Introduction
Navigating the complex landscape of medical device approval, particularly through the FDA’s Premarket Approval (PMA) process, is a critical endeavor for manufacturers. The initial step involves determining whether a PMA is required, based on the device's intended use, technological characteristics, and the associated risk to patients. Understanding the FDA’s criteria and regulatory framework is essential, especially for drug-device combinations and devices with ancillary medicinal substances.
The intricacies of PMA submissions necessitate a thorough grasp of the different types available, such as Traditional PMA and Modular PMA, each with unique requirements and benefits. Pre-submission preparation, including rigorous testing and data collection, is paramount. Utilizing the FDA's Q-Submission Program can provide valuable preliminary feedback, potentially streamlining the approval process.
A comprehensive PMA submission package must include detailed descriptions, performance and clinical data, risk assessments, and post-market surveillance plans, all meticulously documented to meet FDA standards. The review process is rigorous, involving administrative, scientific, and regulatory evaluations, with the FDA providing guidance to ensure devices meet the highest standards of safety and efficacy.
Common challenges in the PMA process, such as inadequate testing and non-compliance with guidelines, underscore the importance of a proactive and well-informed approach. Post-approval requirements, including ongoing surveillance and adherence to regulatory updates, further ensure the device’s continued safety and effectiveness. This article delves into each aspect, offering detailed insights to guide manufacturers through the PMA process, ultimately advancing public health through the development of safe and effective medical products.
Determining if a PMA is Required
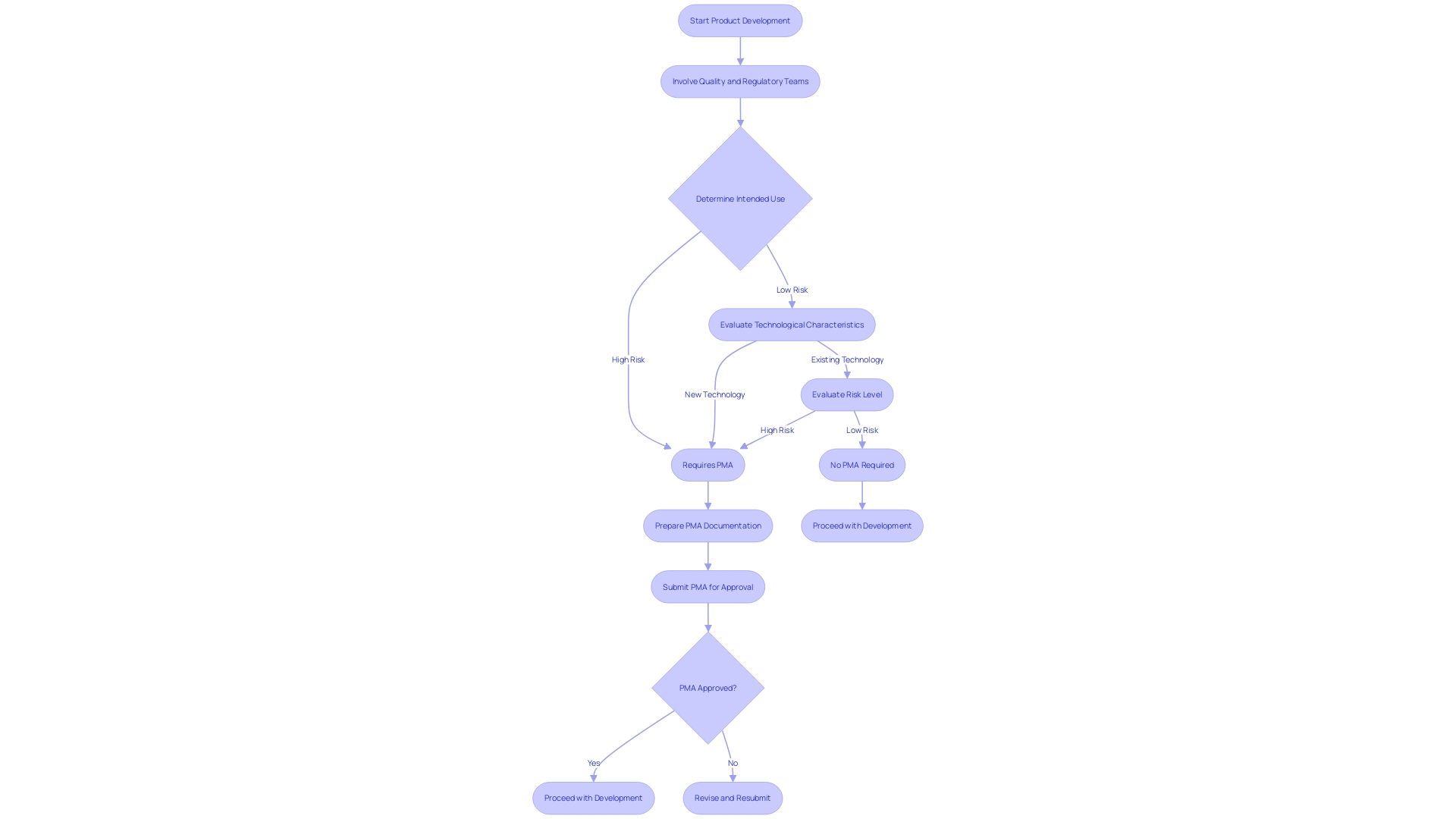
Determining whether a Premarket Approval (PMA) is necessary for your medical product is the first crucial step in navigating the PMA application process. The FDA's specific criteria for PMA necessity are based on the intended use of the product, technological characteristics, and the level of risk it poses to patients. These criteria are designed to ensure that only items with the highest risk or those that are entirely novel in their technology require the rigorous PMA pathway.
