Introduction
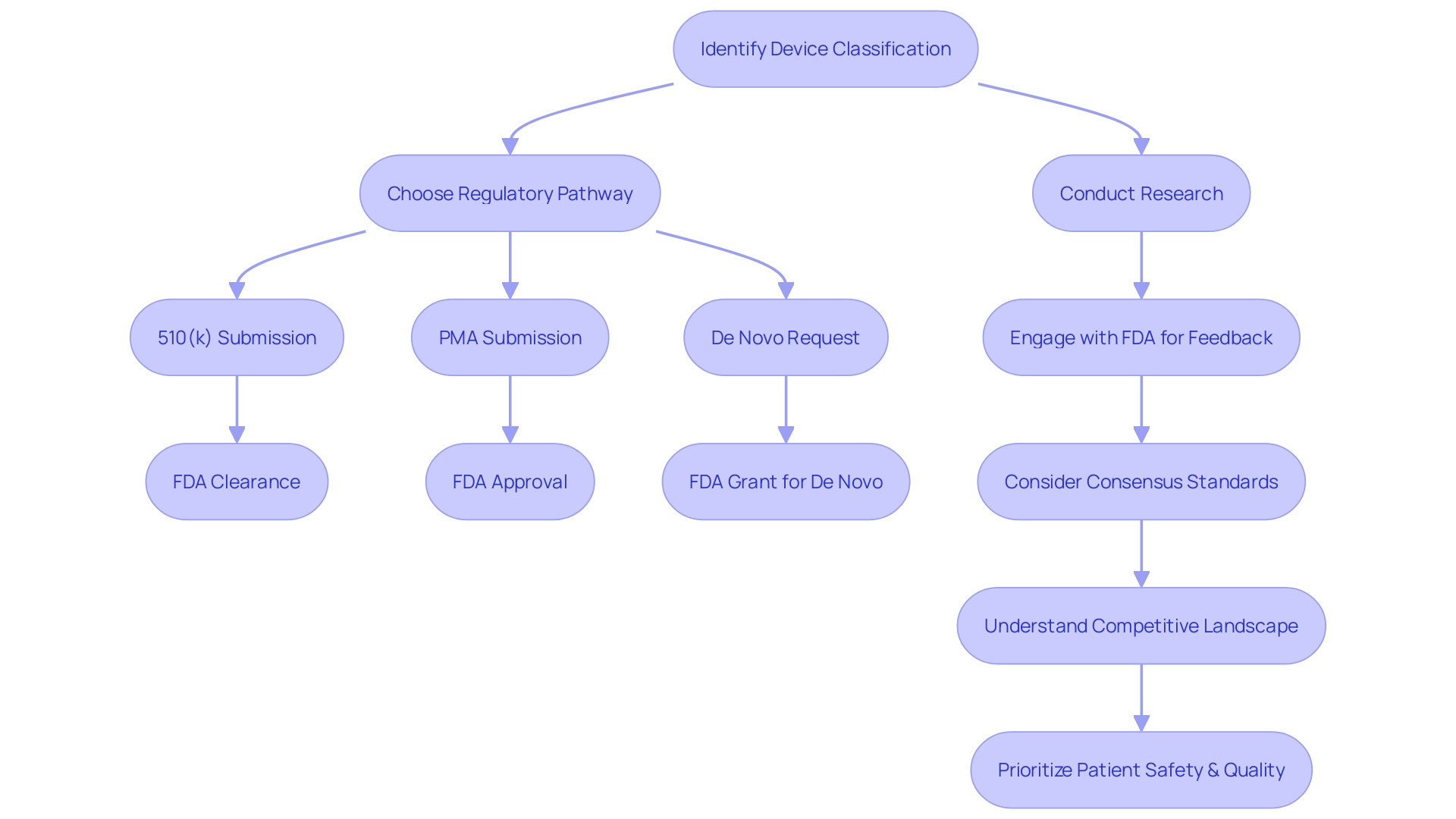
The process of securing Pre-Market Approval (PMA) for a medical device is complex and requires a solid understanding of the FDA's classification system. Devices are categorized based on patient risk, which determines the appropriate regulatory pathway. Prior to marketing a device in the U.S., it must achieve FDA Clearance, Approval, or a Grant for De Novo requests.
Differentiating between these terms is essential to navigate the pre-market process responsibly. Preparation for a PMA submission involves exhaustive research, data compilation, and engagement with the FDA for feedback on regulatory requirements. Familiarity with the target device's users, competitive landscape, and potential predicate devices is crucial for a successful submission.
Maintaining a patient-first approach and prioritizing safety and quality are emphasized by industry experts. Ensuring the exclusion of confidential information when sharing comments with the FDA and conducting diligent strategic planning contribute to a well-prepared PMA process. Overall, the goal is to advance healthcare through the introduction of safe and effective medical devices.
Pre-Submission Preparation and Q-Submission
The pathway to securing Pre-Market Approval (PMA) for a medical device is intricate and requires a solid understanding of the FDA's classification system. Devices are categorized into one of three classes based on patient risk, which determines the appropriate regulatory pathway—whether that be a Premarket Notification (510(k)), PMA, or De Novo process. Prior to marketing a device in the U.S., it must achieve FDA Clearance, Approval, or a Grant for De Novo requests. It's essential to grasp the nuances between these terms; for instance, 'Cleared' typically refers to 510(k) submissions where the device is demonstrated to be substantially equivalent to a legally marketed predicate device. 'Approved' indicates a device has undergone rigorous PMA review and been found to provide reasonable assurance of safety and effectiveness. On the other hand, 'Granted' applies to the De Novo pathway for novel devices that have not been classified and present a low to moderate risk. Understanding these distinctions is key to navigating the pre-market process responsibly.
