that may serve as suitable predicates. It's essential to gather data from research literature, clinical studies, and marketing materials like websites, brochures, and instructions for use. Creating a comparative table to juxtapose your device against potential predicates can clarify the similarities and technological characteristics shared between them, a foundational step in the FDA's evaluation process.
The FDA's modernization efforts have produced a draft guidance document, unveiled on September 7, 2023, that outlines best practices for selecting a predicate device. This document emphasizes the importance of verifying that a potential predicate is legally marketed and registered with the FDA. It also provides direction on how to assess whether the predicate has identical intended use without raising new concerns about safety and effectiveness due to differing technological characteristics. In certain cases, selecting an older predicate can be advantageous, leveraging the accumulation of long-term safety data.
The FDA emphasizes the necessity for manufacturers to thoroughly investigate any issues that may arise during the approval process. A methodical approach, often incorporating tools like 5-whys or fishbone diagrams, is recommended to examine and address potential concerns. Timeliness is also crucial, especially when responding to FDA observations, where a strong, organized response within the 15-business-day window can significantly mitigate further regulatory action.
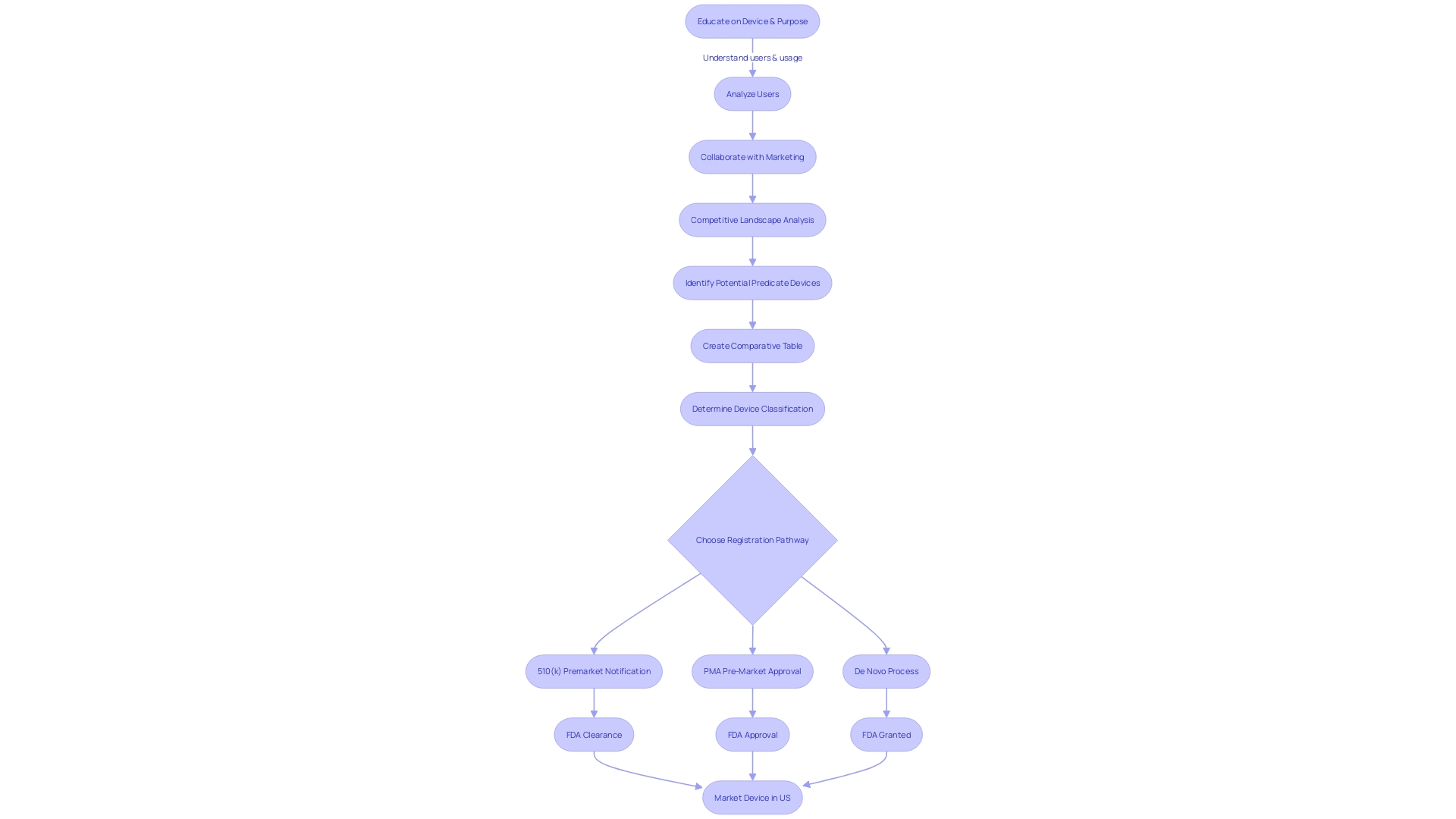
Special and Abbreviated 510(k) Programs
The U.S. Food and Drug Administration (FDA), as an integral part of its mission to protect public health, oversees the safety and effectiveness of medical devices through rigorous regulatory mechanisms. Among these, the 510(k) premarket notification stands as a critical pathway for medical devices to enter the U.S. market. In addition to the conventional 510(k) route, the FDA has instituted alternative pathways, namely the Special and Abbreviated 510(k) processes, tailored for certain medical devices. These alternatives are designed to streamline the submission process while ensuring that devices meet the necessary criteria for substantial equivalence.
In the Special 510(k) program, manufacturers who wish to make modifications to their own FDA-cleared devices can expedite the review process, provided that the changes do not affect the safety and effectiveness of the device. This program emphasizes the use of guidance documents and recognized standards to demonstrate compliance, highlighting the FDA's commitment to transparency and efficiency as stated in their published edition of the eCFR, which is designed for user convenience and regulatory clarity.