Evaluating these criteria involves a thorough understanding of the FDA's regulatory framework. For instance, instruments that combine a medicinal product and a medical instrument, such as pre-filled syringes, are regulated based on their primary mode of action. Likewise, items that include supplementary medicinal substances also have specific consultation procedures as part of their regulatory requirements.
Quality and Regulatory considerations must be integrated from the very start of product development to streamline the approval process. As highlighted by industry experts, early involvement of Quality and Regulatory teams can map out the most efficient strategy for approval, avoiding retrospective changes and potential compliance issues. This proactive approach is crucial, especially given the complex life cycle management of drug-device combinations and the stringent labeling requirements for co-packaged products.
Grasping these aspects and integrating them into the design and development phase guarantees that your product meets regulatory standards, ultimately promoting a smoother PMA application process.
Types of PMA Submissions
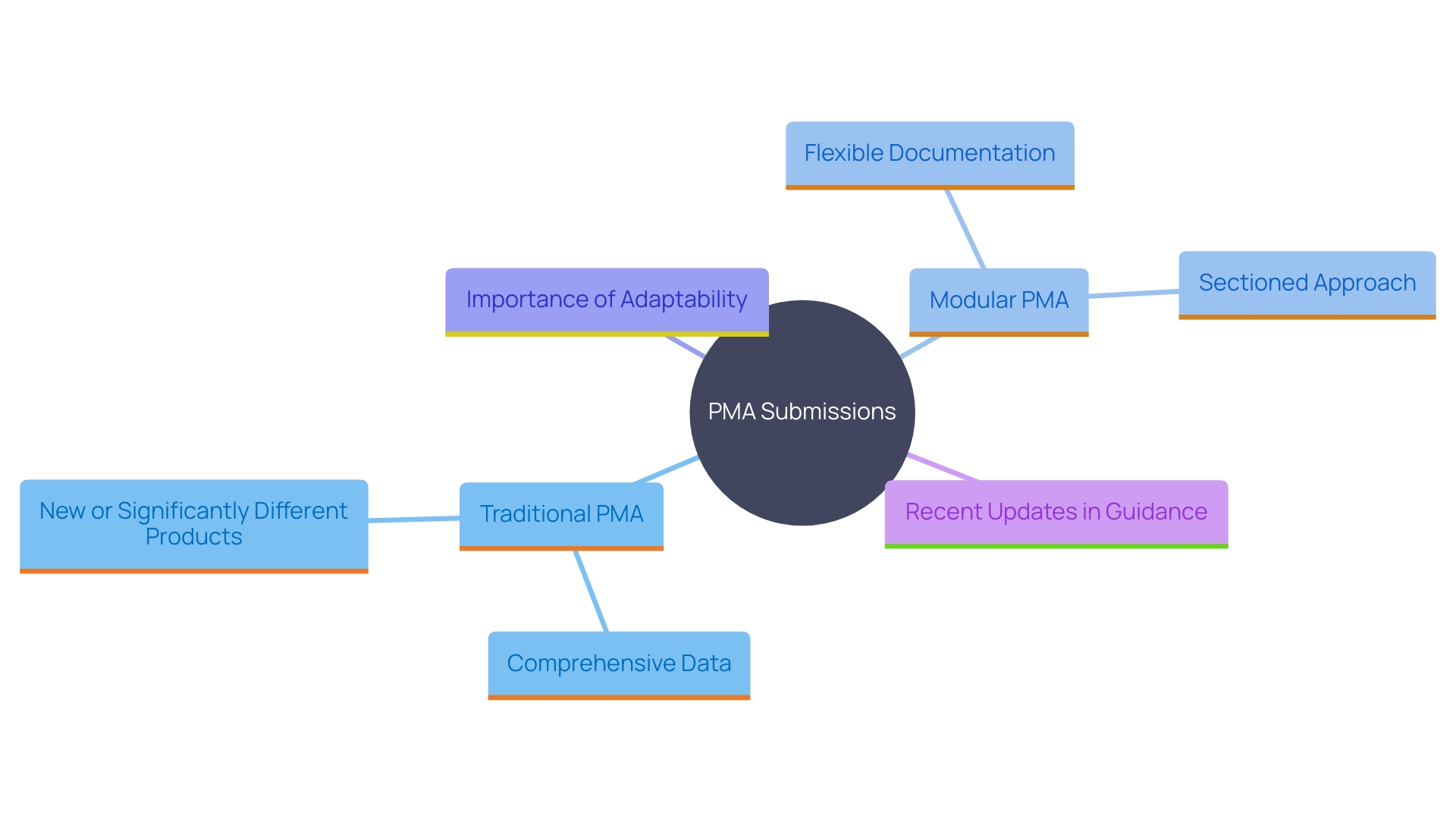
Determining that your equipment requires a PMA is only the first step; understanding the types of PMA submissions is crucial. There are two primary types of PMA submissions: Traditional PMA and Modular PMA. The Traditional PMA is necessary for items that have not been previously cleared through the FDA's 510(k) process or are significantly different from existing products. This type of submission requires comprehensive data and rigorous scientific evidence to demonstrate the safety and efficacy of the product.
On the other hand, the Modular PMA offers a more flexible approach. It enables producers to present their documentation in distinct sections, each concentrating on various features of the product. This method can streamline the process by enabling manufacturers to address specific areas sequentially, which can be particularly beneficial for intricate products requiring detailed and multi-faceted evaluations. Jennifer Mascioli-Tudor highlights the importance of adaptability in regulatory submissions, emphasizing that quality management systems must be robust yet flexible to accommodate various stages of the PMA process.
Additionally, recent updates to regulatory guidance have enhanced the procedural clarity for instruments with integrated medicinal substances and companion diagnostics. This revision reflects the FDA's ongoing efforts to adapt to the evolving landscape of medical technology, ensuring that submissions are evaluated with the most current and relevant standards in mind.
