Introduction
Investigational Device Exemptions (IDEs) are a cornerstone in the realm of clinical trials, enabling sponsors to legally ship investigational devices for clinical studies. This mechanism is crucial for generating the data needed to demonstrate the safety and effectiveness of medical devices, aligning with the FDA's mission to protect public health. IDEs ensure that investigational devices undergo thorough regulatory scrutiny before market entry, fostering the development of innovative medical products.
Clinical research, underpinned by well-structured studies, is vital for advancing public health. The FDA's commitment to harmonizing human subject protection regulations with the Department of Health and Human Services' Common Rule exemplifies efforts to streamline clinical research while safeguarding participants' rights. This regulatory alignment is essential for investigators and sponsors managing IDEs amidst the complexities of global medical device development.
The significant number of injuries and deaths linked to medical devices underscores the necessity of robust regulatory oversight. Adhering to IDE requirements not only enhances the development of safer medical devices but also improves patient outcomes and advances medical knowledge.
Understanding Investigational Device Exemptions (IDEs)
Investigational Device Exemptions (IDEs) play a crucial role in the environment of trials, permitting sponsors to legally transport investigational devices for use in experiments. This mechanism is indispensable as it enables the rigorous collection of data necessary to substantiate the safety and effectiveness of medical devices. As part of the FDA's broader mandate to protect public health, Ideas facilitate the development of innovative medical products by ensuring that investigational devices are subjected to stringent regulatory scrutiny before reaching the market.
Effective, well-structured medical research is essential for enhancing public health, and the FDA actively encourages the creation of trustworthy evidence through these investigations. As one FDA report indicates, the agency is committed to harmonizing human subject protection regulations with the Department of Health and Human Services' Common Rule, aiming to streamline clinical research processes while safeguarding participant rights. This harmonization effort underscores the importance of compliance with FDA regulations, particularly for investigators and sponsors navigating the complexities of IDEs.
The global nature of the medical device industry presents several challenges, including ensuring compliance with varied regulatory standards and addressing safety concerns during product development. Over a ten-year period, more than 1.7 million injuries and 83,000 deaths in the United States were potentially linked to medical devices, highlighting the critical need for robust regulatory oversight. By adhering to IDE requirements, sponsors can contribute to the development of safer and more effective medical devices, ultimately enhancing patient outcomes and advancing medical knowledge.
Types of Device Studies Under 21 CFR Part 812
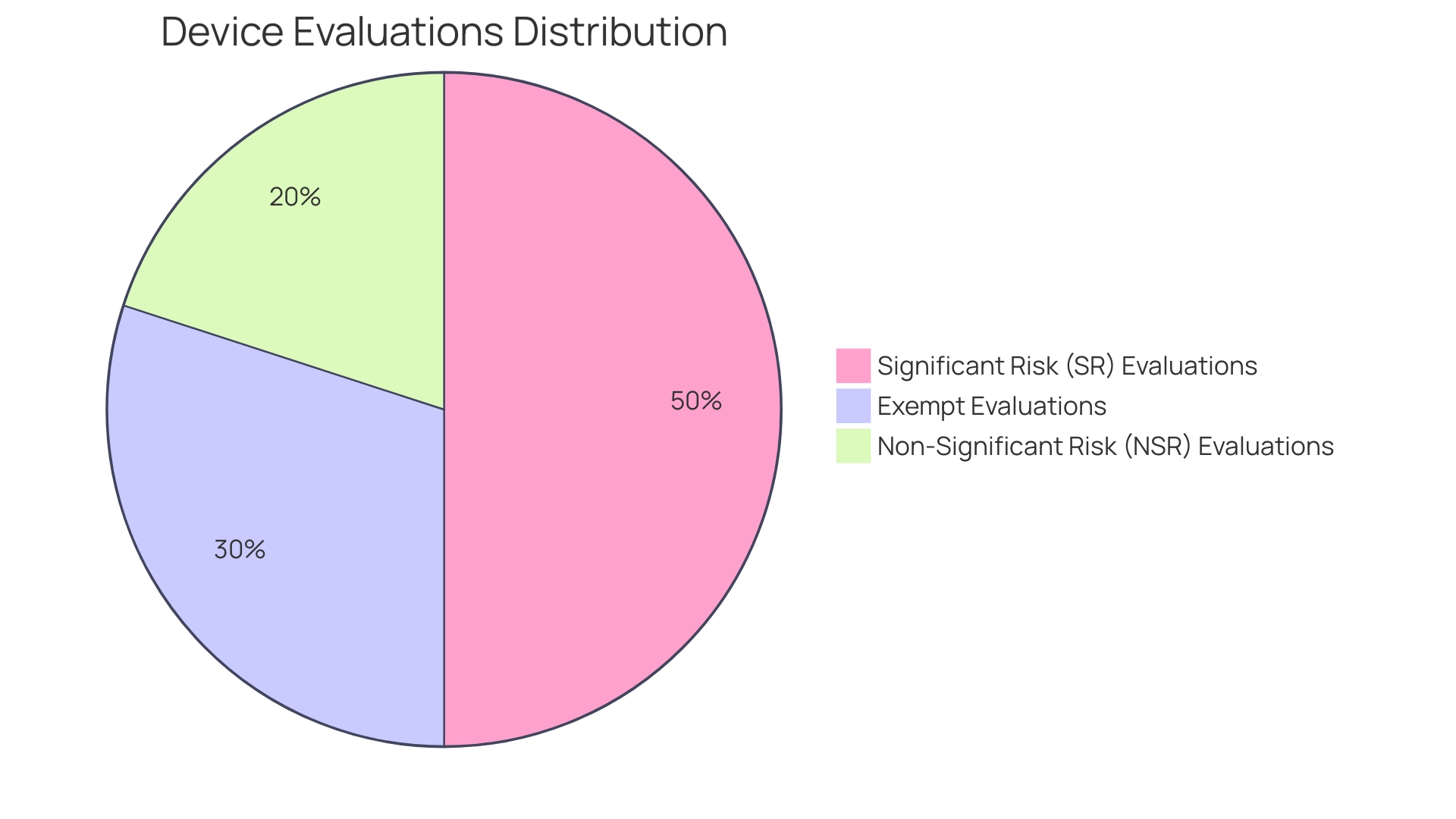
21 CFR Part 812 outlines various categories for device evaluations, specifically exempt evaluations, significant risk (SR) evaluations, and non-significant risk (NSR) evaluations. Each classification has distinct regulatory requirements and oversight. SR examinations are subject to more rigorous scrutiny due to their potential to present a serious risk to the health, safety, or welfare of a subject. In contrast, NSR studies, while still regulated, are considered to pose less risk. Understanding these differences is essential for ensuring compliance with the proper rules and procedures, thus protecting the integrity and safety of research studies.
IDE Application and Approval Process
