Introduction
Navigating the intricate landscape of FDA clearance for medical devices necessitates a thorough understanding of the 510(k) submission process. This pivotal step ensures that new products are as safe and effective as those already available on the market. The 510(k) submission encompasses three primary types: Traditional, Special, and Abbreviated, each designed to address specific scenarios and requirements.
Traditional 510(k) submissions are the most comprehensive, often utilized when there is no predicate device or significant changes are made to an existing product. Special 510(k) submissions are tailored for modifications that do not substantially affect safety or effectiveness, offering a more expedited review process. Abbreviated 510(k) submissions leverage existing guidance documents and standards to streamline the review, particularly when minimal modifications are involved.
Understanding these types, their eligibility criteria, and the essential components of a successful submission is crucial for manufacturers seeking timely FDA approval.
Understanding the Types of 510(k) Submissions
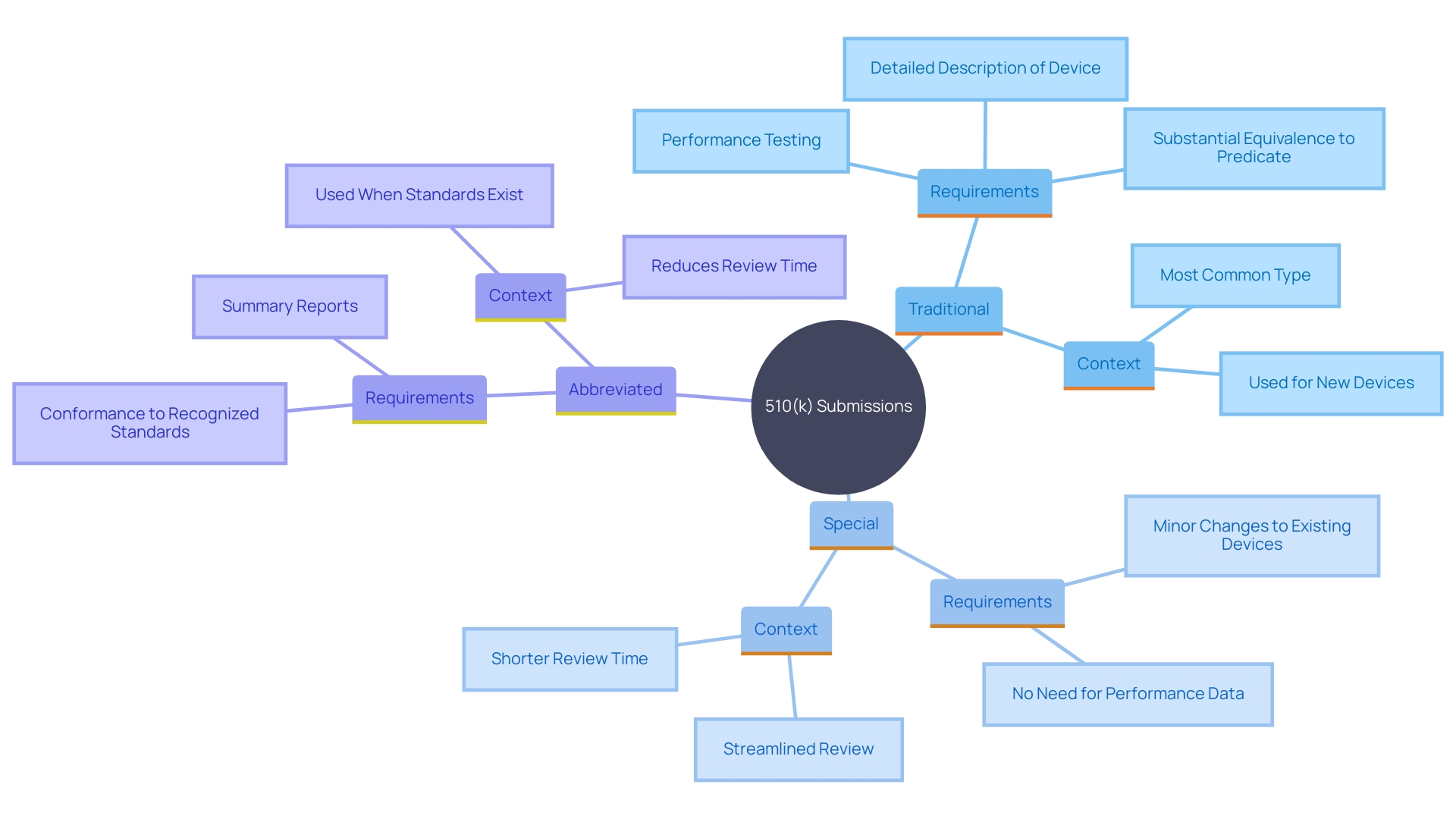
The 510(k) application process is a crucial stage for medical product manufacturers seeking FDA approval, guaranteeing that their offerings are as safe and effective as those currently available. There are three primary types of 510(k) submissions: Traditional, Special, and Abbreviated, each tailored to different situations and requirements.
