Introduction
The Premarket Approval (PMA) process is a crucial pathway for ensuring the safety and effectiveness of medical devices before they are marketed in the United States. It is the most stringent type of device marketing application required by the FDA, and it involves a rigorous scientific review. The PMA Database plays a vital role in providing detailed information about these high-risk devices, aiding healthcare professionals and manufacturers in navigating compliance and market readiness.
In this article, we will explore the importance of the PMA Database, the process of accessing it, and how it serves as a valuable research tool. We will also discuss the significance of understanding device classifications, post-market surveillance, and staying updated with regulatory changes. Join us as we delve into the world of medical device regulation and the role of the PMA Database in ensuring patient safety and driving innovation.
What is the PMA Database?
The Premarket Approval (PMA) process is an essential pathway for ensuring that medical devices intended for human use are safe and effective before they are marketed in the United States. It is the most stringent type of device marketing application required by the FDA and is based on a scientific review. Class III medical devices, which are those that support or sustain human life, are preventatively important or pose a potential unreasonable risk of illness or injury, are subject to this rigorous approval process. These high-risk devices must undergo a PMA, representing approximately 10% of devices regulated by the FDA.
According to experts like Chris, a biomedical engineer with over a decade of experience in the medical device industry, the PMA process can be intricate, involving multiple stages of clinical studies for Class III devices. The PMA Database is a crucial tool that provides access to detailed information about such devices. It not only aids healthcare professionals but also supports manufacturers in navigating the complexities of compliance and market readiness.
Industry observers such as Casey Ross and Bob Herman emphasize the importance of the FDA's role in both innovation and patient protection. In recent years, the FDA has proposed updates to clarify programs like the Breakthrough Devices Program, which aims to accelerate the development and review of innovative medical devices that provide more effective treatment or diagnosis of life-threatening or irreversibly debilitating diseases or conditions.
The FDA's Center for Drug Evaluation and Research (CDER) also plays a pivotal role in advancing healthcare, as it provides clarity to developers on the necessary study design and data needed in the drug application for a comprehensive assessment. Each year CDER approves a range of new drugs and biological products, enhancing treatment options and healthcare advances for the public.
Understanding the impact of regulatory agencies on medical device approval times is crucial. Factors such as the complexity of the device, the medical condition it addresses, and the efficiency of the regulatory process in different regions can affect these timelines. Efforts to streamline regulatory pathways and improve collaboration between agencies and stakeholders have been accentuated by the COVID-19 pandemic, aiming to expedite approvals for devices that meet critical medical needs, especially in areas like digital health and personalized medicine.
The medical device industry is continuously evolving, with a focus on improving the quality of life for patients. Devices range from simple items like tongue depressors to complex technologies like prostheses and in vitro diagnostic tools. The PMA Database stands as a testament to the FDA's commitment to public health, safety, and innovation.
Why is it Important to Navigate the PMA Database?
The Pre-Market Approval (PMA) database is a treasure trove of information that serves as a navigational beacon for stakeholders in the medical device industry. This comprehensive resource sheds light on the intricate regulatory history, clinical data, and vital post-market surveillance (PMS) information of devices that have successfully achieved PMA status. For manufacturers, mastering the PMA database unlocks the potential to mine valuable data on existing devices, scope out the competitive landscape, and crystallize the regulatory benchmarks for their device submissions.
PMS is the vigilant eye that oversees the ongoing safety and efficacy of medical devices once they have entered the market. This vital phase extends beyond pre-market trials, facilitating the continual assessment and reporting of a device's real-world performance. Employing a myriad of methods, such as passive and active surveillance systems, PMS ensures that medical devices are monitored in their natural habitat—the patient-care setting. For instance, the Impella Connect System exemplifies how modern devices incorporate software and hardware components to provide real-time monitoring and critical alerts, reinforcing the importance of PMS in maintaining high standards of patient care.
With the rapid evolution of medical technologies, the journey of a medical device does not culminate with regulatory green-lighting. The post-market phase is where the rubber meets the road, with ongoing vigilance being paramount. The PMA database not only captures this journey but also provides a platform for continuous improvement and safety assurance.
