Introduction
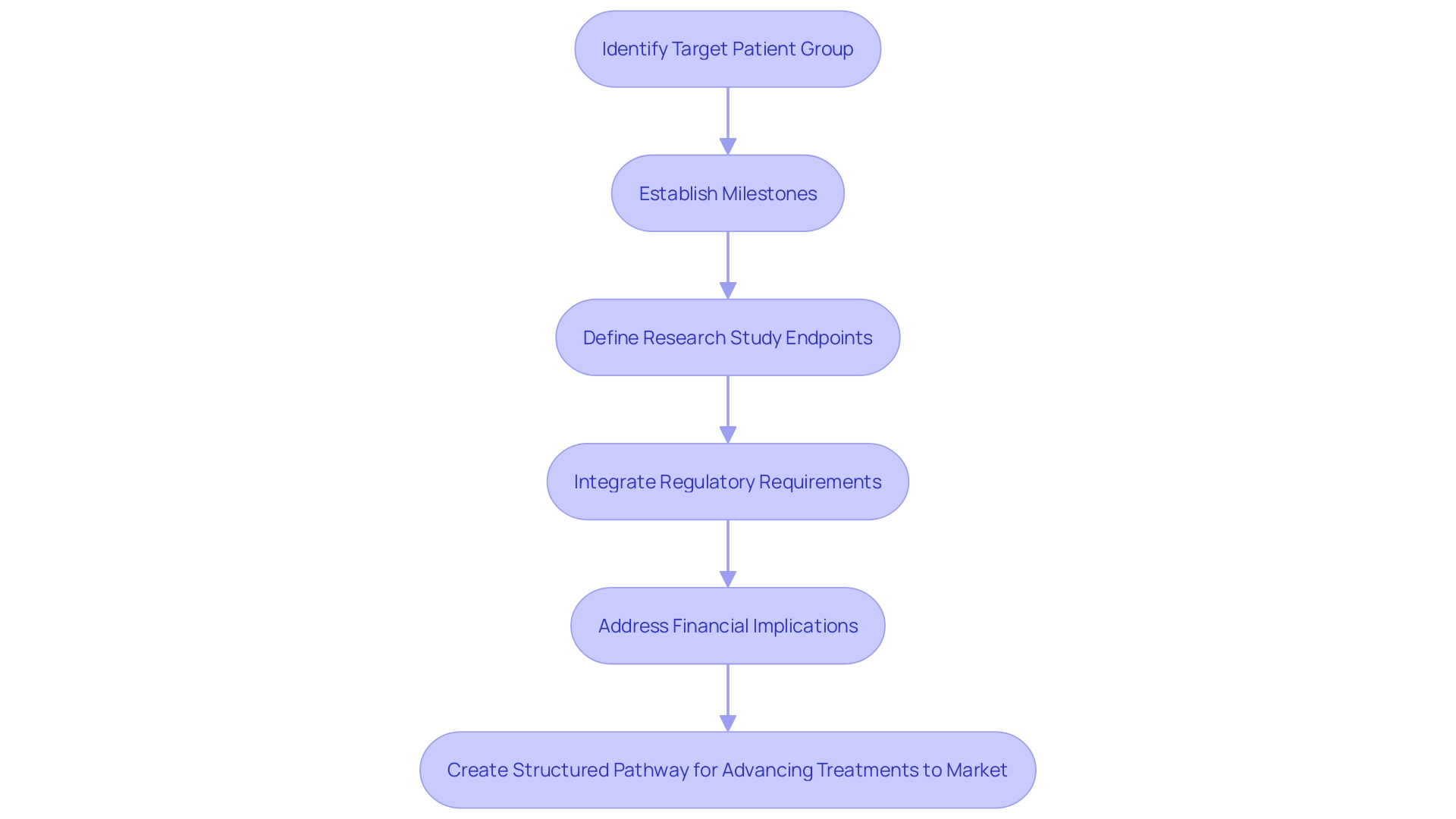
Clinical development planning forms the foundation of progressing new drugs or treatments from conception to market readiness. This intricate process involves setting clear objectives, timelines, and resource allocations, all while meticulously integrating regulatory requirements and financial considerations. At its core, understanding the target patient population shapes the design and methodology of clinical trials, while establishing critical milestones and endpoints ensures meaningful and measurable outcomes.