Understanding these options and the regulatory landscape can significantly impact the efficiency and success of your PMA submission, ultimately leading to better patient outcomes and advancements in medical technology.
Pre-Submission Preparation and Q-Submission Program
Proper pre-submission preparation is essential for a seamless PMA application process. This entails rigorous testing and comprehensive data collection to substantiate the safety and effectiveness of the product. Comprehending the intended users, like clinicians and patients, along with comprehensive instructions, warnings, and cautions, establishes the foundation of this preparation. At the same time, examining the competitive environment and recognizing predicate items with comparable technological traits are essential actions. Utilizing research literature, clinical studies, and marketing materials can aid in creating a comparative table that highlights these similarities.
The FDA's Q-Submission Program offers a strategic advantage by enabling manufacturers to obtain preliminary feedback on their PMA request. This early engagement helps pinpoint potential issues or deficiencies, ultimately conserving valuable time and resources. 'The FDA's commitment to public health ensures that approved products meet the highest standards of safety and efficacy, reinforcing the importance of meticulous preparation and proactive communication with the agency.'.
Key Components of a PMA Submission Package
When preparing your PMA application package, it is imperative to include several key components to ensure a successful submission. These components include a thorough description of the apparatus, detailing its intended use and indications for use. A comprehensive summary of the apparatus's design is also necessary, along with performance testing data and clinical study data, if applicable. Risk evaluation, labeling details, and a suggested strategy for post-market monitoring are all essential components that together illustrate the safety and effectiveness of your product.
Learning about the gadget and its purpose is essential. This involves understanding the users, such as clinicians, physicians, dentists, and patients, and familiarizing yourself with the instructions for use, including warnings and cautions. Furthermore, it's crucial to examine the competitive environment by assessing research literature, clinical studies, and promotional content of rival products. Recognizing possible predicate devices with comparable technological features and intended purpose can assist in generating a comparative table, which is a crucial part of your submission.