Medical device development is an intricate dance that intertwines concept generation, design, engineering, and regulatory compliance, leading up to the grand finale of market launch. Stakeholders in search of a development partner often seek entities that offer a comprehensive suite of services to ensure a smooth transition between phases, ultimately saving time, resources, and enhancing the success of the project. The ideal partner brings to the table not only technical know-how but also a profound grasp of the regulatory terrain and market dynamics.
The medical device sector is characterized by its relentless pursuit of innovation, with technological advancements spearheading progress and better patient outcomes. From sophisticated diagnostic tools and surgical instruments to advanced imaging systems and wearable health technologies, these devices are instrumental in improving patient health and quality of life. Project managers in this field must possess a unique blend of traditional project management skills and specialized knowledge of medical device industry practices to successfully navigate this complex landscape, always with a patient-first approach.
Accessing the PMA Database
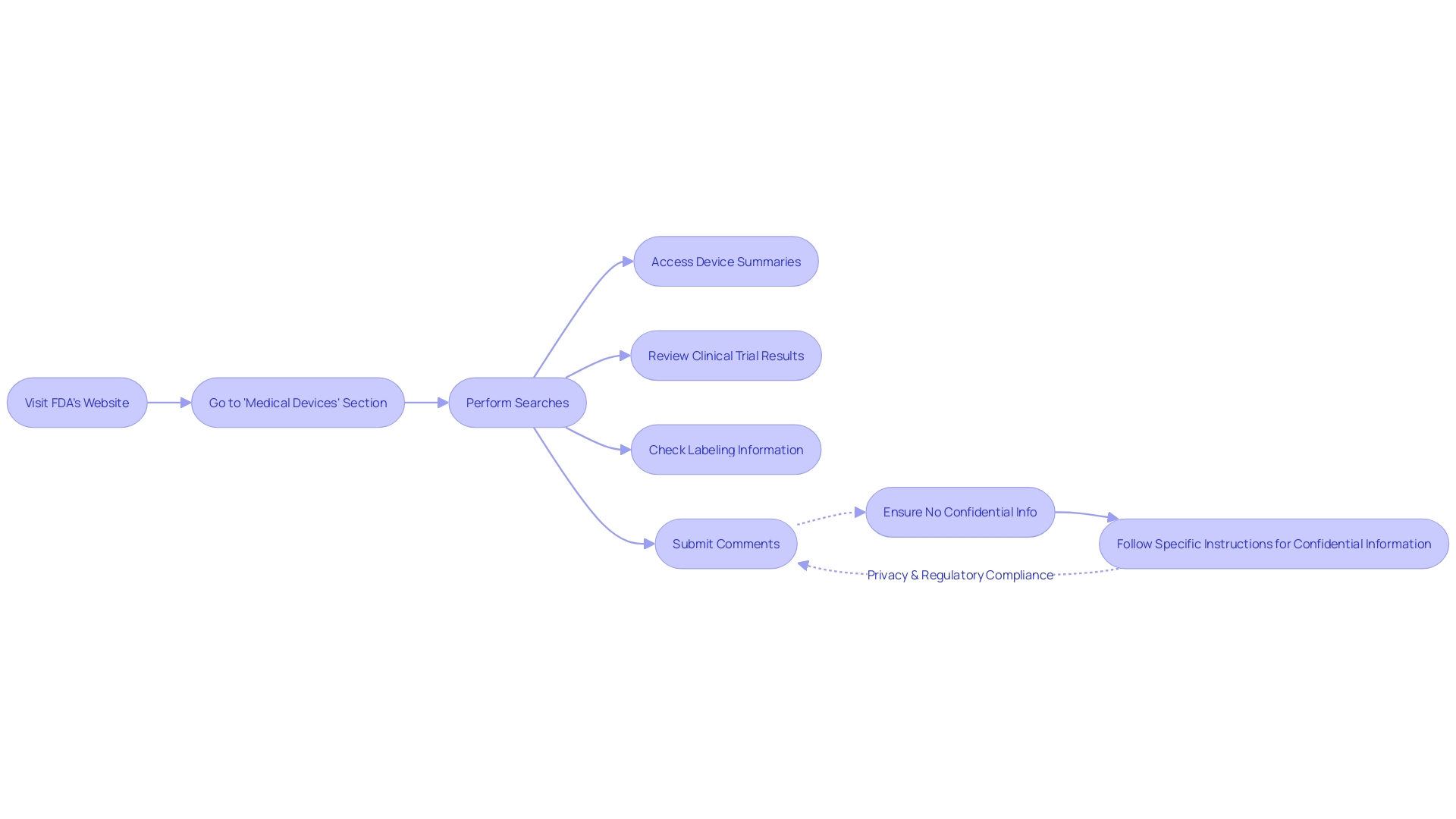
Navigating the FDA's PMA database can be a crucial task for professionals in the field of clinical research and medical device regulation. To find the necessary information, one would start by visiting the FDA's official website. Once there, the 'Medical