Navigating the evolving landscape of regulatory compliance is paramount, as stringent legislation demands thorough review and adherence. Financial strategy plays a crucial role, particularly with the global emphasis on sustainable clinical trials, where innovative approaches like the Carbon Emission Index are gaining traction. Additionally, ethical, legal, and social considerations must be addressed transparently to ensure that patient outcomes and risks are communicated effectively.
A well-structured clinical development plan, therefore, is multifaceted and requires meticulous alignment with regulatory, financial, and ethical standards. This comprehensive approach not only facilitates the advancement of new treatments but also enhances patient outcomes and drives forward medical innovation.
Key Components of Clinical Development Planning
Clinical development planning is a comprehensive strategy that delineates the pathway for advancing a new drug or treatment to market. This process is pivotal for setting clear objectives, timelines, and resource allocations. Key elements involve identifying the target patient group, establishing important milestones, and defining endpoints for research studies. Additionally, it is essential to integrate regulatory requirements and financial considerations to ensure alignment with the overall business strategy.
Comprehending the intended group is essential, as it affects the planning and approach of research studies. Defining key milestones helps in tracking progress and making necessary adjustments. Setting clear endpoints guarantees that the research studies yield significant and quantifiable results.
Including regulatory requirements is vital, considering the changing environment of laws and policies overseeing research. For instance, gaining market approval and ensuring compliance with regulatory bodies are top priorities for medical device leaders, as highlighted by a study where 47% emphasized the importance of regulatory compliance. As legislation becomes more stringent, the volume of literature to review increases, posing a significant challenge.