The Abbreviated 510(k) pathway, on the other hand, offers a more streamlined submission for devices that conform to FDA-recognized consensus standards or special controls. As outlined by the Office of Management and Budget (OMB) Circular A-119, consensus standards are developed through a process that ensures transparency, open participation, and balanced representation—qualities that contribute to regulatory quality and public trust. Conformity assessment, as part of a strong regulatory framework, is crucial in demonstrating that a product meets these standards, which can significantly simplify the 510(k) submission process.
Navigating these programs requires a deep understanding of the device in question, its intended users, and the competitive landscape. It's imperative to identify the proper classification of the device, as this determines the appropriate regulatory pathway—be it 510(k), PMA, or De Novo. Each classification correlates with a distinct level of patient risk, guiding the depth and breadth of evidence needed to support the claim of substantial equivalence.
For medical device professionals, it is essential to gather comprehensive data on predicate devices with similar intended uses and technological characteristics as part of the submission process. Detailed comparisons, backed by research literature, clinical studies, and instructions for use, form the backbone of a compelling 510(k) submission. This preparation, combined with a clear understanding of the FDA's expectations and the nuances of the submission process, is key to achieving a favorable outcome.
To ensure a submission is in line with FDA standards, applicants are encouraged to consult the FDA’s resources, which clarify the requirements and provide step-by-step guidance on the submission process. The challenge lies in compiling the necessary information to support the application within the allotted time frame and to the FDA's satisfaction, aiming for a determination of substantial equivalence.
Submitting comments or information to the FDA requires careful consideration to avoid including confidential or sensitive details that should not be made public. The FDA explicitly cautions submitters to exclude confidential business information or personal data from their comments, unless these are submitted through secure means as detailed in their instructions for written submissions. This ensures the integrity and confidentiality of proprietary information throughout the regulatory process.