In preparation for a PMA submission, it’s imperative to conduct exhaustive research and compile data from pre-clinical and clinical studies, ensuring alignment with FDA guidelines. A strategic step in this process is the Q-Submission, which allows you to engage with the FDA for feedback on regulatory requirements. Furthermore, engagement with consensus standards can enhance regulatory quality, as these are established by SDOs adhering to principles of transparency, openness, and due process, as outlined in OMB Circular A-119 and ANSI Essential Requirements. Moreover, familiarity with the target device's users, competitive landscape, and potential predicate devices is crucial to inform your submission. It's also important to maintain a patient-first approach, prioritizing safety and quality, as underscored by industry experts who emphasize the direct impact of medical devices on patient lives and the unique responsibilities that project managers bear in this sector.
When sharing comments with the FDA, be mindful to exclude confidential information, as these submissions become part of the public record. This level of diligence and strategic planning ensures that you are well-prepared for the PMA process, ultimately contributing to the advancement of healthcare through the introduction of safe and effective medical devices.
Key Components of a PMA Application
Crafting a successful Pre-Market Approval (PMA) application is a finely tuned process that necessitates a meticulous presentation of the medical device, backed by comprehensive evidence of its safety and efficacy. Critical to this endeavor is the inclusion of detailed device descriptions and manufacturing processes, supported by robust pre-clinical and clinical data, clear labeling, and precisely defined indications for use.
To navigate this intricate process, it's imperative to blend traditional project management skills with specialized knowledge of the medical device industry's regulations and patient-centric practices. As highlighted by the experiences of industry professionals such as Chris, a biomedical engineer with over a decade in the field, and Etienne Nichols, a seasoned Medical Device Guru, understanding the user perspective is instrumental in steering the decision-making process and prioritizing patient safety and product quality.
The FDA, which governs the safety and efficacy of medical devices in the U.S., divides these devices into three classes based on risk, with Class III devices like pacemakers undergoing the most stringent evaluation due to their critical role in sustaining life. These high-risk devices, which account for about 10% of the FDA-regulated devices, face an extensive approval process.
Recent initiatives have sought to streamline the regulatory pathways, a movement accelerated by the urgent needs of the COVID-19 pandemic, aiming to shorten approval times for groundbreaking devices in emerging fields such as digital health. Regulatory agencies play a pivotal role in the time frame for device approval, with variables including device complexity and the specific medical conditions they address.
It's essential for professionals in the medical device sector to stay abreast of the latest FDA guidelines and standards, like those recently published regarding direct-to-consumer advertising, which emphasize the use of consumer-friendly language and dual modality presentation for clarity. By adhering to these guidelines and leveraging insights from case studies and market research, such as MMIT's evaluation of asthma treatment products, manufacturers can enhance their PMA applications and ultimately contribute to advancing patient outcomes in the healthcare landscape.
The 4-Step PMA Review Process
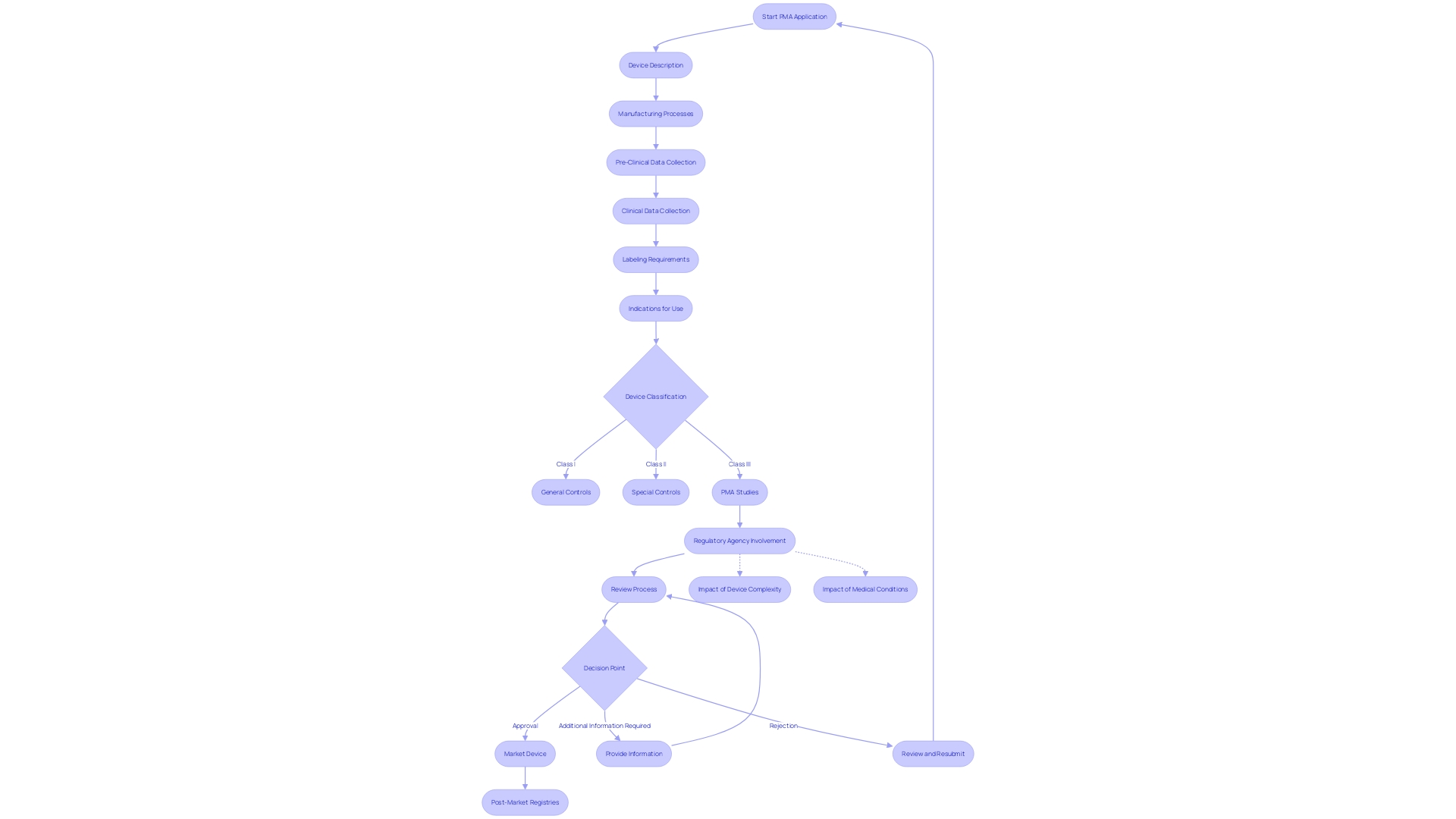
Navigating the Pre-Market Approval (PMA) process for medical devices is a multi-step journey that requires meticulous attention to detail and an in-depth understanding of regulatory requirements. The PMA is the FDA's rigorous method for evaluating Class III medical devices—those that support or sustain human life and carry the highest risk. This process demands comprehensive scientific and regulatory assessment to ensure the safety and effectiveness of a device before it can be marketed in the United States.