Devices' section is the gateway to access the PMA Database. This comprehensive resource is designed to be intuitive, allowing users to perform searches based on various criteria such as device names, manufacturers, or specific PMA numbers.
The database offers an extensive array of details for each PMA-approved device. Users can expect to find summaries that shed light on the safety and effectiveness data, which are critical for making informed decisions. Moreover, clinical trial results are available, providing insights into the efficacy of the devices. Labeling information is also included, which is essential for understanding the intended use and the operational guidelines of the medical device in question.
It's important to note, when submitting comments or information to the database, that anything provided electronically, including attachments, will be made publicly available and posted to the docket without changes. Therefore, it's incumbent upon the submitter to ensure that no confidential information is included in the comments, such as private medical details, Social Security numbers, or proprietary business information.
Professionals, like Chris — a biomedical engineer with over a decade of experience managing clinical studies for Class III devices — understand the significance of the data housed within the PMA database. He leverages his expertise as a Solutions Engineer to navigate these resources effectively.
As the FDA is committed to assuring the safety, effectiveness, and security of medical devices, the PMA database serves as a critical tool in this endeavor. It supports the agency's broader responsibilities, which encompass the public health protection of food supply, cosmetics, dietary supplements, products emitting electronic radiation, and oversight of tobacco products.
For those looking to engage with the PMA database, it's advised to submit comments with discretion, considering the public nature of the information. In instances where confidential information needs to be submitted, following the detailed instructions for written/paper submissions is necessary to ensure privacy and compliance with regulatory standards.
Understanding the PMA Approval Process
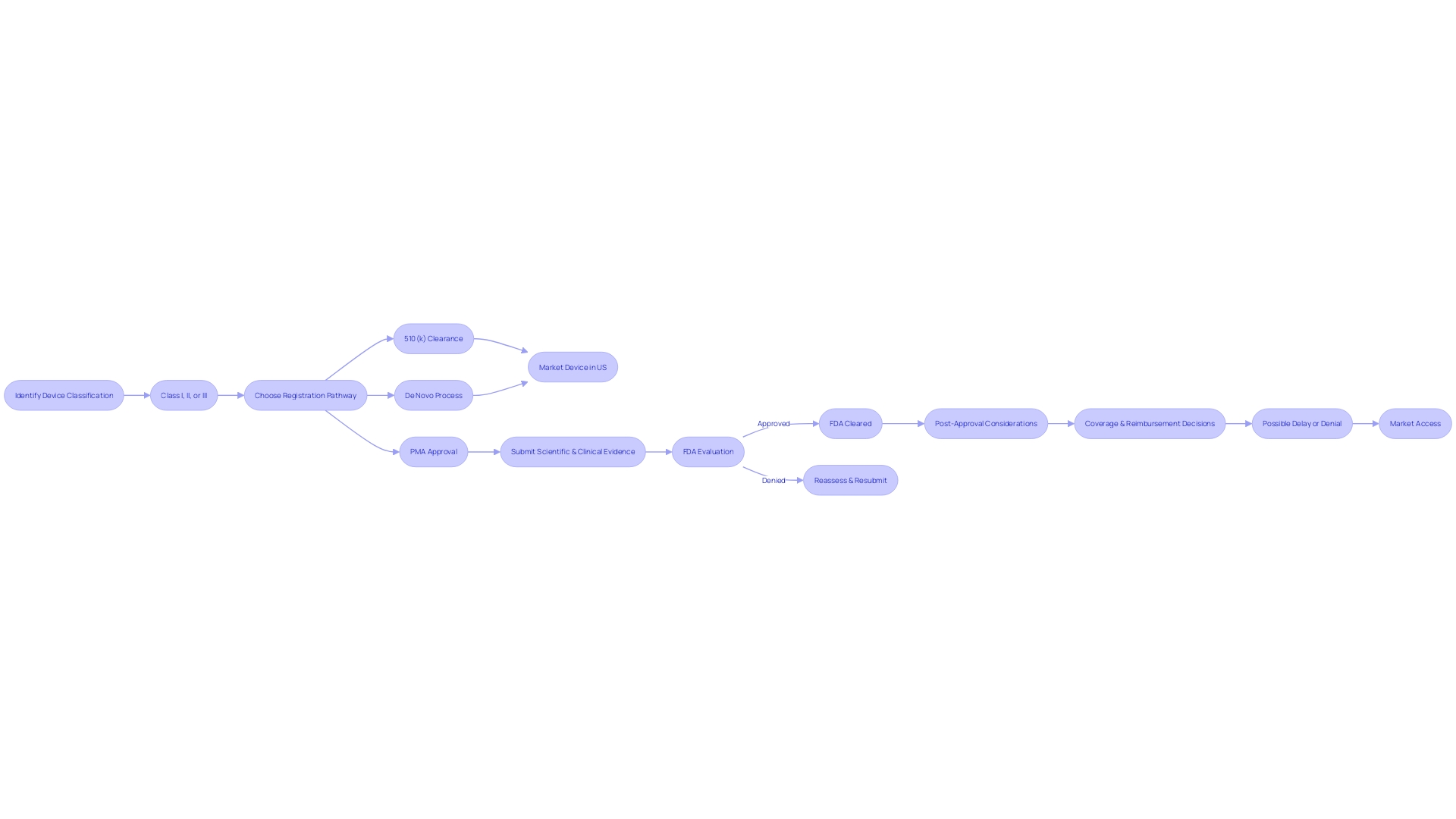
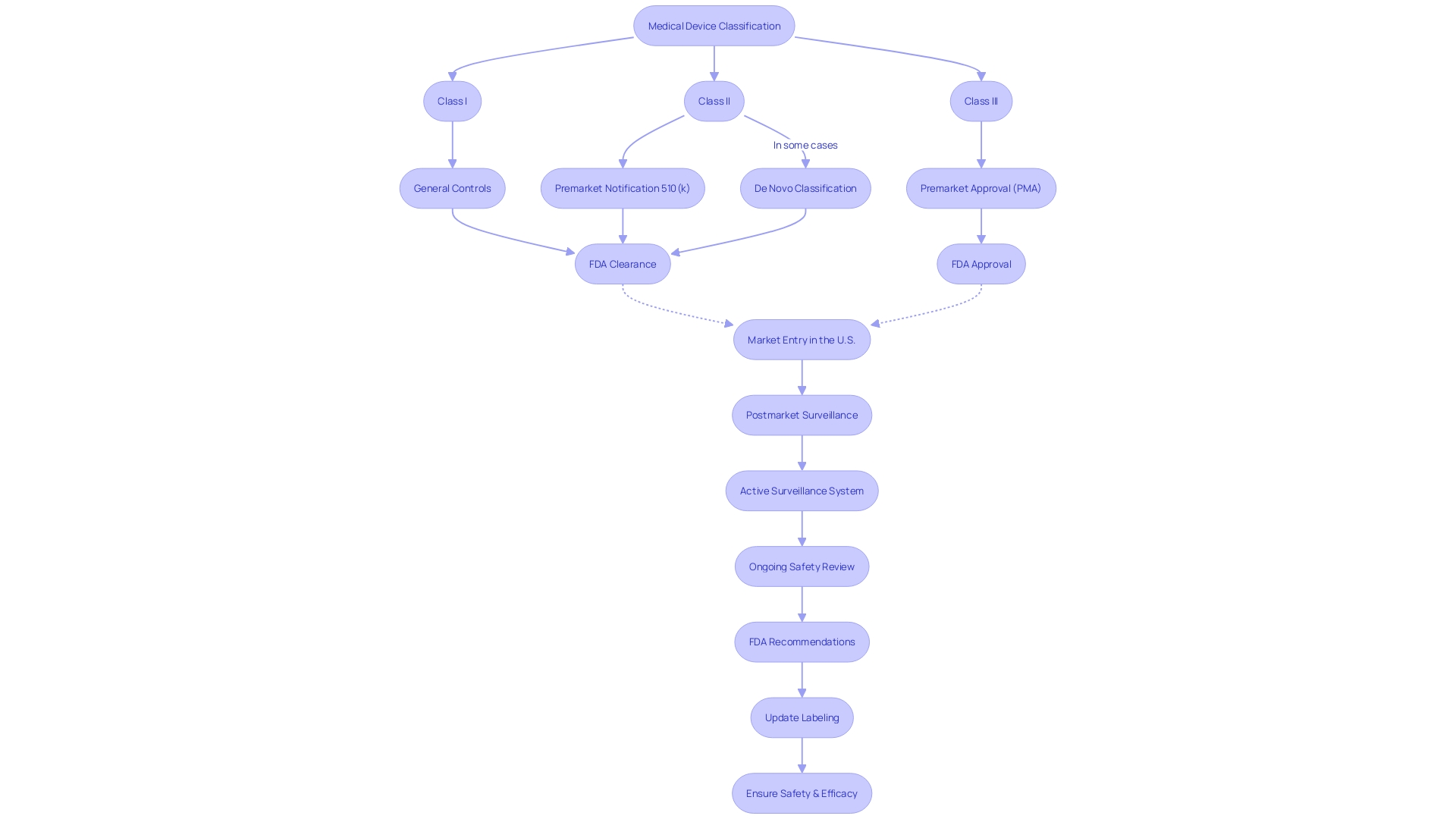
To comprehend the Pre-Market Approval (PMA) process thoroughly, it's essential to recognize that it is the FDA's most rigorous path to evaluate medical device safety and effectiveness. Manufacturers embarking on this process are obliged to present a substantial body of scientific and clinical evidence to establish the device's safety and efficacy. However, the journey to PMA approval begins with the correct classification of the device based on the level of risk it poses to patients. The FDA categorizes devices into three classes, with Class III devices, such as life-sustaining pacemakers, undergoing the most stringent evaluation due to their high-risk nature. These devices must comply with the PMA pathway and represent roughly 10% of all devices regulated by the FDA.
Understanding the differences between the terms 'Registered,' 'Cleared,' 'Approved,' and 'Granted' is crucial for regulatory professionals, as each signifies a different level of FDA evaluation and endorsement. For example, 'Cleared' typically refers to devices that have successfully undergone the 510(k) process, which involves comparing the new device to a previously approved one. On the other hand, 'Approved' devices have met the more stringent requirements of the PMA process.
Moreover, the PMA process is not solely about FDA approval. Post-approval, healthcare providers and payors such as CMS and private health plans determine coverage and reimbursement decisions, which are not always straightforward. The data needed for FDA approval may not align with what payors require for coverage determinations, potentially leading to delays or denials in device utilization.
Remaining abreast of the latest FDA rules and guidelines, including the use of consumer-friendly language and terms, is a duty shared by all stakeholders in the medical device industry. Such standards ensure that information is accessible and comprehensible to the public. Thus, navigating the PMA Database becomes more than an exercise in regulatory compliance; it is about gaining a deep understanding of the intricate requirements and standards that govern medical device approvals, ultimately impacting patient care and health outcomes.