The FDA's approval process relies heavily on well-designed clinical research and reliable data. Over time, there has been a noted decline in the quality of clinical trials, which underscores the importance of presenting robust evidence in your PMA submission. The FDA aims to strengthen innovation through higher approval standards and greater transparency in clinical trials, ensuring that medical products are both safe and effective for patients.
Including these elements in your PMA submission not only aligns with FDA guidelines but also supports the agency's mission to advance public health by promoting the development of safe and effective medical products. Every element of your software plays a crucial role in supplying the required proof to show the safety and effectiveness of your device, ultimately aiding its approval and ensuring its successful entry into the market.
Preparing the PMA Application
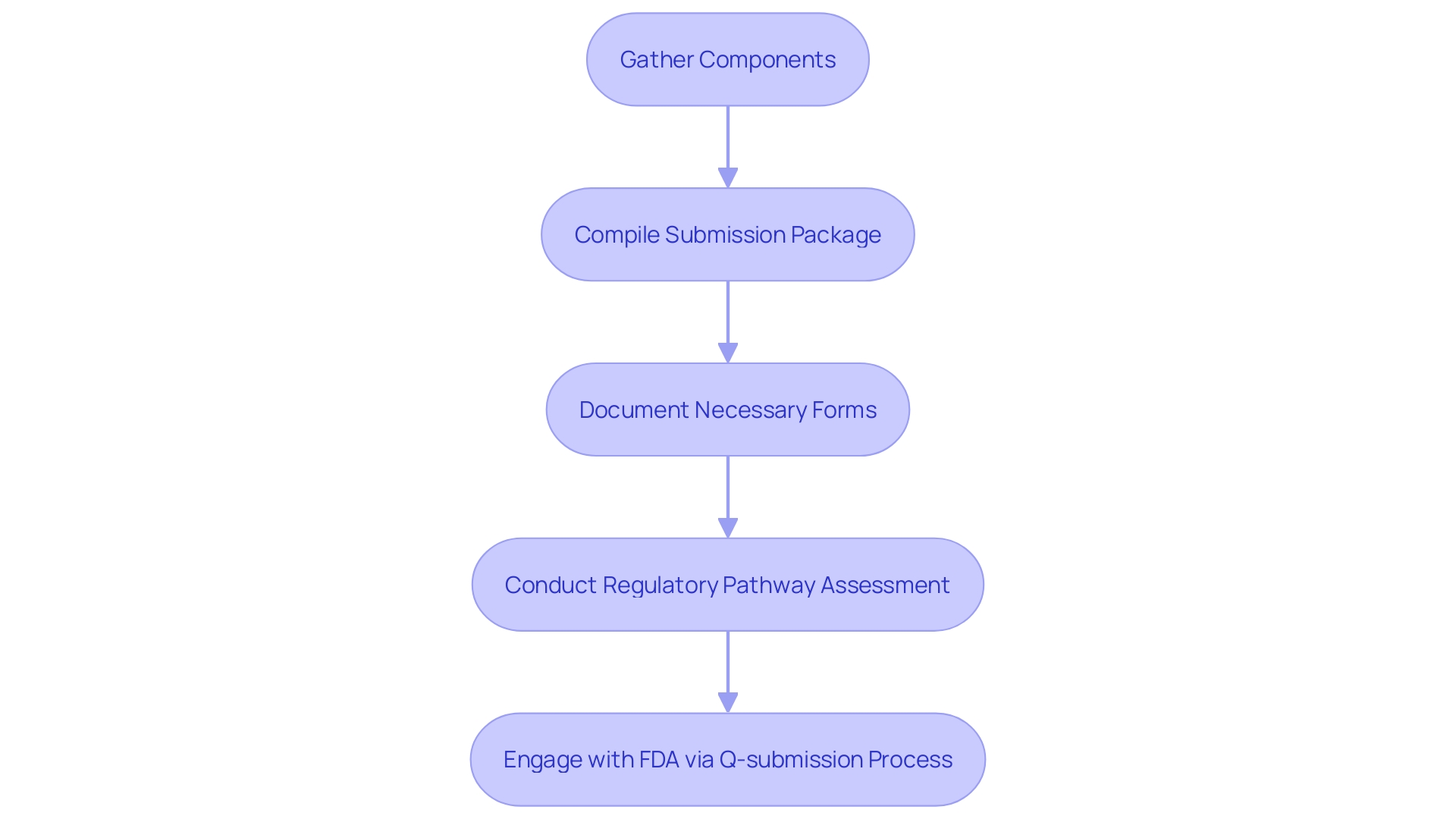
Once all necessary components are gathered, preparing the PMA request involves compiling and organizing the required information into a comprehensive submission package. This package should be meticulously documented, including all necessary forms and supporting data in accordance with FDA guidelines. The software must adhere to the FDA's formatting requirements to ensure completeness and accuracy. Regulatory professionals benefit from conducting a thorough regulatory pathway assessment, which serves as the foundation of a regulatory strategy. This includes scavenging FDA databases to understand the product code, submission pathways, and necessary exemptions. Engaging with the FDA through the Q-submission process can also provide clarity on specific questions and requirements.