Financial implications cannot be overlooked. The strategic allocation of resources ensures that the development process remains financially viable. This is especially relevant considering trends such as the global movement towards sustainability in clinical studies. Pioneering approaches to reduce the environmental impact, like using the Carbon Emission Index in trial designs, demonstrate the intersection of financial planning and ethical considerations.
Moreover, ethical, legal, and social issues must be addressed. Each case study often begins with vignettes to highlight these issues, structured by guiding questions that probe into market incentives, intellectual property, and the broader social goals of the research. Clarity in the development process is essential, ensuring that information affecting risks and outcomes is communicated clearly.
In summary, a well-structured development plan is multifaceted, requiring meticulous planning and alignment with regulatory, financial, and ethical standards. This holistic approach ensures the successful advancement of new treatments, ultimately enhancing outcomes for individuals and advancing medical knowledge.
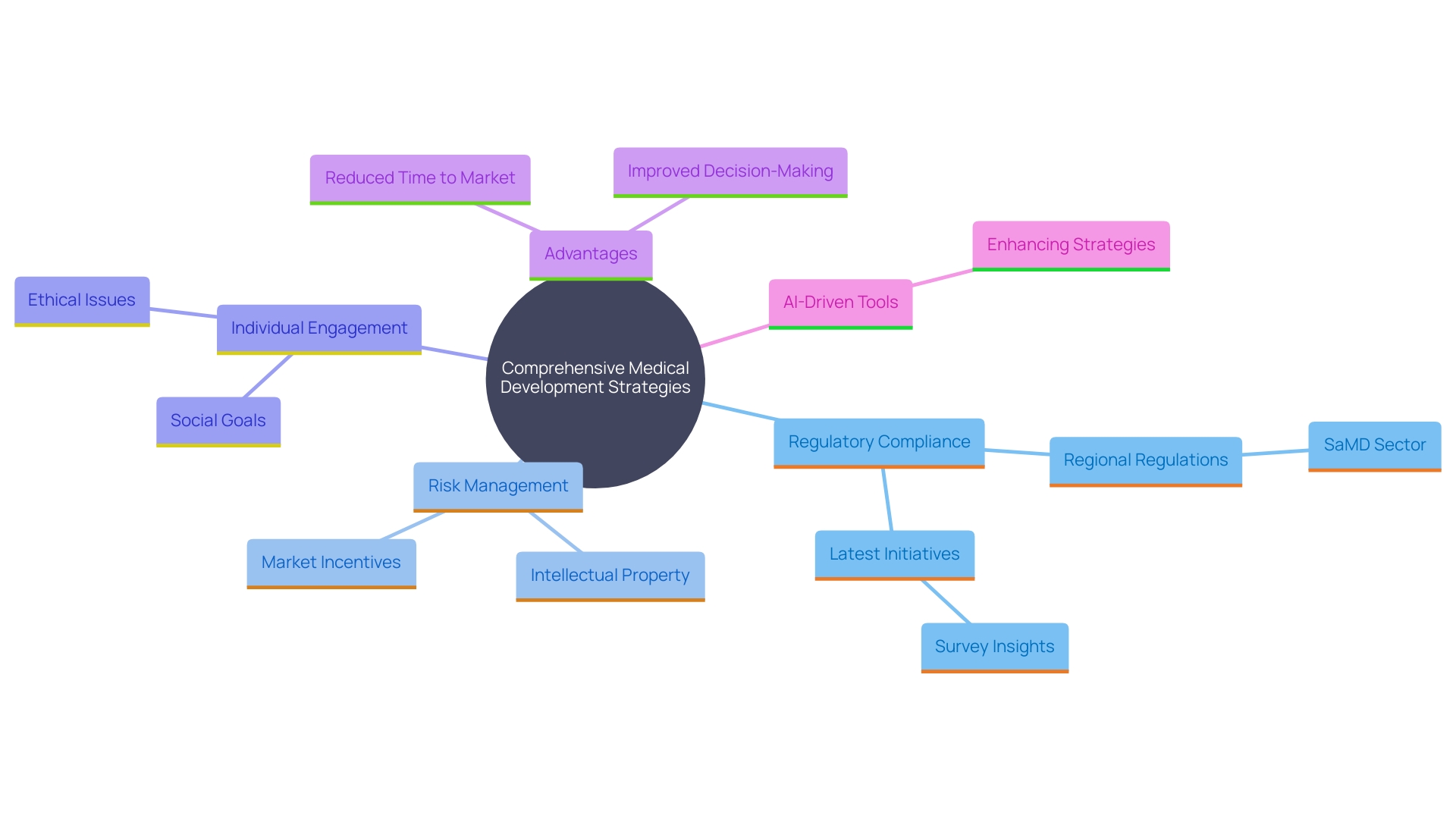
Benefits of Comprehensive Clinical Development Strategies
Comprehensive medical development strategies offer numerous advantages, including reduced time to market and optimized resource utilization. By integrating various elements such as regulatory compliance, risk management, and individual engagement, these strategies enhance the likelihood of successful outcomes. For instance, adhering to Who's definition of a clinical trial ensures standardized protocols, which are crucial for global regulatory acceptance and safety of individuals. These strategies also promote improved decision-making during the development process, enabling flexibility in reaction to new information or market changes. The evolving landscape of medical device regulations, particularly in the burgeoning Software as a Medical Device (SaMD) sector, underscores the importance of understanding regional nuances and compliance processes. Leveraging AI-driven regulatory tools can streamline these processes and alert professionals to global updates, thereby reducing risks and expediting market entry. Ultimately, a holistic approach can lead to improved patient access to innovative therapies, driven by transparent partnerships and rigorous data analysis.