A Traditional 510(k) is the most comprehensive, often utilized when there is no predicate item or when substantial changes are made to an existing product. On the other hand, a Special 510(k) is suitable for products undergoing modifications that do not significantly affect safety or effectiveness. This type benefits manufacturers by offering a reduced review time. For instance, regulatory consultants can assist in determining the eligibility for a Special 510(k), ensuring a more streamlined process.
The Abbreviated 510(k) leverages guidance documents, special controls, and recognized consensus standards to expedite the review process. It is usually used when there is a clear predicate element and the changes made are minor.
A noteworthy example is iRhythm Technologies, which recently received its first 510(k) clearance. The CEO, Quentin Blackford, highlighted the structured approach they adopted, submitting their applications sequentially to ensure compliance and timely approvals.
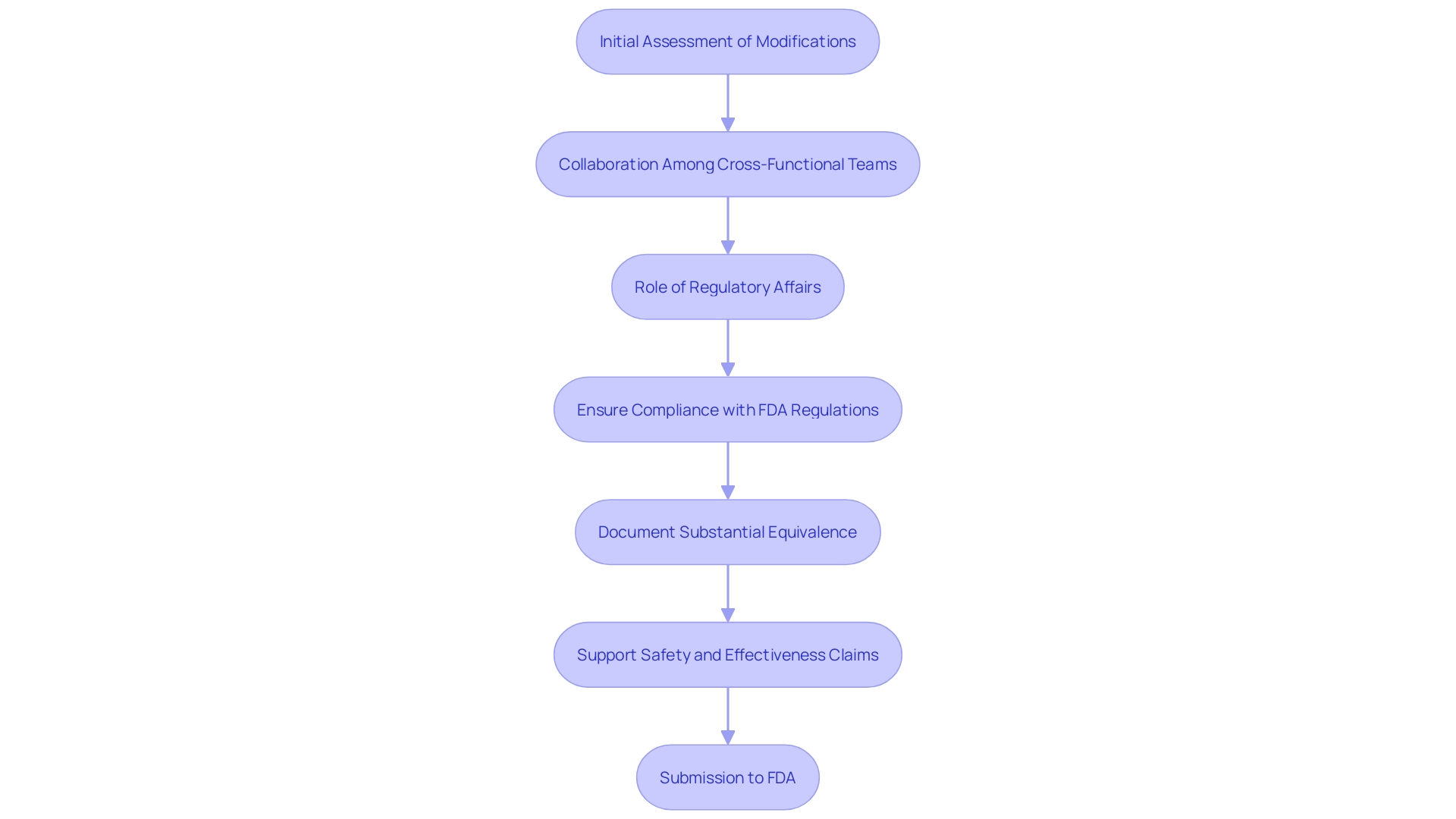
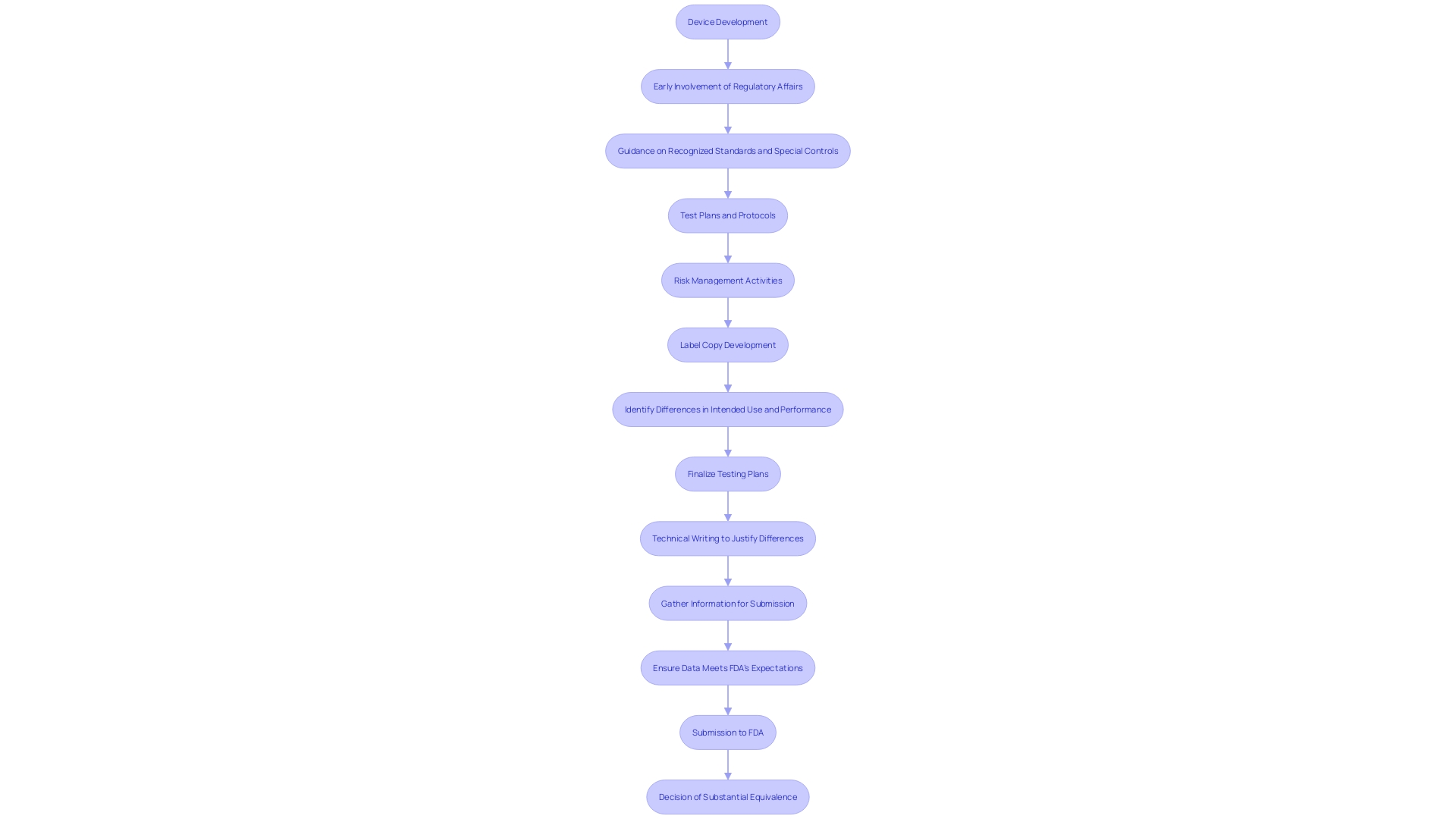
