Introduction
Ensuring the swift and efficient approval of research proposals is a critical aspect of advancing medical science, particularly when it involves the Institutional Review Board (IRB) expedited review process. This article delves into the eligibility criteria and categories of research that qualify for expedited review, highlighting the types of studies that benefit from this streamlined approach. Furthermore, it outlines the expedited review process itself, detailing the necessary documentation and notification requirements that researchers must adhere to.
By understanding common pitfalls and adopting best practices, researchers can navigate the regulatory landscape more effectively, thereby contributing to timely medical advancements and improved public health outcomes.
Eligibility Criteria for Expedited Review
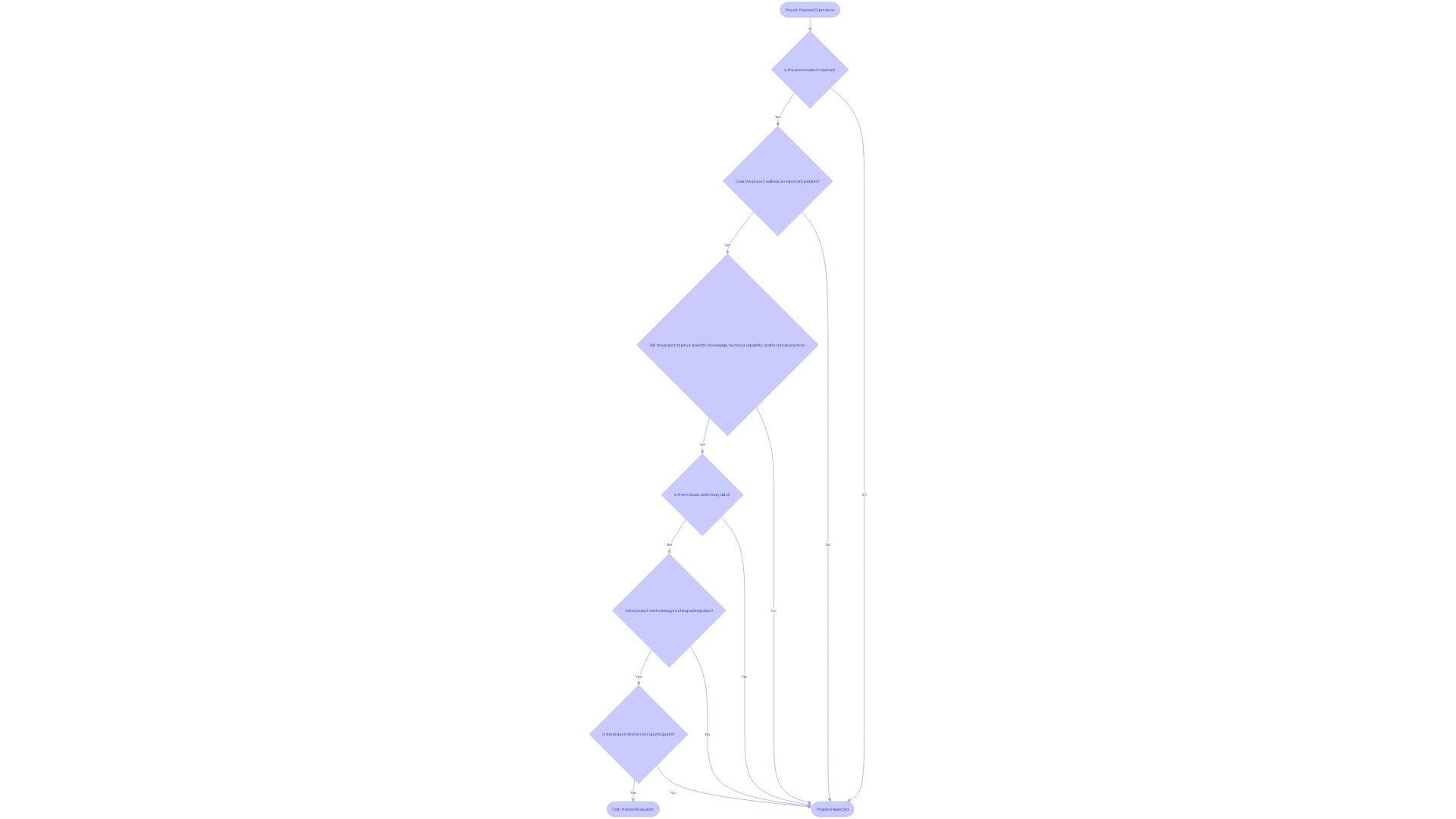
For a project proposal to be eligible for fast-tracked evaluation by the Institutional Review Board (IRB), it must comply with certain standards aimed at ensuring safety and efficiency. 'These criteria typically encompass investigations that pose minimal risk to participants and involve certain types of methodologies, such as studies on existing data or minimal interaction with subjects.'. For instance, the Center for Biologics Evaluation and Research (CBER) program provides accelerated procedures for serious conditions, requiring that the drug product addresses unmet medical needs or demonstrates significant improvement over existing therapies. Furthermore, proposals should be supported by robust preliminary data or literature to justify the scientific rationale.