FDA Review Process
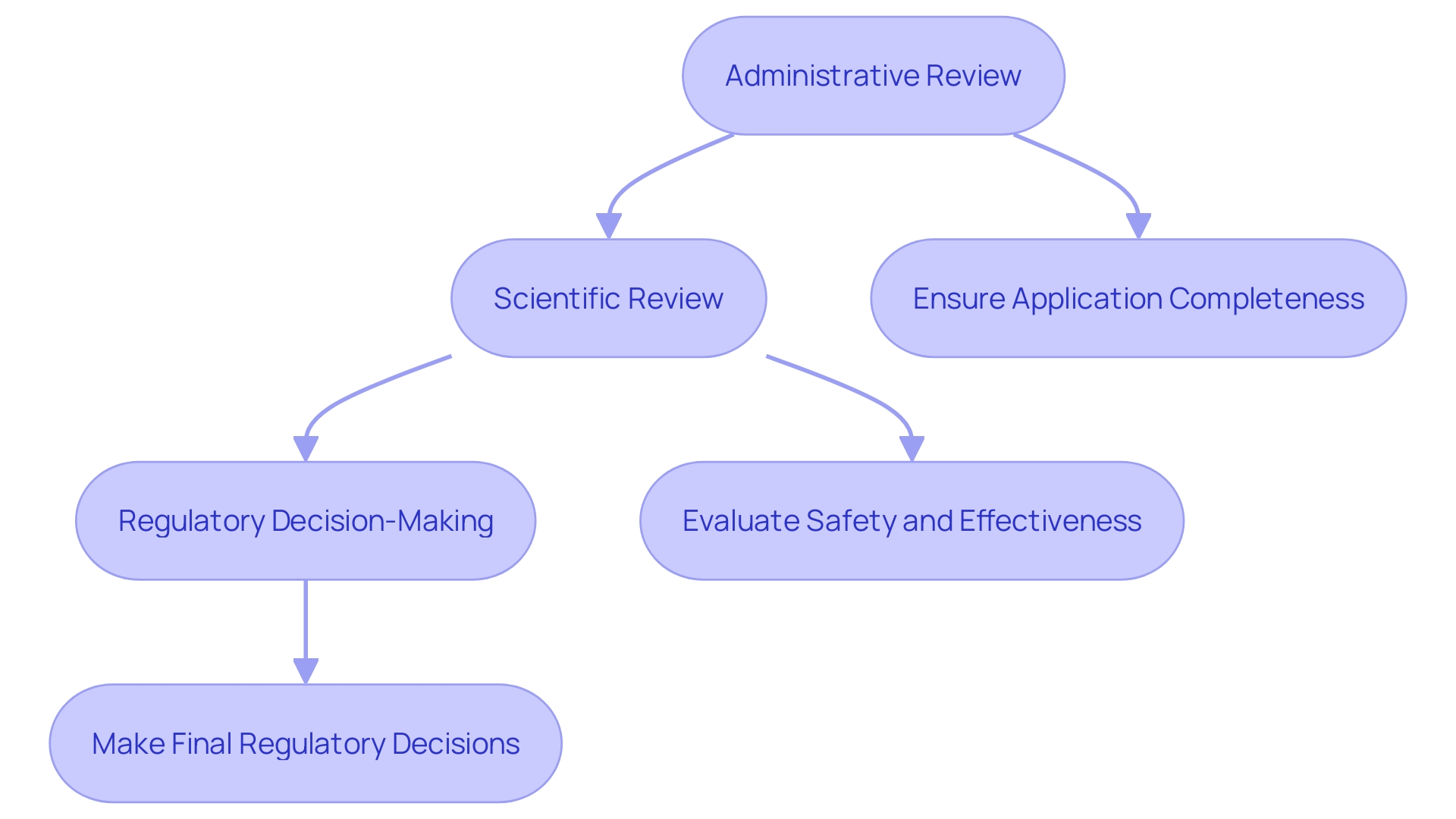
Once you submit your PMA request, it undergoes a meticulous review process by the FDA, consisting of several critical stages: administrative review, scientific review, and regulatory decision-making. During the administrative review, the FDA ensures that your application is complete and meets all submission requirements. Following this, the scientific review evaluates the safety and effectiveness of your product, leveraging extensive data from clinical trials and other relevant studies. This stage often involves a detailed analysis of the data provided and may include the use of large data networks like Sentinel to investigate safety concerns about FDA-regulated products.