Exploring Device Classifications and Indications
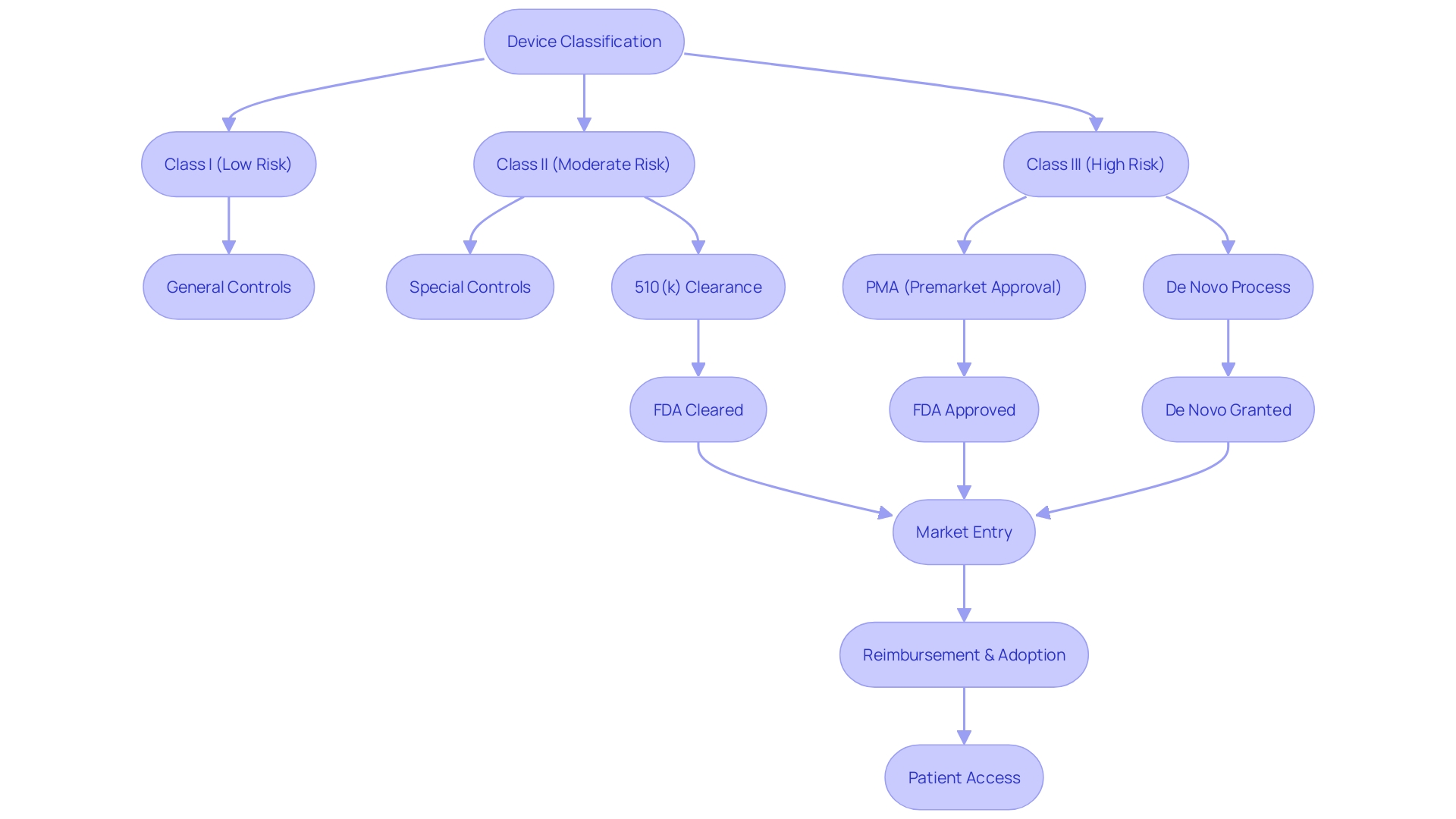
Navigating the PMA database is a crucial step in understanding the FDA's regulatory framework for medical devices. Devices are classified by the FDA into three categories reflecting the level of risk they pose to patients. To legally market a device in the U.S., the device must be FDA Cleared, Approved, or, in the case of the De Novo process, Granted. Understanding these distinctions is essential for manufacturers to strategically approach the market and adhere to regulatory standards.