The initial step in the PMA process involves the correct classification of the device according to the FDA's three-tier system, which categorizes devices based on risk. Class I devices pose the least risk to patients, while Class III devices, such as implantable pacemakers, are subject to the most stringent scrutiny due to their critical role in patient care. It's essential to distinguish between being 'Registered', 'Cleared', 'Approved', or 'Granted' by the FDA, as these terms signify different levels of regulatory consent.
Once classified, the manufacturer must prepare a PMA application that includes extensive preclinical and clinical data demonstrating the device's safety and efficacy. This data must satisfy not only the FDA's rigorous standards but also the separate, and sometimes divergent, requirements of payers like CMS and private health plans, which assess the device's eligibility for coverage and reimbursement.
The PMA review by the FDA involves an exhaustive examination of the submitted data, after which the agency may request additional information or studies. With only approximately 10% of medical devices falling into the high-risk Class III category, the approval times can be protracted, reflecting the critical importance of these devices in clinical applications.
Finally, if the FDA determines that the evidence sufficiently supports the device's safety and effectiveness, it will grant approval. However, it's important to note that approval does not guarantee immediate coverage or widespread clinical use, as payers conduct their evaluations based on different data sets. These subsequent decisions by payers can affect the device's integration into the healthcare system, potentially leading to delays or denials in coverage.
Recent regulatory updates, such as those announced by the UK's MHRA, aim to harmonize international standards and facilitate access to medical devices, acknowledging the rapid evolution of medical technology and the need for patient-centered regulations.
Initial Review and Filing
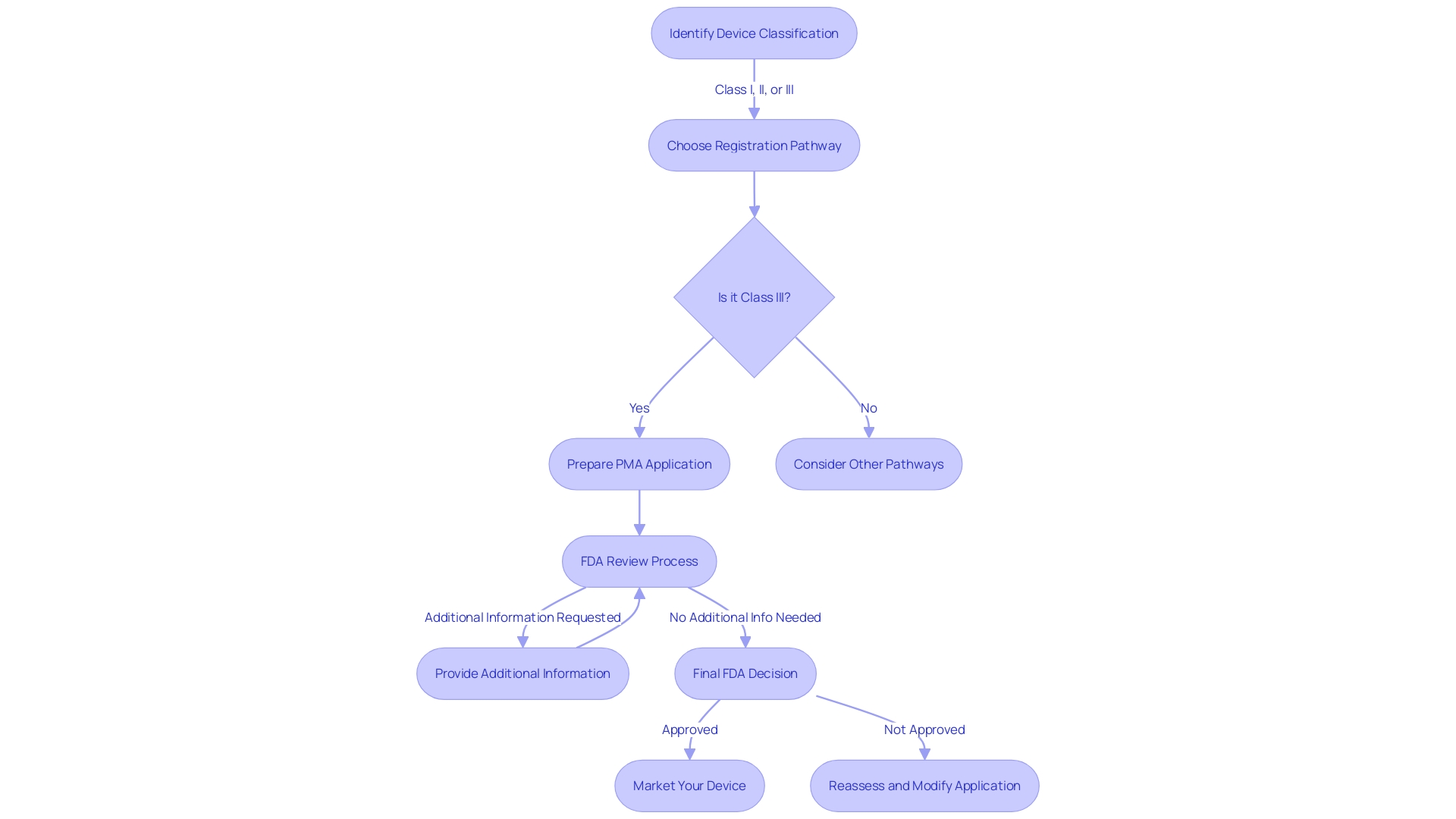
The FDA's initial review of a PMA application is a critical step that requires manufacturers to provide an extensive array of information to demonstrate the safety and effectiveness of their medical device. This documentation encompasses a detailed device description, the manufacturing processes, and comprehensive pre-clinical and clinical data. Additionally, clear labeling and precise indications for use must be included. It's essential to understand that a device's FDA approval does not guarantee immediate coverage or payment from healthcare payors; discrepancies between the data required by the FDA and that needed by payors can lead to delays or denials in coverage, impacting patient access to new devices. Thus, it is paramount for manufacturers to ensure their PMA submissions are complete and adhere to all regulatory requirements, setting the stage for further critical evaluation by the FDA. The PMA process is particularly stringent for Class III medical devices, which are typically those that support or sustain human life and are subject to the highest level of regulatory control due to their increased risk profile.