The regulatory decision-making stage is where the FDA makes its final conclusions. They may request additional information or clarification to ensure the equipment meets all regulatory standards. The FDA's role is not only to authorize or deny requests but also to offer advice and suggestions, making sure that the products entering the market are safe and effective for public use. The whole procedure highlights the FDA's dedication to public health by upholding stringent criteria for medical product approval.
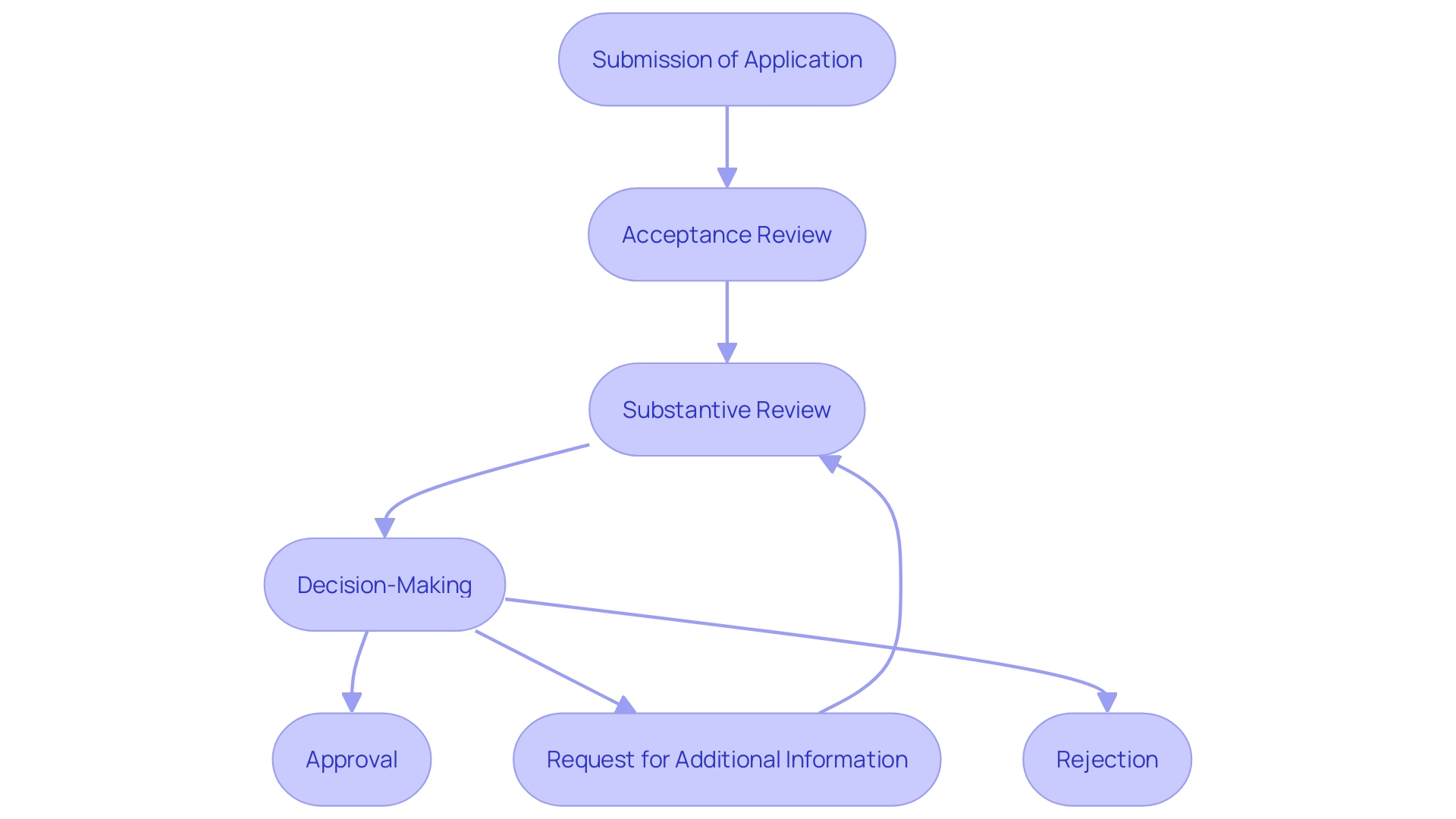
Steps in the FDA Review Process
The FDA review process involves several critical steps: acceptance review, substantive review, and decision-making. Initially, during the acceptance review, the FDA ensures the application is complete and meets all fundamental requirements. The next phase, substantive review, is where the FDA meticulously evaluates the scientific data and conducts an in-depth assessment of the product's safety, efficacy, and quality. This phase is essential as it relies on rigorous scientific analysis to determine the potential impact on patient health. The final step, decision-making, involves making a determination based on the comprehensive review findings. The FDA then determines whether to authorize or deny the PMA request, ensuring that only products meeting the highest standards of safety and effectiveness reach the market.