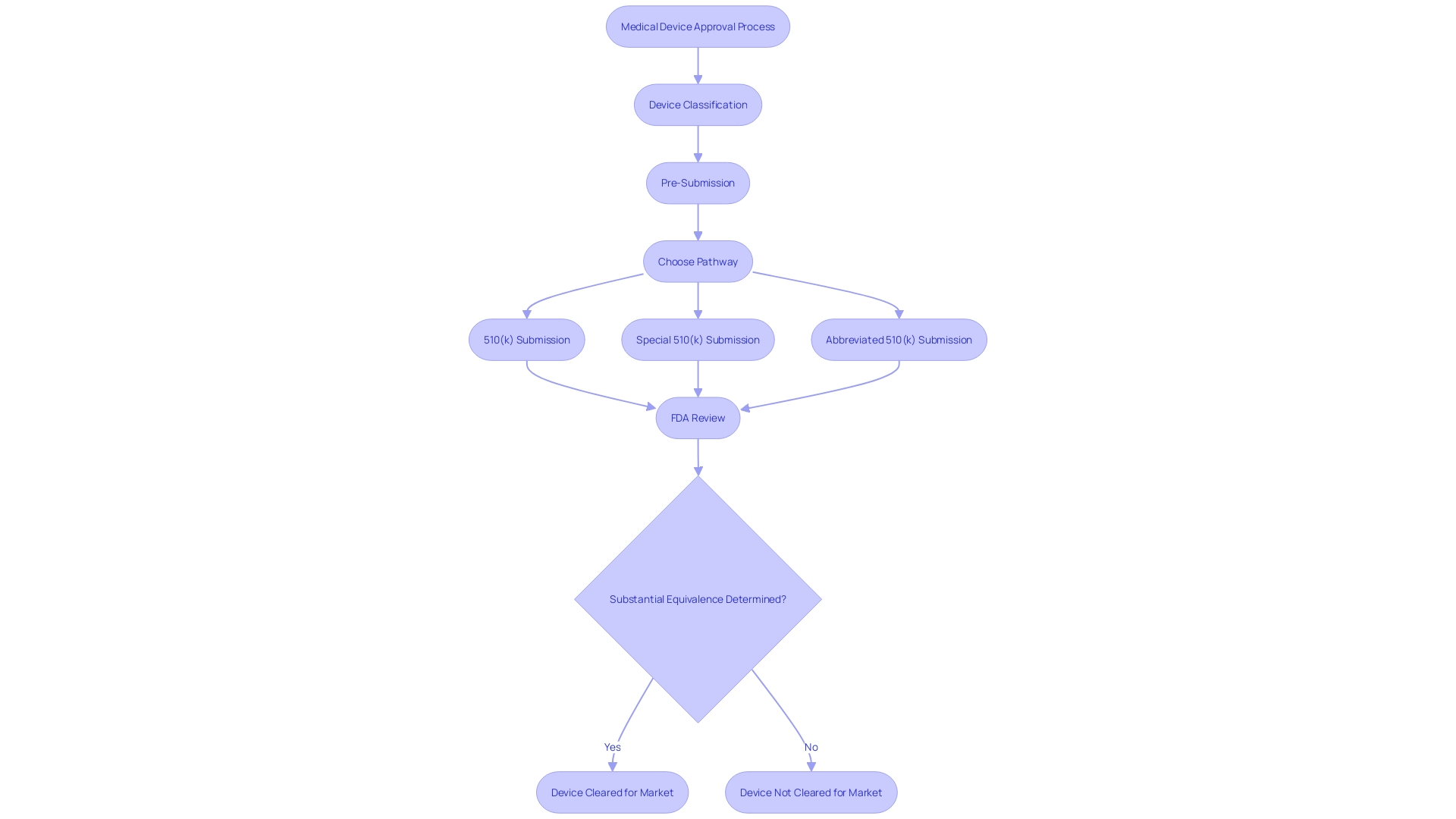
Post-Decision Procedures and Clearances
Following the FDA's decision on a 510(k) submission, manufacturers must initiate several post-decision procedures to ensure ongoing compliance and safety of the medical device. This involves obtaining marketing clearance, which signifies the FDA’s formal approval for the device to be sold within the United States. However, the obligations of manufacturers extend far beyond this initial clearance.