Substantive Review and Day-100 Meeting
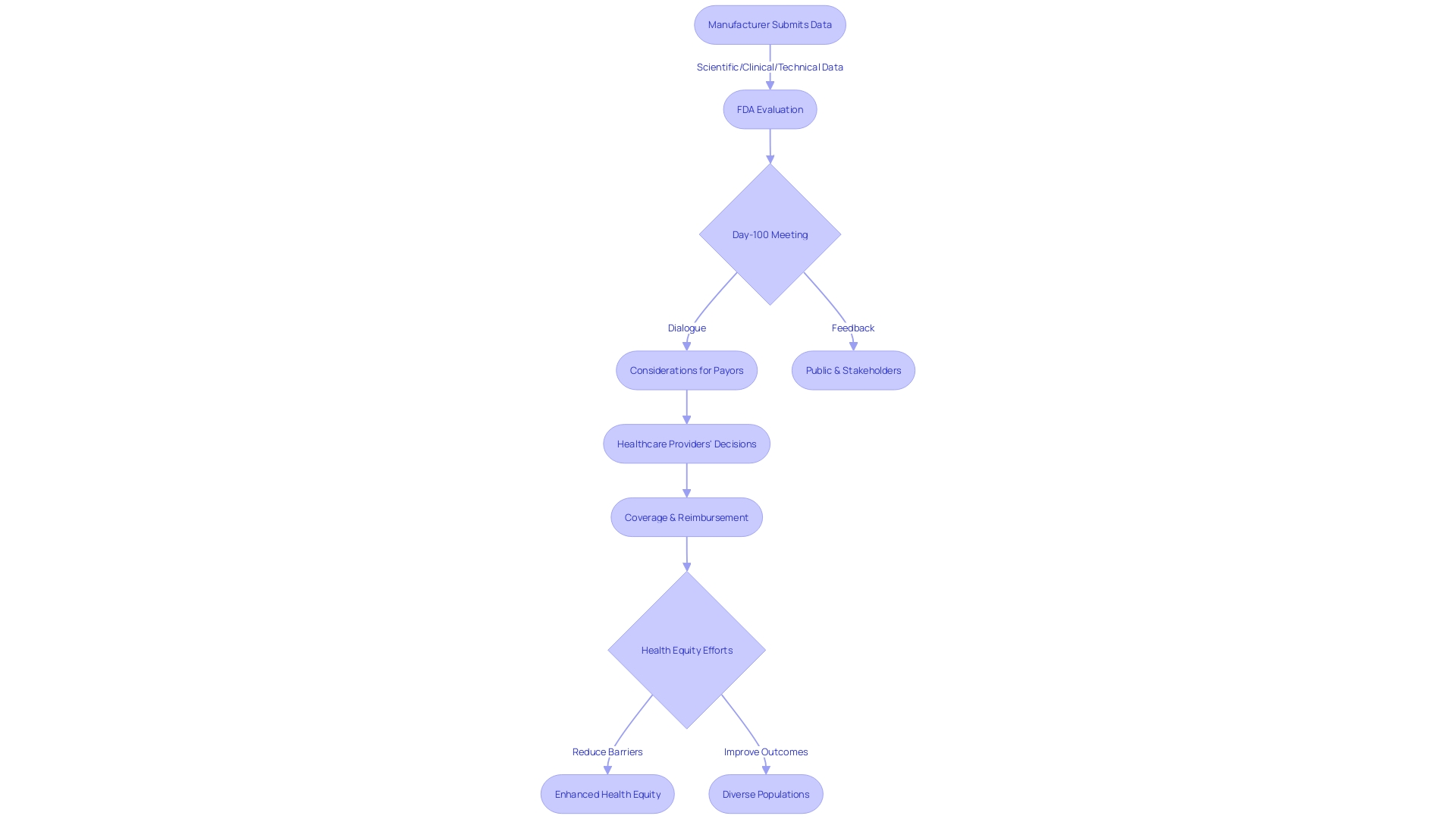
The Premarket Approval (PMA) process involves a thorough examination by the FDA of the scientific, clinical, and technical data submitted by medical device manufacturers. This rigorous evaluation is crucial as it ensures that high-risk medical devices, such as life-sustaining implantables, meet the stringent standards for safety and efficacy before they can be marketed in the United States. During the PMA review, the FDA meticulously analyzes the data to confirm that the benefits of the device outweigh any potential risks.
In line with the FDA's commitment to public health and safety, a critical milestone in this process is the Day-100 meeting. This meeting provides a platform for open dialogue between the FDA and the device sponsor. It is an opportunity to address substantive questions, clarify issues, and discuss any significant concerns that have emerged during the review. The goal is to resolve any outstanding matters that could impact the approval process, ensuring that the device is safe for use and performs as intended.
The FDA's evaluation extends beyond the initial approval, with considerations given to how the device will be received by other stakeholders, including payors and healthcare providers. It is acknowledged that the data submitted for FDA approval may not always align with the information required by payors, such as CMS and private health insurers, for coverage and reimbursement decisions. Consequently, even after FDA clearance, there may be delays or denials in patient access to new medical devices due to coverage determinations. This underscores the importance of comprehensive and relevant data submission to facilitate timely patient access to medical innovations.