Understanding these criteria is crucial for researchers aiming to expedite their proposals. Efficiently designed studies and reliable data not only facilitate quicker approvals but also align with the FDA's mission to advance the development of safe and effective medical products. Moreover, aligning human subject protection regulations with the U.S. Department of Health and Human Services Common Rule can simplify clinical activities while ensuring participant safety. By meeting these rigorous standards, researchers can significantly contribute to medical advancements and public health improvements.
Categories of Research Eligible for Expedited Review
Accelerated assessment procedures can greatly aid project submissions that belong to particular classifications. 'Studies focused on educational practices, surveys, interviews, and the analysis of existing data or specimens are prime candidates for fast-tracked review.'. This streamlined approach facilitates quicker approvals and ensures that valuable research can proceed without unnecessary delays. The Center for Biologics Evaluation and Research (CBER) program, for example, includes a streamlined program for serious conditions, allowing for quicker approval of drug products intended to address unmet medical needs. The Office of the Chief Scientist is also creating guidance documents to further assist these accelerated procedures, aiming to publish by the end of December 2024. By determining if a proposal aligns with these classifications, researchers can maneuver the evaluation phase more effectively, aiding the progress of medical understanding and patient treatment.
Expedited Review Process
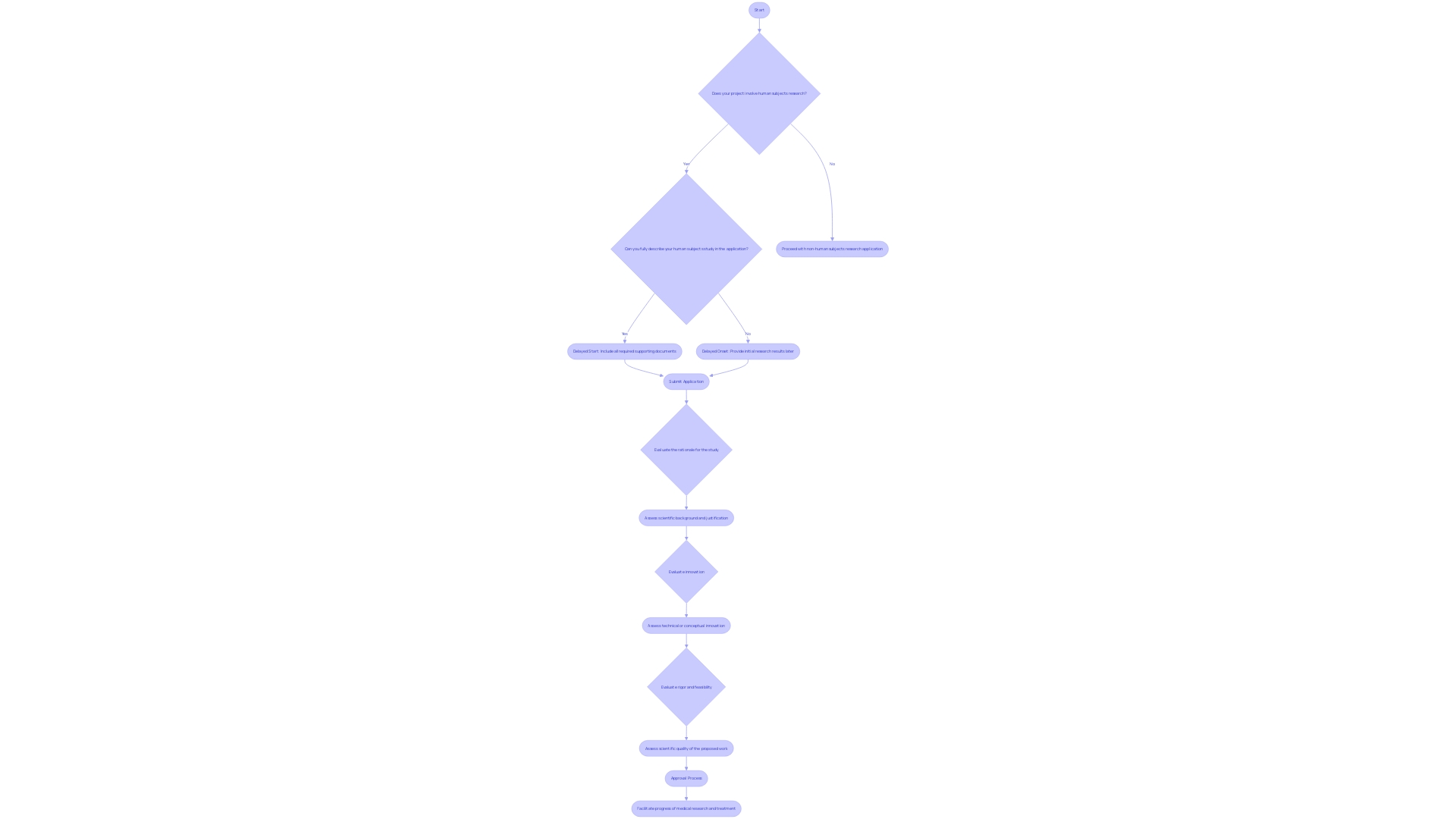
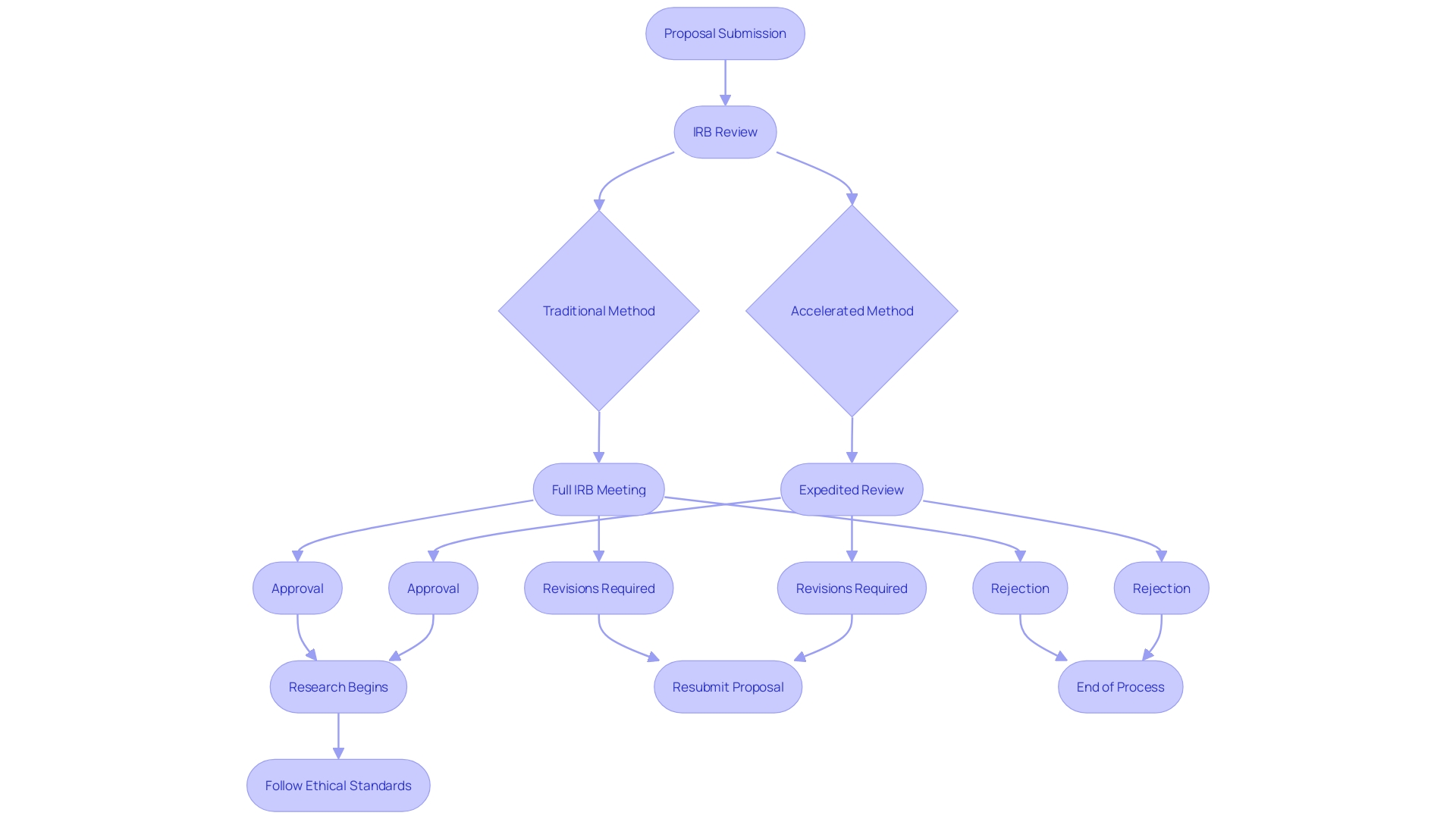
The accelerated evaluation method provides a streamlined approach where one or more members of the Institutional Review Board (IRB) assess the research proposal, instead of the whole board. 'This method can greatly shorten the duration needed for authorization, which is vital considering that conventional IRB evaluations can span weeks or even months, depending on the frequency of IRB meetings and the intricacy of the proposal.'