Common Challenges and Pitfalls
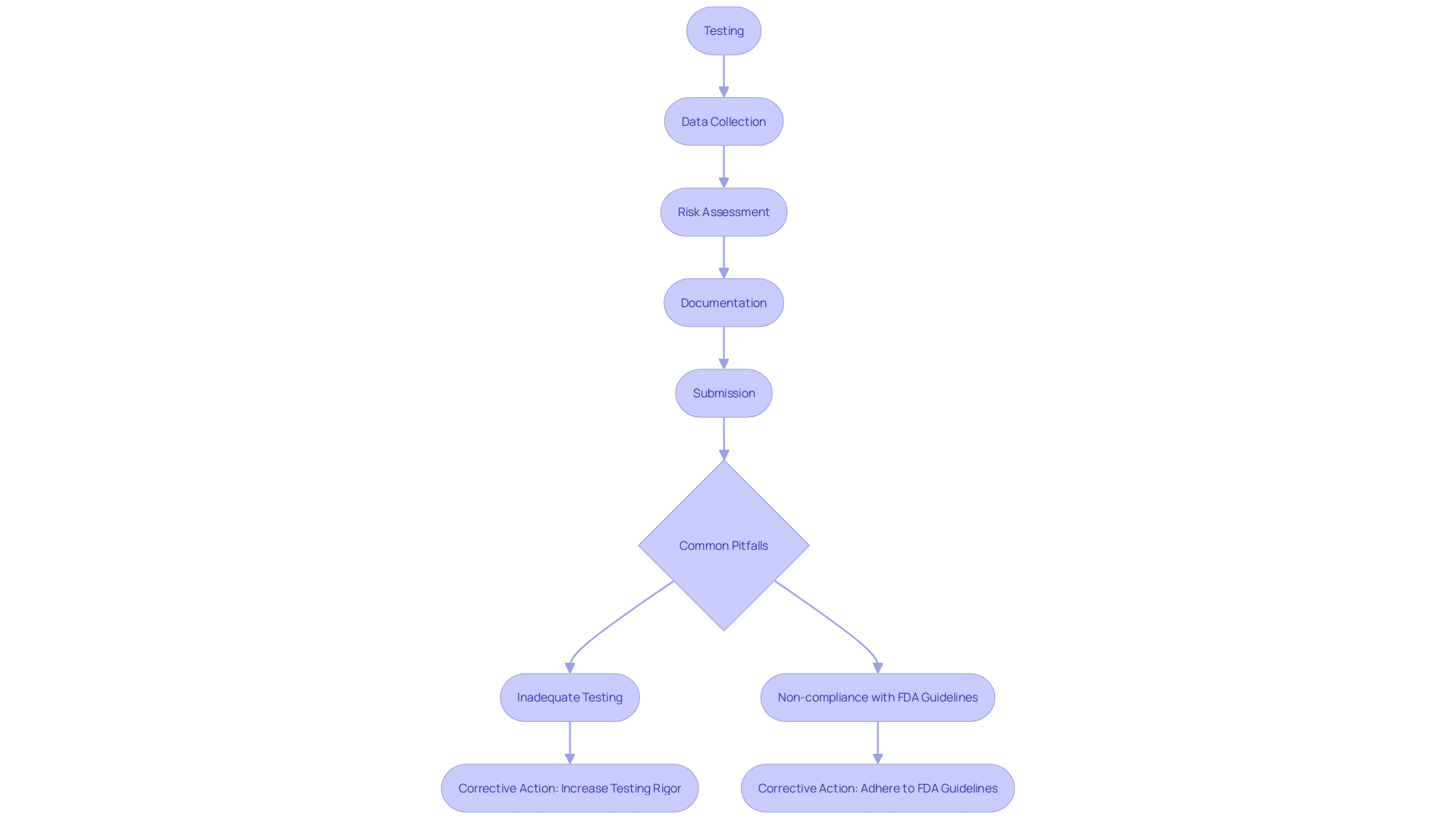
Navigating the PMA submission process is intricate, filled with numerous potential pitfalls that can derail a request if not meticulously addressed. Common issues include inadequate testing and data collection, which can result in an incomplete risk assessment and insufficient evidence of safety and efficacy. Absence of clarity in the submission often results in confusion and hold-ups, as the FDA demands accurate and thorough information to assess a product effectively.