In its efforts to enhance health equity and ensure that clinical studies reflect the intended use population, the FDA has initiated discussions on reducing barriers and improving outcomes across diverse populations. Recognizing that disease burden, physiology, and technology can influence the design of a clinical study, the FDA seeks public input on these considerations to refine guidelines and ensure that clinical data are generalizable to the broader patient community that the device is intended to serve.
Panel Review and Advisory Committee
The Pre-Market Approval (PMA) process for medical devices, particularly those classified as high-risk Class III, involves a stringent review by the FDA to ensure safety and effectiveness. A significant facet of this review is the convening of an advisory committee, comprising experts who offer their unbiased insights on the device in question. These experts, selected by the Commissioner from a pool of knowledgeable authorities in the field of digital health, assess various dimensions, such as scientific validity, clinical utility, and technical soundness.
A quintessential example of such a committee is the FDA's Digital Health Advisory Committee, which is instrumental in evaluating emerging technologies like AI, machine learning, and wearables. The committee provides the FDA with a comprehensive understanding of the potential benefits, risks, and clinical outcomes of Digital Health Technologies (DHTs). Their guidance is crucial, especially when it pertains to the use of DHTs in clinical trials or post-market studies regulated by the FDA.
The advisory committee's role extends beyond mere consultation; their recommendations have significant implications for the FDA's decision-making process. As such, these experts are a core component in navigating the intricacies of digital health regulation, ensuring that any policy or regulatory action by the FDA is well-informed and considers the rapid innovation in this sector.
This collaborative dynamic between advisory committees and the FDA underscores the agency's commitment to public health, as seen in its recent initiatives. For instance, the FDA's new rule on direct-to-consumer prescription drug advertisements emphasizes the clear and conspicuous presentation of a drug's major side effects in TV and radio ads, using consumer-friendly language and dual modality. Such measures reflect the FDA's dedication to transparency and consumer understanding, principles that are echoed in the PMA review process.
In conclusion, the advisory committee's contribution is a critical element in the FDA's holistic approach to medical device regulation, ensuring that each decision is backed by authoritative expertise and a thorough evaluation of all pertinent aspects of the device.

Final Decision and Approval
Upon concluding the rigorous review process, the U.S. Food and Drug Administration (FDA) arrives at a pivotal decision regarding the Pre-Market Approval (PMA) of medical devices. This determination is predicated on a comprehensive evaluation of data, including the substantive review findings and advisory committee insights. Should the device in question fulfill the stringent criteria, demonstrating both safety and efficacy, the FDA endorses it with a PMA approval. This endorsement is a green light for the device's introduction to the U.S. market. Understanding the FDA's classification system, which categorizes medical devices into three risk-based classes, is essential prior to seeking approval. Each classification dictates a specific regulatory pathway—be it 510(k) clearance, PMA, or the De Novo process—tailored to the device's risk profile. For medical device manufacturers and regulatory professionals, clarity on the distinct meanings of terms like Registered, Cleared, Approved, and Granted is crucial, as each represents a different compliance status in the FDA's lexicon.
FDA Actions on PMA Applications
During the PMA approval process for medical devices, the FDA meticulously evaluates submissions to ascertain whether the medical device complies with rigorous standards for safety and effectiveness. The actions that the FDA may take include:
- Reviewing all submitted comments, which are publicly accessible once posted to the docket, while ensuring that no confidential information is inadvertently disclosed.
- Providing guidance on how to submit comments with confidential information through written/paper submissions as detailed in the agency's instructions.
- Engaging with manufacturers during the review process to clarify any issues that arise from the submitted data or to request additional information necessary for the evaluation.
The FDA is committed to safeguarding public health by ensuring that only medical devices that meet their standards are approved. As expressed by Regeneron Pharmaceuticals, the definition of non-interventional studies used by the FDA differs from the European Medicines Agency (EMA), calling for a more harmonized approach between international regulatory bodies to avoid discrepancies in study classifications.
Moreover, recent news has highlighted the FDA's ongoing efforts to regulate the market effectively, including its rapid surveillance and targeted investigations of products appealing to youth, such as certain e-cigarette brands. These efforts underscore the agency's comprehensive approach to public health, extending beyond medical devices to include other products under its jurisdiction.
In the backdrop of regulatory scrutiny, it's noteworthy that some medical devices have been approved through the FDA's 510(k) clearance process, which has been criticized for not always requiring clinical trials. This process has been under examination, as seen in the 2018 documentary 'The Bleeding Edge', which revealed instances where devices approved through this pathway caused patient injuries. The FDA continues to evolve its processes in response to such findings, ensuring that patient safety remains paramount.
Approval Order and Conditions of Approval
Upon meticulous review by the FDA, if a PMA application is deemed to meet all essential criteria, the FDA will issue an approval order. This document is more than a mere formality; it delineates the specific conditions under which the medical device is allowed to be marketed, as well as any post-approval obligations, including additional studies that might be required. Adherence to these conditions is of paramount importance for the continuation of the device's approved status. The significance of this phase in the regulatory process cannot be overstated, as evidenced by past challenges in the medical device industry. For instance, a 2018 documentary highlighted the risks associated with devices that were cleared through the FDA's 510(k) pathway, which does not always require clinical trials – some of these devices were later associated with severe patient harm. This underscores the criticality of rigorous post-approval monitoring, a responsibility that the FDA has been enhancing through the development of an active postmarket surveillance system, in response to past incidents where over 1.7 million injuries and 83,000 deaths were potentially linked to medical devices over a decade. The FDA's commitment to safety is a continuous endeavor, ensuring that once a device is on the market, it is subject to ongoing scrutiny to protect public health.