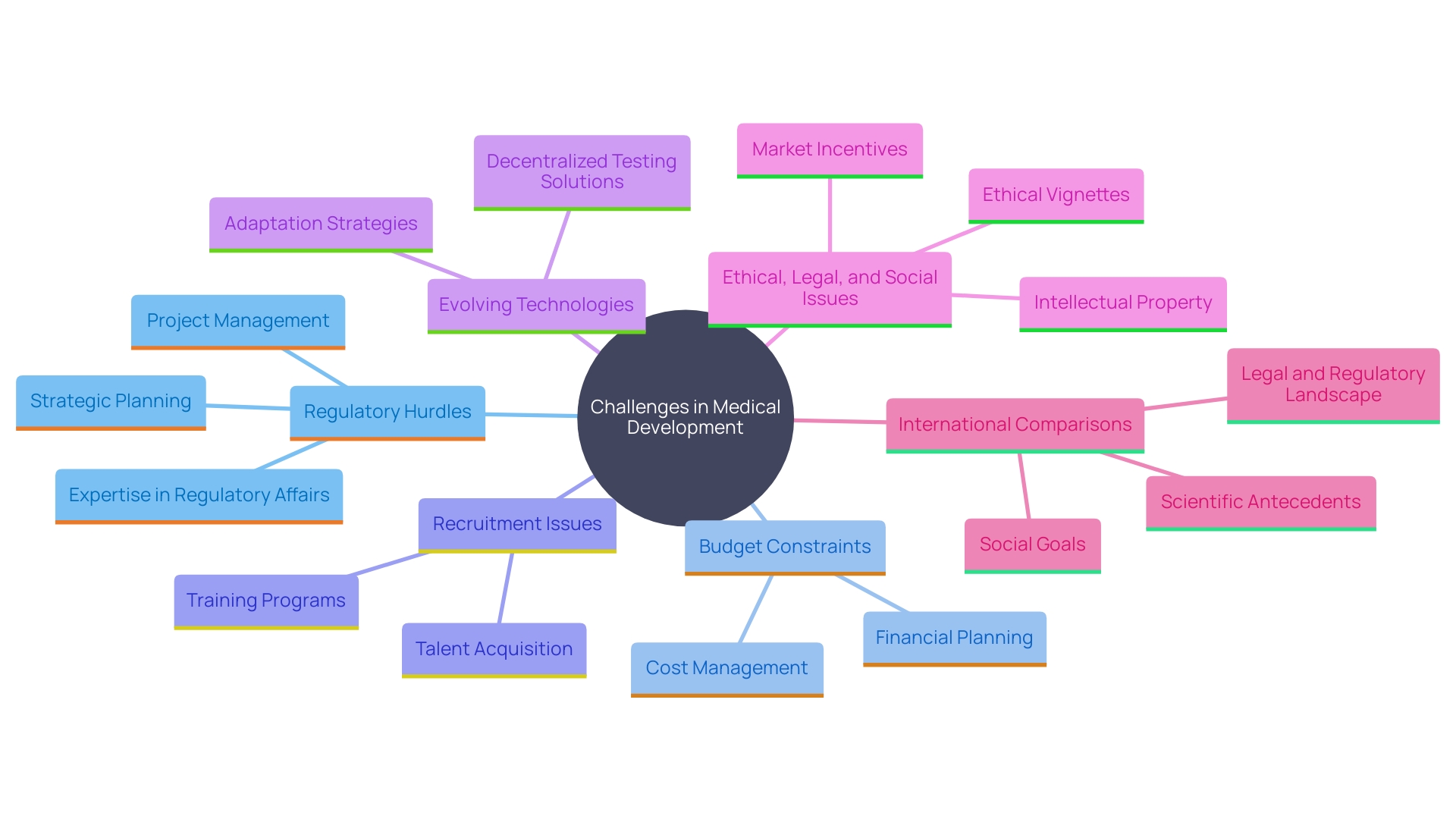
Challenges in Clinical Development and How Consulting Services Help
'The landscape of medical development is fraught with challenges, including regulatory hurdles, budget constraints, and recruitment issues.'. Consulting services play a crucial role in addressing these obstacles by providing expertise in Regulatory Affairs, strategic planning, and project management. For instance, a case study by Cardinal Health demonstrated how a comprehensive regulatory strategy facilitated timely and thorough Investigational New Drug (IND) submissions. Moreover, the 2023 Study Activation Survey by Advarra showed that almost 60% of research sites have experienced a rise in study volume since 2018, further highlighting the necessity for effective project management.