Another significant challenge is the failure to adequately address potential risks and their mitigations. This oversight not only jeopardizes patient safety but also violates FDA guidelines, leading to probable rejection. Non-compliance with FDA regulations and guidelines is a critical pitfall that can arise from insufficient knowledge or failure to stay updated with evolving standards. As mentioned in recent updates to regulatory guidance, the combination of medical instruments with medicinal substances, such as pre-filled syringes, has brought about new responsibilities and requirements for manufacturers.
To successfully navigate these challenges, it is essential to adopt a proactive approach. This requires thorough testing and data gathering to provide strong proof of safety and efficacy, clear and detailed submission documents, comprehensive risk evaluations with well-documented mitigation strategies, and strict adherence to FDA guidelines. The recent establishment of advanced testing facilities, like the UL Solutions laboratory in Rochester Hills, Michigan, exemplifies how manufacturers can leverage state-of-the-art resources to meet stringent regulatory requirements and enhance product safety and performance.
Post-Approval Requirements
Once your PMA application is approved, there are several post-approval requirements that must be fulfilled to ensure the ongoing compliance and safety of the medical product. These requirements include conducting post-market surveillance to monitor the performance and safety of the product. This involves gathering real-world data to detect any potential issues early and ensure patient safety. Periodic reports must be submitted to the FDA, detailing the product's performance and any adverse events.
Additionally, it is essential to comply with any labeling or manufacturing changes mandated by the FDA. For example, if your apparatus is an integral drug-apparatus combination, such as a pre-filled syringe, you must adhere to specific labeling guidelines to ensure proper usage. The FDA may also specify additional post-approval requirements tailored to your product's unique characteristics and intended use.
Maintaining approval status requires constant vigilance and adherence to regulatory guidance. This includes staying updated with any revisions in FDA regulations based on new experiences and cases encountered. By actively managing these post-approval obligations, you can ensure that your device remains safe, effective, and compliant with all FDA requirements.
Conclusion
Navigating the FDA's Premarket Approval (PMA) process is a multifaceted endeavor that requires careful planning and thorough understanding of regulatory requirements. The journey begins with determining whether a PMA is necessary, influenced by the device's intended use, technological characteristics, and associated risks. Manufacturers must integrate quality and regulatory considerations from the outset to streamline approval and avoid compliance issues.
Understanding the various types of PMA submissions—Traditional and Modular—is essential for tailoring the application strategy. Each type serves distinct purposes, with Traditional PMA requiring comprehensive data for novel devices, while Modular PMA allows for a more flexible approach. Pre-submission preparation, including rigorous testing and leveraging the FDA's Q-Submission Program, can significantly enhance the chances of a successful application.
The PMA submission package must include crucial components such as a detailed device description, performance data, risk assessments, and post-market surveillance plans. The FDA's review process is thorough, encompassing administrative, scientific, and regulatory evaluations to ensure devices meet stringent safety and efficacy standards. Common challenges, such as inadequate testing and non-compliance, highlight the importance of a proactive approach to avoid pitfalls during the application process.
Post-approval requirements are equally vital, encompassing ongoing monitoring and compliance with regulatory updates to maintain device safety and effectiveness. By understanding and addressing these critical aspects, manufacturers can navigate the PMA process successfully, ultimately contributing to the advancement of public health through the introduction of safe and effective medical products.