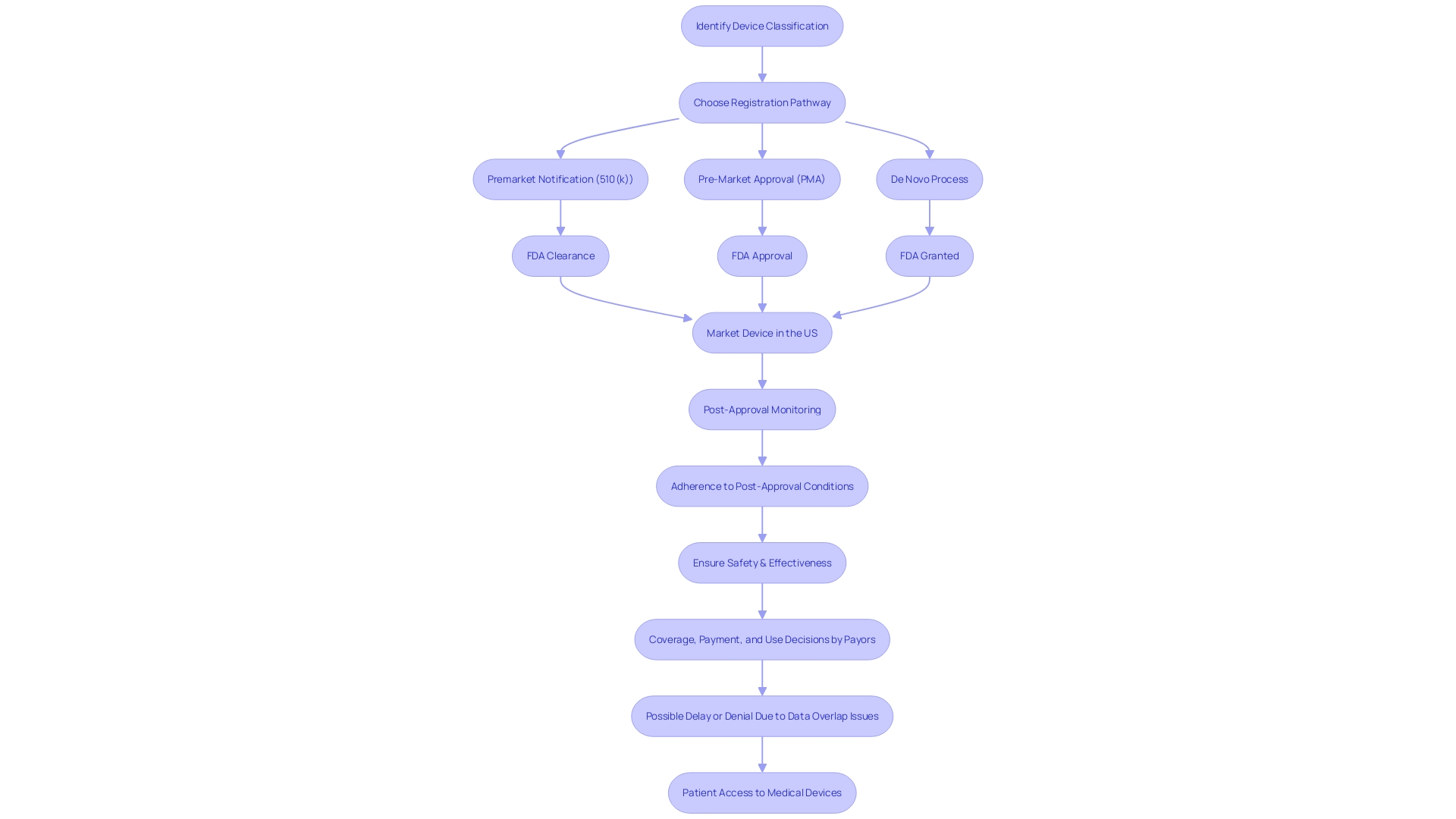
Approvable, Not Approvable, and Denial Letters
Navigating the FDA's Pre-Market Approval (PMA) process is a multi-faceted journey, requiring a thorough understanding of device classifications and regulatory pathways. Each medical device is classified based on the level of risk it poses to patients, ranging from low-risk Class I to high-risk Class III devices. Class I and II devices typically follow a less stringent 510(k) clearance process, while Class III devices, which often sustain or support life, must undergo the more rigorous PMA pathway. The latter, representing about 10% of devices regulated by the FDA, demands comprehensive evidence of safety and efficacy due to the critical nature of their applications.
Upon submission of a PMA application, the FDA's feedback can take several forms. An 'approvable' letter signals that the application is on track for approval, pending specific additional information or clarifications. Conversely, a 'not approvable' letter indicates that the application currently falls short of the regulatory requirements. In the event that an application is deemed to not sufficiently demonstrate the device's safety and effectiveness, the FDA will issue a 'denial' letter. Each type of correspondence from the FDA requires a strategic response, with a comprehensive understanding of the distinctions between 'Registered,' 'Cleared,' 'Approved,' and 'Granted' statuses being pivotal for advancing through the regulatory landscape.
Recent cases, such as that of the Impella Connect System, illustrate the importance of discerning FDA terminology and processes. The system, encompassing both hardware and software components, had to meet specific criteria under section 201(h) of the Federal Food, Drug, and Cosmetic Act due to its role in critical care settings. Its ability to provide patient-specific medical information and generate vital alarms necessitated premarket authorization, underscoring the intricacies of FDA regulations and the significance of complying with them for patient safety and public health assurance.
This regulatory framework, designed to protect public health, requires meticulous adherence to FDA guidelines. Whether a device is intended for remote monitoring, like the Impella Connect System, or is part of a more traditional medical device category, understanding and responding to the FDA's feedback is essential for successful market entry and patient care.