Additionally, consulting companies help in recognizing possible obstacles early, creating backup plans, and optimizing procedures, which ultimately boosts the effectiveness of studies and enhances the quality of information gathered. The FDA’s evolving regulatory landscape, as highlighted in recent news, underscores the importance of specialized knowledge to navigate complex compliance requirements. This is especially pertinent as the sector moves towards more decentralized testing solutions, utilizing advanced technologies such as electronic information capture and interactive voice response systems to handle the increasing amount of information.
'The growing adoption of connected gadgets and wearable tech in research studies offers more comprehensive insights, allowing for more informed choices in drug development.'. However, this also introduces additional burdens on research sites, as noted in Advarra's survey, making the role of consulting services even more critical. By combining different information sources and employing artificial intelligence-based approaches, consulting firms can assist sponsors and research organizations in establishing effective data strategies, ensuring safety and data quality are upheld throughout the process.
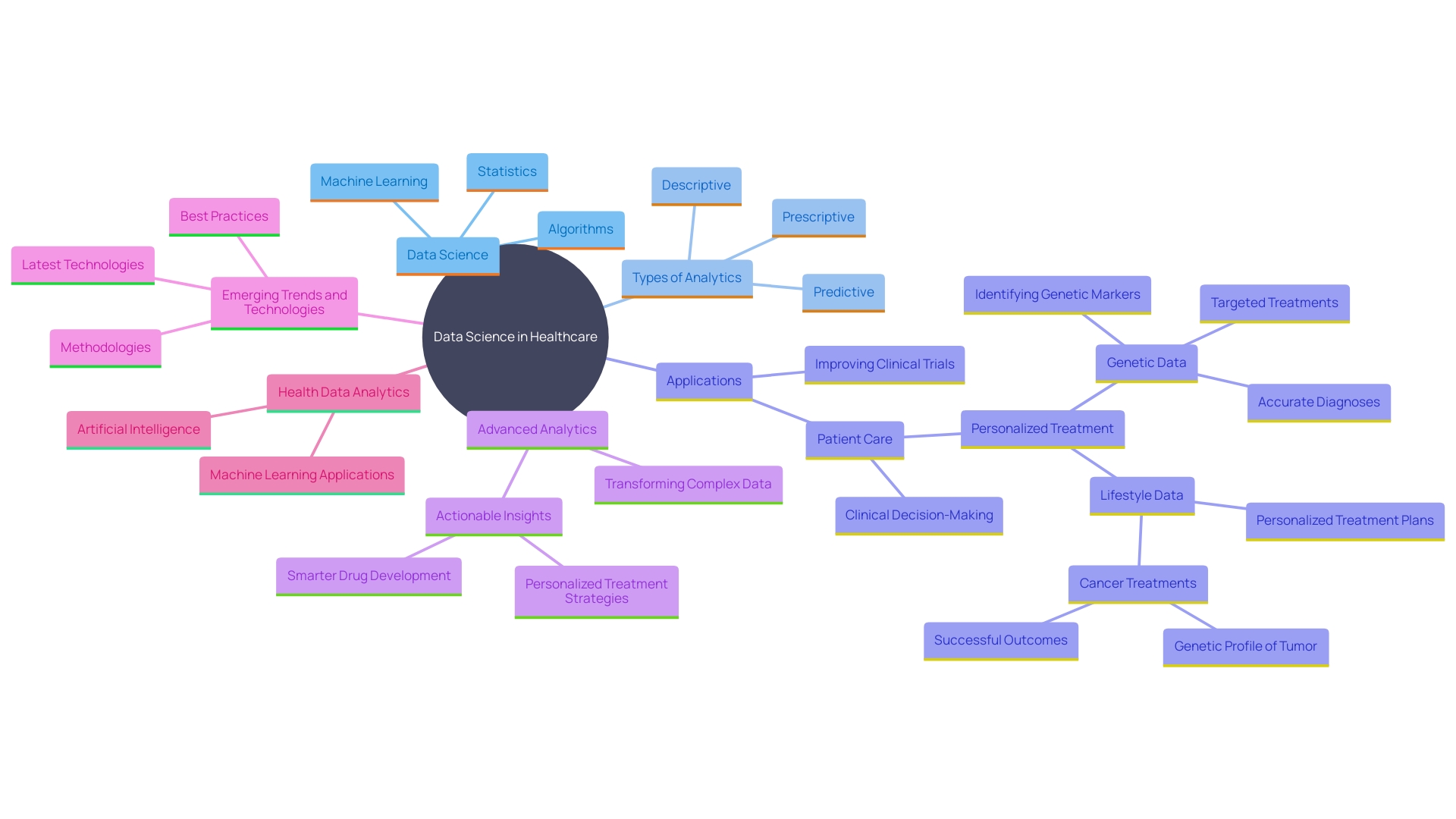
Leveraging Data Science and Advanced Analytics in Clinical Development
Data science and advanced analytics are transforming development in healthcare by allowing more informed decision-making and improving operational efficiency. Advanced analytical instruments enable organizations to acquire deeper understanding of demographics, treatment responses, and study results. As stated by the FDA, employing AI to examine large quantities of information from both clinical experiments and observational research is essential for updating clinical studies. This approach optimizes trial designs, improves participant stratification, and enhances engagement strategies.
One notable case study highlights the transformation of vast, complex information sets into actionable health solutions, particularly for rare disease patients. By leveraging new methodologies, organizations can quickly translate research observations into practical health solutions. This is becoming ever more essential as healthcare produces unparalleled volumes of information from electronic health records, medical imaging, and genomics. When effectively utilized, this information accelerates the drug development process and unlocks innovative treatments.
'Real-world information science case studies underscore the importance of evidence-based strategies.'. 'For instance, information from connected devices and wearables in decentralized trials provide richer insights and patient behavioral trends, informing smarter drug development decisions.'. However, this requires a well-defined information strategy to manage the flow of traditional and digital sources efficiently.