The IDE application process is a critical step in initiating clinical trials for medical devices. This procedure necessitates submitting a comprehensive plan that details the aims, methodology, and adherence to regulatory standards. The FDA meticulously reviews this application to ensure that the proposed research aligns with the required safety and efficacy criteria. Familiarity with the FDA’s regulations and guidance documents is paramount, particularly concerning the Investigational Device Exemption (IDE). An IDE allows the utilization of non-cleared devices in trials to collect crucial safety and effectiveness information. Overcoming challenges in the IDE submission, such as administrative or substantive review errors, is vital for securing timely approval. This process not only facilitates pre-market research but also contributes significantly to the development and evaluation of medical devices' post-market performance.
Institutional Review Board (IRB) Approval and Oversight
Institutional Review Boards (IRBs) are crucial ethics committees tasked with the protection of participants' rights and welfare in clinical trials. These boards meticulously review research protocols, informed consent documents, and ongoing adherence to ethical standards. The significance of IRBs can't be overstated as they ensure that human subjects are treated with respect and care throughout the research process.
IRBs were officially established under the National Research Act of 1974, a response to unethical research practices, such as the infamous Tuskegee Syphilis Study. Today, approximately 2,300 IRBs exist in the U.S., overseeing both academic and commercial research. Their primary role is to evaluate the purpose of the research, procedures, risks and benefits, and consent forms, ensuring compliance with federal regulations and institutional criteria.
Obtaining IRB approval is a fundamental step in conducting ethical medical research. 'Without it, research cannot legally continue, underscoring the board's critical function in safeguarding public health and maintaining trust in medical research.'.
Participant Safety and Informed Consent
Ensuring participant safety is a cornerstone of clinical studies. The informed consent process is designed to provide potential participants with comprehensive information about the study, including risks, benefits, and the voluntary nature of participation. Recent guidance emphasizes the importance of presenting key information in a clear and concise manner at the beginning of the consent document. This includes the purpose of the research, expected duration, procedures, and compensation for research-related injuries.
The National Organization for Rare Disorders (NORD) has praised efforts to make informed consent more accessible, highlighting the need to address language barriers, sensory impairments, and health literacy levels. Innovative approaches, such as videos, have been recommended to tailor the process to individual needs, ensuring participants fully understand the risks and benefits.
Clinical studies often involve participants who may not directly benefit from the research but contribute to future advancements. For example, elderly patients with transthyretin-mediated amyloidosis engage in studies hoping to assist future generations. Despite the personal toll, including invasive tests and new side effects, their motivation often stems from a desire to improve outcomes for others.
Addressing the complexity of informed consent documents is crucial, as they have become increasingly burdensome. Originally intended to help potential participants in making educated choices, these papers now frequently surpass twenty pages and are composed at an advanced reading level, creating barriers to enrollment, particularly among marginalized groups.
The draft guidance encourages the use of key information as a guide to support discussions between investigators and potential participants. This approach aims to facilitate comprehension and ensure ethical compliance, building trust between researchers and participants.