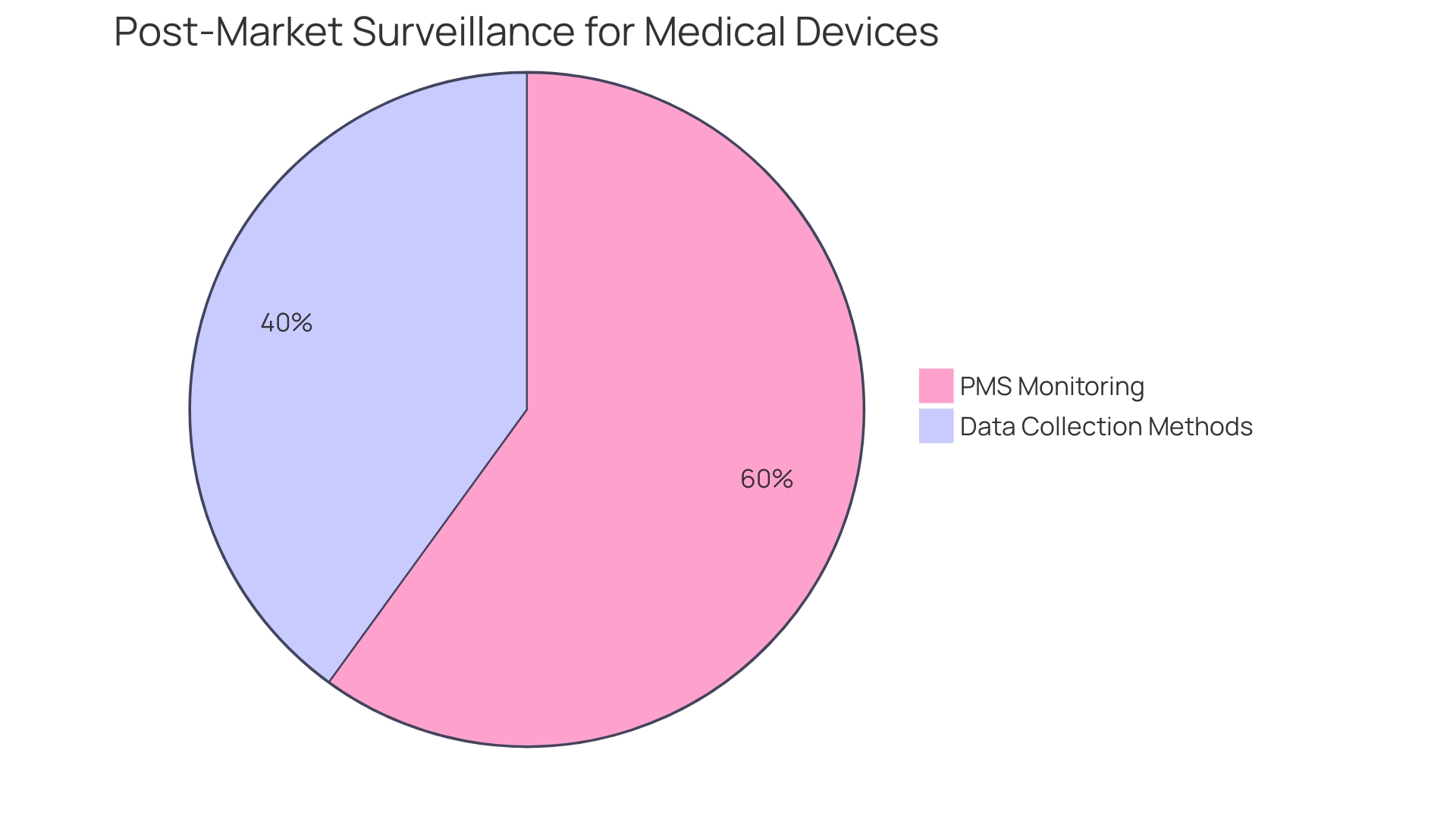
Post-market surveillance (PMS) becomes a pivotal component of the manufacturer's responsibilities. PMS is a rigorous process that entails the continuous monitoring of the device's performance and safety after it has been released to the market. This proactive approach is vital for identifying potential safety issues that may not have been apparent during pre-market testing. The FDA actively encourages the use of various data collection methods, including the spontaneous reporting by healthcare professionals and patients, active surveillance systems such as registries or dedicated studies, and leveraging electronic health records and administrative databases. These methods are designed to capture real-world data, providing insights into the long-term safety and effectiveness of the device.