'Healthcare analytics methodologies such as descriptive, predictive, and prescriptive analytics further illustrate the potential of information science.'. Descriptive analytics aids in summarizing historical information, while predictive analytics anticipates future results based on prior information. Prescriptive analytics advances by suggesting particular measures to attain intended outcomes, such as tailored treatment plans based on an individual's genetic profile and medical history.
In summary, the power of data science and advanced analytics in clinical development lies in their ability to transform complex data into meaningful insights, driving innovation and improving patient outcomes.
Conclusion
The intricate process of clinical development planning is integral to the successful advancement of new drugs and treatments. By establishing clear objectives, timelines, and resource allocations, stakeholders can navigate the complexities of regulatory compliance and financial considerations effectively. Understanding the target patient population is essential, as it directly influences trial design and methodology.
The identification of critical milestones and endpoints plays a crucial role in ensuring that the outcomes of clinical trials are both meaningful and measurable.
Comprehensive clinical development strategies present numerous benefits, such as reduced time to market and optimized resource utilization. By integrating regulatory compliance, risk management, and patient engagement, these strategies enhance the probability of favorable outcomes. The use of advanced technologies and AI-driven tools further streamlines regulatory processes, ensuring that organizations remain agile in response to evolving market demands.
This holistic approach not only improves patient access to innovative therapies but also fosters transparent partnerships and rigorous data analysis.
Despite the advantages, the landscape of clinical development is fraught with challenges, including regulatory hurdles and recruitment issues. Consulting services provide invaluable support in navigating these obstacles, offering expertise in strategic planning and project management. By leveraging advanced data science and analytics, stakeholders can derive deeper insights from complex data sets, facilitating smarter decision-making throughout the drug development process.
As the industry continues to evolve, the integration of innovative methodologies will be vital in enhancing operational efficiency and ultimately improving patient outcomes.