Before a device can be marketed, its classification must be accurately determined, which will dictate the registration pathway to follow: Premarket Notification (510(k)), PMA, or De Novo process. This classification system allows the FDA to ensure patient safety while also streamlining the approval process for lower-risk devices. The process of establishment registration requires manufacturers to provide detailed information on their devices and activities to the FDA.
Recent FDA actions, such as the final rule on direct-to-consumer drug advertisements, underscore the importance of clear and consumer-friendly communication. This translates to the medical device industry, where clarity in the classification, risks, and intended use of a device is paramount for the protection of public health. Moreover, the data submitted to the FDA to demonstrate a device's safety and efficacy may differ from what payors require for coverage decisions. This can lead to delays or denials in coverage, impacting the device's market entry and patient access.
Device manufacturers need to be mindful that while FDA approval is a significant milestone, it is one step in a broader journey that includes reimbursement and adoption by healthcare providers. Therefore, a comprehensive understanding of the FDA's classification and approval processes is not just regulatory compliance, but a strategic business necessity.
Analyzing Post-Market Surveillance Data
Post-market surveillance (PMS) is an essential, continuous process that monitors the safety and effectiveness of medical devices after they have been approved for market entry. This phase extends the scrutiny of medical devices beyond pre-market testing, providing a real-world assessment of device performance and identifying potential safety issues that may not have been evident during the pre-market phase. The PMS system incorporates a variety of data collection methods, including passive surveillance systems like spontaneous reports from healthcare professionals and patients, active surveillance via registries or studies, and analysis of electronic health records and administrative databases. These methods collectively form a comprehensive approach to evaluate medical devices continuously, yielding valuable insights into their safety and long-term efficacy.
Through PMS, manufacturers gain access to vital post-market data, such as reports on adverse events, device malfunctions, and other safety-related information. By analyzing this data, they can detect potential safety concerns and understand how their devices perform outside of controlled clinical environments. This analysis is pivotal for making informed decisions about device development, as well as refining post-market surveillance strategies. With over 1.7 million injuries and 83,000 deaths potentially linked to medical devices in a 10-year period in the United States, robust PMS is not just regulatory compliance—it's a critical component of patient safety and device innovation. The FDA recognizes the importance of PMS and is actively working to overcome challenges such as funding and patient identification to ensure that the surveillance system is effective and can progressively include more devices over time.
Staying Updated with Regulatory Changes
For medical device manufacturers, the significance of the Premarket Approval (PMA) process cannot be understated. Continuous engagement with the FDA's PMA database is imperative not only for compliance but also for maintaining a competitive edge in the dynamic landscape of medical device regulation. Updates to the database include critical information on newly approved PMAs, modifications to existing product labeling, and vital safety communications. Biomedical professionals, like Chris, with over a decade of experience in Class III medical device clinical studies, emphasize the importance of integrating these updates into the company's regulatory strategies. In light of recent FDA publications, such as the final rule on direct-to-consumer prescription drug advertisements, it is clear that the agency is escalating its efforts to ensure public health safety and transparency in medical communications. Adhering to these evolving standards demands that manufacturers not only monitor but also interpret and implement changes in a timely manner to uphold the integrity of their products and the safety of the patients they serve.
Utilizing the PMA Database as a Research Tool
The Pre-Market Approval (PMA) Database not only plays a critical role in the regulatory landscape but also stands as a pivotal research instrument. Healthcare professionals and researchers have the opportunity to delve into a wealth of clinical data within this resource, examining the design and outcomes of studies tied to devices that have met PMA standards. This treasure trove of information is instrumental in guiding evidence-based decisions, propelling research efforts, and spurring innovation in the realm of medical devices.