The importance of PMS is underscored by statistics revealing that over a recent 10-year period, more than 1.7 million injuries and 83,000 deaths in the United States were potentially linked to medical devices. These figures demonstrate the critical need for ongoing vigilance in monitoring medical devices post-market to prevent such adverse events. The FDA has recognized this necessity and is in the process of building an active postmarket surveillance system, starting initially with a select few devices and planning to scale over time.
Additionally, manufacturers may also be required to make labeling or manufacturing changes as directed by the FDA to ensure the continued safety and efficacy of the device. These changes are often the result of insights gained through PMS and other post-approval studies. It is essential for manufacturers to stay well-informed about their device and its competitive landscape, seeking a deep understanding of the device's users, usage instructions, warnings, and cautions. This knowledge is not only crucial for compliance but also for maintaining a competitive edge in the market.
Overall, the post-decision phase of the 510(k) process is a dynamic and ongoing commitment to patient safety, requiring manufacturers to be vigilant and responsive to any new data or directives from the FDA.
Conclusion
The FDA's 510(k) Premarket Notification process is vital for medical device manufacturers to gain FDA approval and bring their products to market in the United States. By demonstrating substantial equivalence to a legally available device, manufacturers can navigate this complex process effectively.
To create a successful 510(k) submission, manufacturers must thoroughly understand the device, its user base, usage instructions, and potential risks. Adhering to FDA guidelines and following best practices is essential for market readiness and better healthcare outcomes.
The submission process offers different types, including traditional, special, and abbreviated, each with specific requirements. Manufacturers should select the appropriate submission type and safeguard confidential information.
A comprehensive submission includes detailed device information, performance specifications, and relevant clinical data. It should consider the device's users, instructions for use, and the competitive landscape. Comparative analysis with the predicate is crucial.
Content requirements for a successful submission include comprehensive device labeling, indications for use, and performance testing results. Manufacturers should provide a list of components, manufacturing procedures, and specifications for device quality.
The strategic selection of a predicate device is vital. Manufacturers should understand the subject device, review instructions for use, and collaborate with marketing teams. The FDA's database is valuable for identifying predicates.
Electronic submission formats, such as eSTAR and eCopy, require meticulous attention to detail and exclusion of confidential information.
During the acceptance review process, manufacturers must meet administrative criteria, address deficiencies, and maintain transparent communication with the FDA.
The substantive review stage involves rigorous evaluation of safety and effectiveness. Manufacturers should provide comprehensive evidence and address any issues identified during inspections.
Navigating the FDA review process requires a profound understanding of the device and collaboration with marketing teams. Timely communication with the FDA is crucial.
Manufacturers must initiate post-decision procedures to ensure ongoing compliance and safety. This includes marketing clearance, post-market surveillance, and potential changes based on FDA directives.
In conclusion, manufacturers can navigate the FDA's 510(k) process effectively by understanding the device, following guidelines, and collaborating with marketing teams. By adhering to best practices, manufacturers contribute to better healthcare outcomes for the public.