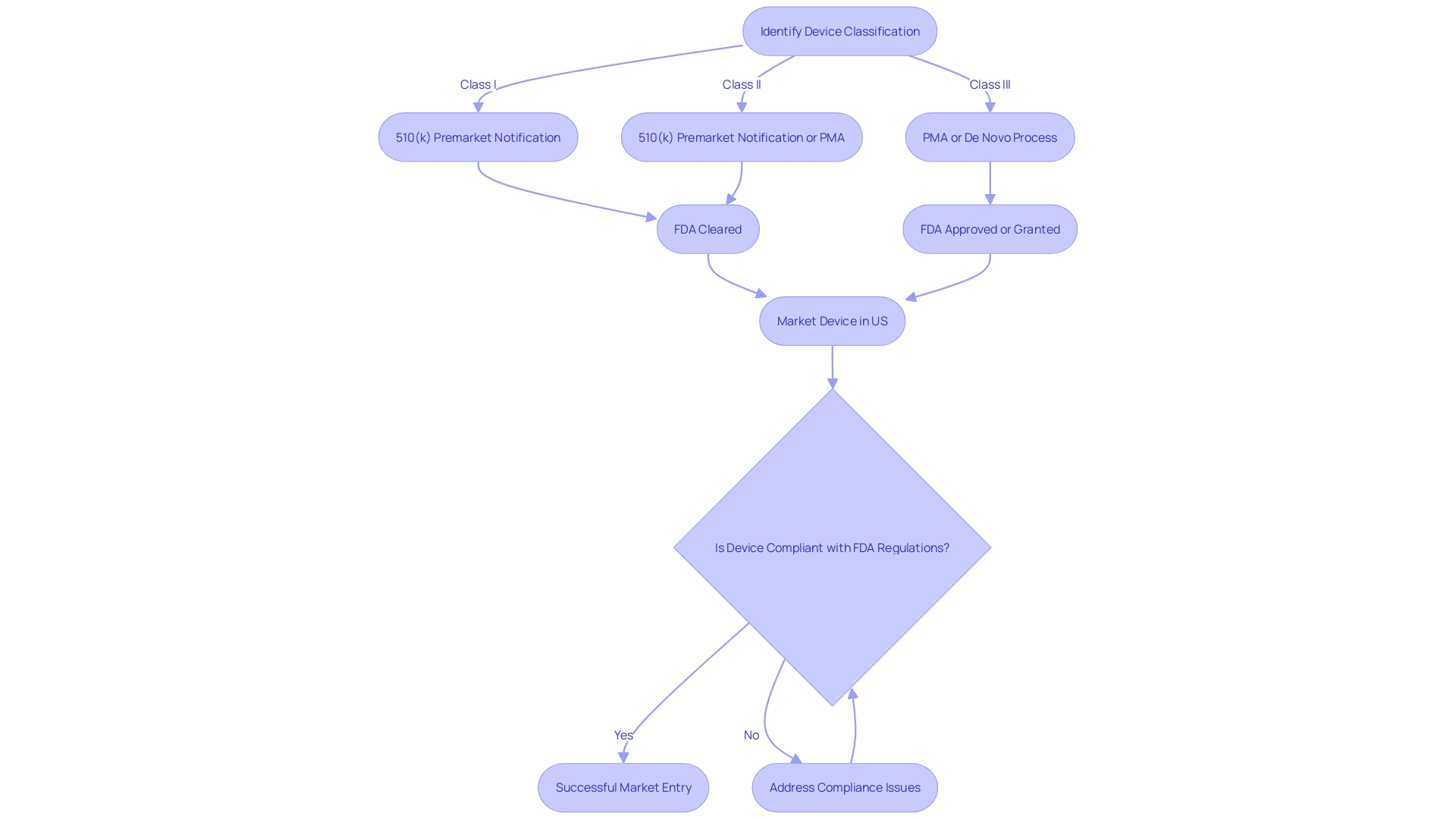
Post-Approval Requirements and Compliance
Achieving Pre-Market Approval (PMA) for a medical device is a significant milestone, but the journey doesn't end there. The FDA requires ongoing compliance through post-approval measures to guarantee the safety and effectiveness of the device in the real-world setting. This includes active participation in Post-Market Surveillance (PMS) to collect data on device performance, reporting any adverse events, upholding quality control systems, and updating labeling or manufacturing processes as needed.
Understanding the device's market scope is crucial since it determines the breadth of regulatory compliance. Whether a medical device has a global reach or is limited to specific regions, companies often opt for universal compliance to navigate the complexities of varying regulations. For instance, the RoHS directive, which affects electronics supply chain activities such as Printed Circuit Board Assemblies (PCBAs), is one such regulation that companies may need to adhere to depending on their device's components and distribution.
In addition to market understanding, maintaining complete transparency in the device's Bill of Materials (BOM) is imperative. Full visibility, including component serialization and lot traceability, is essential for risk management and regulatory compliance. Changes in regulations may place previously acceptable components at risk, necessitating quick identification of their use and distribution.
The role of the supply chain is also vital in ensuring compliance. Given the complexity and multi-level nature of medical device manufacturing supply chains, companies often rely on upstream suppliers to certify the compliance of parts or materials. This practice aids in streamlining the process and ensuring that every component meets regulatory standards.
The importance of these post-approval obligations is underscored by the FDA's recent actions to address product misinformation and ensure device safety. For example, the recall notice sent by Medtronic for its MiniMed pump system demonstrates the critical need for timely and effective post-market measures.
In conclusion, the PMA approval is just the beginning. The post-approval phase is a dynamic and critical period for medical devices, where manufacturers must remain vigilant and proactive in meeting regulatory obligations to ensure patient safety and device efficacy.