Post-Market Surveillance (PMS) extends the oversight of medical devices beyond preliminary testing, emphasizing ongoing assessments and reporting to ensure devices perform safely and effectively once available to the public. PMS employs a combination of active and passive surveillance systems, including the exploitation of electronic health records and other databases, to collect essential data. This continuous monitoring is vital for revealing long-term safety and effectiveness.
Medical devices, as defined by WHO, encompass a broad range of products from basic tools to sophisticated implants and prostheses, pivotal in diagnosing, preventing, and treating diseases, ultimately enhancing patient quality of life. The development of these devices, a significant facet of mechanical engineering, involves intricate design processes that are heavily regulated to ensure patient safety.
In the United States, the FDA categorizes medical devices into three classes based on risk, with Class III devices such as pacemakers requiring PMA due to their significant role in life support. These devices, although comprising about 10% of FDA-regulated devices, undergo the most stringent approval processes. Meanwhile, the integration of software in medical devices presents additional layers of complexity, necessitating specialized project management skills that align with patient safety priorities and regulatory demands.
The insights from industry experts and the rigorous regulatory frameworks underscore the importance of not only adhering to FDA guidelines but also understanding the broader implications of medical devices in clinical settings. The PMA Database, therefore, serves as a nexus of valuable data for those in the field, offering insights that drive the evolution of medical technology and patient care.
References
Navigating the U.S. Food and Drug Administration's (FDA) regulatory framework for medical devices is a critical step for manufacturers aiming to enter the U.S. market. The FDA classifies medical devices into three categories based on the level of risk they pose to patients, with Class I devices posing the least risk and Class III the most. Each classification requires a different pathway for market entry: Premarket Notification (510(k)), Premarket Approval (PMA), or the De Novo classification process.
The PMA process is the FDA's rigorous method of scientific and regulatory review to evaluate the safety and effectiveness of Class III medical devices. It is intended for devices that support or sustain human life, are of substantial importance in preventing impairment of human health, or present a potential, unreasonable risk of illness or injury.
Manufacturers seeking to market their medical devices in the U.S. must receive FDA clearance, approval, or grant (in the case of De Novo) before selling their product. These terms—Registered, Cleared, Approved, and Granted—are often used interchangeably in the industry, but they have distinct legal and regulatory meanings. For instance, the 510(k) clearance allows a device to be marketed based on its substantial equivalence to a legally marketed predicate device that does not require a PMA.
In recent news, the FDA has continued to emphasize the importance of clear communication in consumer-directed materials. The newly published standards for direct-to-consumer prescription drug advertisements underscore the agency's commitment to ensuring information is presented in a clear, conspicuous, and neutral manner.
The FDA's Center for Drug Evaluation and Research (CDER) also plays a pivotal role in the approval of innovative drugs and biological products, shedding light on the required study design and data essential for comprehensive drug applications. This contributes to the introduction of new treatment options and enhances healthcare for the American public.
Understanding the differences between these regulatory pathways and terms is essential for regulatory professionals and manufacturers to ensure compliance and to successfully navigate the complexities of the FDA's system.
Conclusion
The Pre-Market Approval (PMA) process and the associated PMA Database are crucial for ensuring the safety and effectiveness of medical devices in the United States. Accessing the PMA Database is vital for healthcare professionals and manufacturers to navigate compliance and market readiness. Post-market surveillance (PMS) is essential for monitoring device performance and improving safety.
Understanding device classifications and staying updated with regulatory changes are key to success in the medical device industry. In conclusion, the PMA Database plays a vital role in ensuring patient safety and driving innovation. It supports evidence-based decision-making, helps navigate regulatory changes, and enhances compliance.
The PMA process, along with the PMA Database, is essential for healthcare professionals and manufacturers in the dynamic landscape of medical device regulation.
Stay informed and succeed in the medical device industry with our regulatory updates and expertise.