Record Keeping and Reporting Requirements
Maintaining accurate records and adhering to reporting requirements are critical components of compliance with 21 CFR Part 812. Sponsors must keep detailed documentation of all study-related activities, including adverse events and protocol deviations. This guarantees the integrity of clinical experiments by offering a thorough account of the research's execution and any problems faced. Regular reporting to the FDA and IRB is essential for transparency and accountability. During the last 25 years, the criteria for information components in research documentation have developed, mirroring alterations in study reporting regulations and guidelines. Consequently, older records on platforms like ClinicalTrials. Gov may lack information that has since become mandatory. Modernized reporting systems now require extensive information submissions to ensure thorough oversight. To further enhance public health protection, regulatory agencies like the FDA focus their compliance and risk management processes on high-impact areas. This approach is designed to maintain flexibility, enabling rapid and effective responses to emerging public health threats.
Quality Assurance and Quality Control Measures
Implementing quality assurance (QA) and quality control (QC) measures is indispensable for maintaining the integrity of clinical trials. These measures ensure that studies comply with regulatory standards and that the information gathered is both reliable and valid. The World Health Organization (WHO) estimates there are two million different kinds of medical devices globally, each potentially impacting millions of lives. Clinical information management is crucial in this context since it involves human subjects and devices that may be widely used. Article 62 of the European Union Medical Device Regulation (EU MDR) emphasizes that clinical investigations must prioritize the rights, safety, dignity, and well-being of participants while ensuring that the information generated is scientifically valid, reliable, and robust.
Clinical trials often utilize time- and labor-intensive methods for information collection, creating burdens on clinicians and patients. This is further complicated by the absence of simultaneous information from other sources, which could provide significant insights into a participant’s health. For instance, information from daily activities or thorough patient-reported details is often absent, impacting the validity and generalizability of the findings.
Public and private investments over the past two decades have significantly advanced electronic health record (EHR) adoption, health information interoperability, and information standards. These advancements offer a strong basis for enhancing healthcare information management practices. However, there is still an urgent need to build on this infrastructure to support reusable research trial capabilities. The FDA also encourages the use of automated processes for information validation, steering away from manual methods that are prone to errors.
Outstanding management of medical information is essential for business to, establishing the foundation of submissions to regulatory authorities. In the US, approximately 10-15% of successful 510(k) submissions for Class II devices depend on study data, and all Class III devices necessitate extensive evaluations to establish safety and effectiveness. Thus, comprehending and implementing strong QA and QC practices are essential for the overall success of research and the following approval and marketability of medical devices.
Monitoring and Compliance with 21 CFR Part 812
Ensuring adherence to 21 CFR Part 812 regulations requires ongoing supervision of research studies. This involves a multitude of activities such as regular audits, inspections, and reviews of study processes and data. Effective monitoring practices are crucial in identifying potential issues early, thereby allowing for timely interventions and maintaining the integrity of the clinical study. 'Clinical studies are pivotal in testing and establishing the safety and efficacy of new treatments or interventions, making stringent monitoring indispensable.'.
ClinicalTrials.gov functions as an essential archive for summarizing research protocols and results information, which must be regularly updated by sponsors and investigators. Throughout the last 25 years, the criteria for information components on this platform have developed, mirroring alterations in research reporting regulations and guidelines. Given the importance of accurate and comprehensive data, ongoing monitoring ensures that study records are consistent with current standards.
Furthermore, the FDA plays a vital role in protecting public health by assuring the safety, effectiveness, and security of medical products. This encompasses supervising research studies and making certain they comply with set standards. For example, the FDA's recent release of the "Direct-to-Consumer Prescription Drug Advertisements" final rule highlights the agency's dedication to clear and transparent communication of drug information, which is essential to the public's trust in medical research.
Monitoring also involves the use of Data Monitoring Committees (DMCs), which provide an additional layer of oversight. These committees are essential in assessing the safety and efficacy data during a study, making recommendations on whether to continue, modify, or terminate the research based on interim findings. This guidance is part of the FDA's broader efforts to enhance regulatory processes, foster innovation, and advance public health protection.
Ultimately, the role of effective monitoring cannot be overstated. It ensures that clinical trials are conducted to the highest standards, safeguarding both the integrity of the research and the well-being of participants.
Conclusion
The exploration of Investigational Device Exemptions (IDEs) highlights their critical function in the landscape of clinical trials. These exemptions not only allow sponsors to legally ship investigational devices but also ensure that thorough data collection occurs to validate the safety and effectiveness of medical devices. The FDA's commitment to regulatory oversight is paramount, particularly in light of significant concerns regarding medical device-related injuries and fatalities.
By adhering to IDE requirements, sponsors can enhance patient outcomes and contribute to medical knowledge, fostering the development of safer medical products.
Understanding the different categories of device studies under 21 CFR Part 812, as well as the IDE application and approval process, is essential for compliance and the integrity of clinical trials. The role of Institutional Review Boards (IRBs) in safeguarding participant rights cannot be overstated, as they ensure ethical standards are upheld throughout research. Furthermore, the informed consent process must be transparent and accessible, addressing the diverse needs of potential participants to facilitate comprehension and trust.
Record keeping, reporting requirements, and the implementation of quality assurance and quality control measures are vital for maintaining the integrity of clinical trials. Continuous monitoring and compliance with regulatory standards ensure that clinical research adheres to established guidelines, ultimately safeguarding public health. By focusing on these aspects, the medical device industry can not only navigate the complexities of clinical trials but also contribute to the advancement of innovative and effective medical solutions.