. Researchers should submit a complete proposal along with any necessary documentation, clearly indicating their request for prompt consideration. This includes detailed descriptions of the investigation's purpose, procedures, risks, benefits, and consent forms. By bypassing the necessity for a complete board evaluation, the accelerated process not only conserves precious time and resources but also guarantees that ethical standards are upheld, effectively addressing matters of consent and risk management.
Documentation and Notification Requirements
Researchers are required to compile thorough documentation to substantiate their expedited review requests. This includes informed consent forms, study protocols, and any pertinent appendices. The guidance from the FDA emphasizes that informed consent should begin with key information about the study presented in a clear and concise manner to facilitate understanding. Key information should cover the purpose of the research, potential risks and benefits, and the study’s duration and procedures. This approach helps participants make well-informed decisions about their involvement in the study. After the IRB's decision, it is crucial to promptly inform researchers of the outcome and communicate any conditions or modifications needed for approval. The inclusion of key information in consent documents is also seen as beneficial for ongoing study participants.
Common Pitfalls and Best Practices
Navigating the accelerated assessment procedure for Institutional Review Board (IRB) approval often presents several challenges for researchers. Frequent traps consist of insufficient reasoning for the fast-tracked evaluation and incomplete paperwork, both of which can considerably postpone project schedules. To mitigate these issues, thorough preparation is essential. Researchers should thoroughly examine IRB guidelines to ensure all requirements for accelerated assessment are fulfilled. Early and proactive communication with the IRB can also clarify expectations and streamline the submission process.