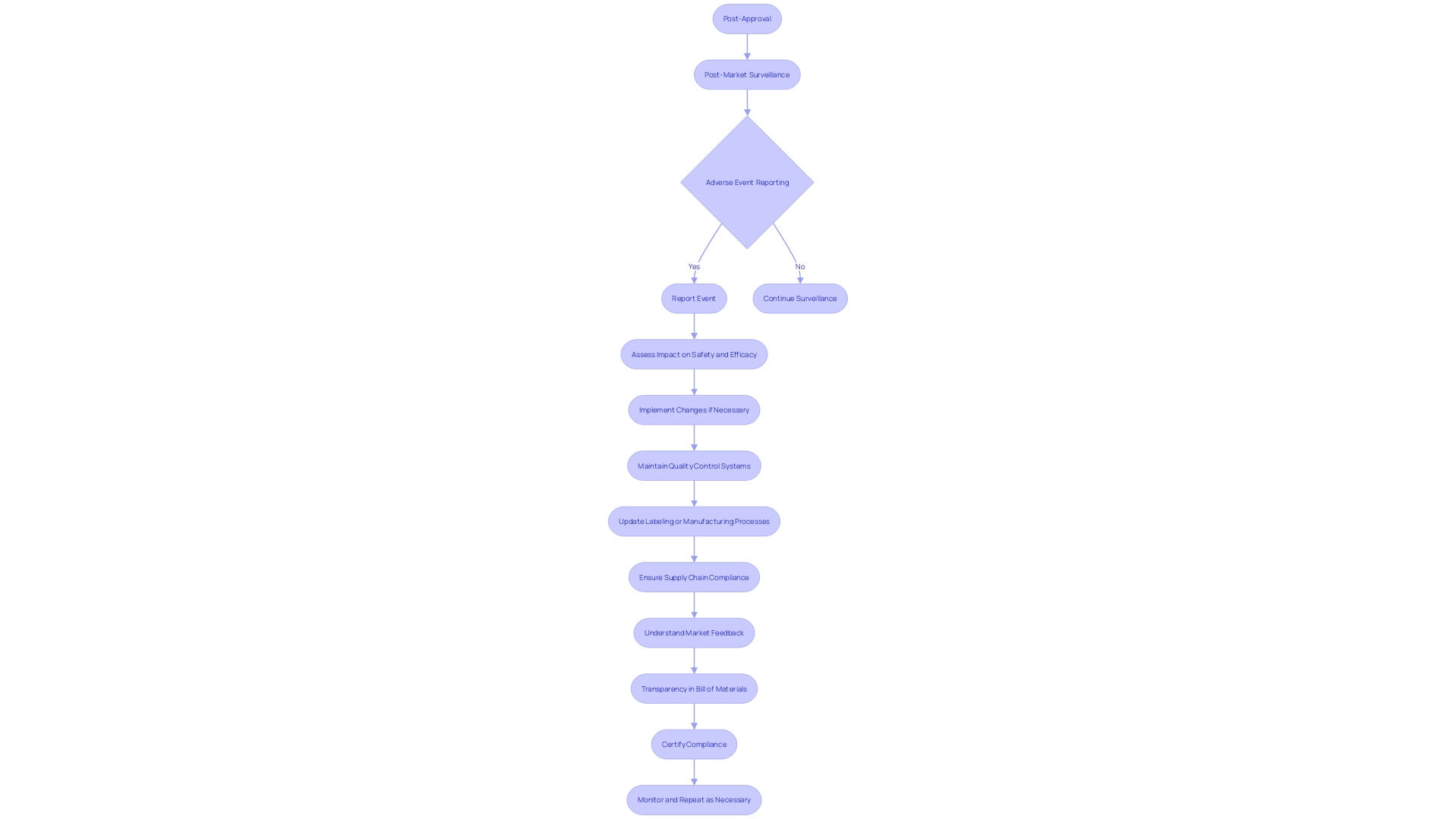
Common Challenges and Pitfalls in PMA Submissions
The Pre-Market Approval (PMA) process for medical devices is a critical pathway that requires meticulous attention to detail and adherence to FDA guidelines to ensure a successful application. The PMA pathway is specifically designed for Class III medical devices, which are considered high risk due to their significant role in sustaining or supporting life, such as implantable devices like pacemakers. Given that these devices represent about 10% of the FDA-regulated medical devices, they are subject to a more rigorous regulatory process compared to Class I and II devices, which may be cleared through the 510(k) pathway.
A clear understanding of the device's intended use, patient risks, and proper classification is paramount before choosing the registration pathway. Once the classification is determined, the PMA process involves submitting comprehensive evidence to demonstrate the safety and efficacy of the device, including robust pre-clinical and clinical data. This is in contrast to the 510(k) clearance, which relies on demonstrating substantial equivalence to an already cleared predicate device.
A common pitfall in the PMA submission process is the inadequacy of supporting data and misalignment with the FDA's regulatory expectations. A fully realized submission should include meticulously organized documentation and address all FDA feedback promptly and thoroughly. This proactive approach is crucial because Class III devices, due to their complexity and high risk, undergo a longer approval time.
The medical device landscape is evolving with a push towards streamlined regulatory pathways, and efforts are underway to foster collaboration between regulatory agencies and industry stakeholders. These initiatives, accelerated by the COVID-19 pandemic, aim to expedite the approval process for novel devices meeting urgent medical needs, particularly in emerging fields like digital health and personalized medicine.
It is crucial for applicants to educate themselves on their device, the competitive landscape, and the regulatory environment. A deep understanding of the users, the device's instructions for use, and potential predicate devices can inform a more strategic PMA submission. In doing so, applicants can significantly improve their chances of navigating the PMA process successfully and bringing their Class III medical devices to market, ultimately enhancing patient care and quality of life.

Conclusion
In conclusion, securing Pre-Market Approval (PMA) for a medical device is a complex process that requires thorough research, data compilation, and engagement with the FDA. Prioritizing patient safety, product quality, and adhering to regulatory requirements are crucial for a successful PMA submission.
Crafting a successful PMA application involves presenting comprehensive evidence of the device's safety and efficacy. Understanding the user perspective and incorporating their needs into the decision-making process is vital.
The PMA review process entails the correct classification of the device, thorough preparation of the application with extensive data, and a rigorous FDA review. The FDA's evaluation extends beyond initial approval to consider the device's reception by payers and healthcare providers.
The advisory committee's insights play a significant role in the FDA's decision-making process, ensuring well-informed policies and regulations. The FDA's commitment to transparency and consumer understanding is evident in recent initiatives, such as guidelines for direct-to-consumer advertising.
Post-approval obligations, including active participation in post-market surveillance and compliance with regulatory requirements, are essential for maintaining the device's approved status.
Navigating the FDA's PMA process requires a deep understanding of device classifications and regulatory pathways. The FDA's actions during the approval process, such as reviewing comments and engaging with manufacturers, ensure compliance with rigorous standards.
In summary, achieving PMA for a medical device requires meticulous attention to detail, adherence to FDA guidelines, and a comprehensive understanding of regulatory requirements. Prioritizing patient safety, maintaining transparency, and fulfilling post-approval obligations are crucial for successful market entry and patient care. The FDA's commitment to safety and transparency underscores its dedication to public health and the advancement of safe and effective medical devices.