Moreover, organizing all submission materials meticulously—ensuring completeness and coherence—can enhance the likelihood of a successful expedited review. This approach aligns with the FDA's broader mission to advance clinical research efficiently while safeguarding the rights of participants. By adopting these best practices, researchers can better navigate the regulatory landscape, ultimately contributing to the timely and effective advancement of medical knowledge.
Conclusion
The expedited review process by the Institutional Review Board (IRB) serves as a vital mechanism for accelerating the approval of research proposals that pose minimal risk to participants. Understanding the eligibility criteria is essential, as it encompasses studies that involve existing data or minimal interaction with subjects. By ensuring that research aligns with the rigorous standards set forth by regulatory bodies, researchers can effectively contribute to the advancement of medical science and public health.
Certain categories of research, including educational practices and surveys, are particularly suited for expedited review. This streamlined process not only facilitates quicker approvals but also enhances the efficiency of clinical research. The ongoing efforts by organizations such as the Center for Biologics Evaluation and Research (CBER) to refine and support these expedited processes will further benefit researchers seeking to address unmet medical needs.
The expedited review process itself is designed to save time and resources while maintaining ethical standards. By submitting a comprehensive proposal and adhering to documentation requirements, researchers can navigate this process more effectively. Clear communication of the research's purpose, risks, and benefits is crucial for securing informed consent and ensuring participant understanding.
Common pitfalls, such as inadequate justification for expedited review and incomplete documentation, can hinder the approval process. By implementing best practices—such as proactive communication with the IRB and meticulous organization of submission materials—researchers can mitigate these challenges. Ultimately, a thorough understanding of the expedited review process empowers researchers to advance medical knowledge efficiently while safeguarding participant rights.