The importance of meticulous documentation and adherence to FDA guidelines cannot be overstated. As highlighted by regulatory specialists, the primary aim of the 510(k) content is to offer the FDA with reasonable assurance of substantial equivalence to the predicate product. Consequently, early participation of Regulatory Affairs (RA) in the development process is vital for a successful filing, ensuring that all recognized standards and special controls are meticulously followed.
Eligibility Criteria for Special 510(k) Submissions
To meet the criteria for a Special 510(k) application, the item in question must be a modification of a legally marketed product, adhering strictly to section 513(I)(1)(A) of the FD&C Act. These modIfIcatIons should not change the Intended use, fundamental technology, or Its safety and effectIveness. Manufacturers must ensure that the changes can be substantiated with existing data and align with all regulatory requirements.
The FDA emphasizes that meeting the 510(k) standard does not inherently guarantee the safety and effectiveness of the product. It is essential for the planning of a 510(k) application to start early in the product development process, with Regulatory Affairs (RA) playing a key role. RA must provide guidance on recognized standards, applicable special controls, test plans, and protocols to ensure the data generated supports substantial equivalence.
The preparation of a 510(k) application is a collaborative effort, necessitating inputs from cross-functional team members, including subject matter experts (SMEs). This comprehensive approach ensures that all aspects of the apparatus's design, performance, and associated risks are thoroughly considered and documented, thereby facilitating a robust justification for the modifications made to the predicate apparatus.
The FDA's draft guidance on the 510(k) Third Party Review Program and Third Party Emergency Use Authorization (EUA) Review underscores the importance of providing reasonable assurance of substantial equivalence. The technical writing within the document must convincingly argue that any differences from the predicate device do not introduce new safety or effectiveness concerns. This approach is essential for gaining FDA clearance, as highlighted by industry experts in various discussions and podcasts.
Key Components of a Special 510(k) Submission
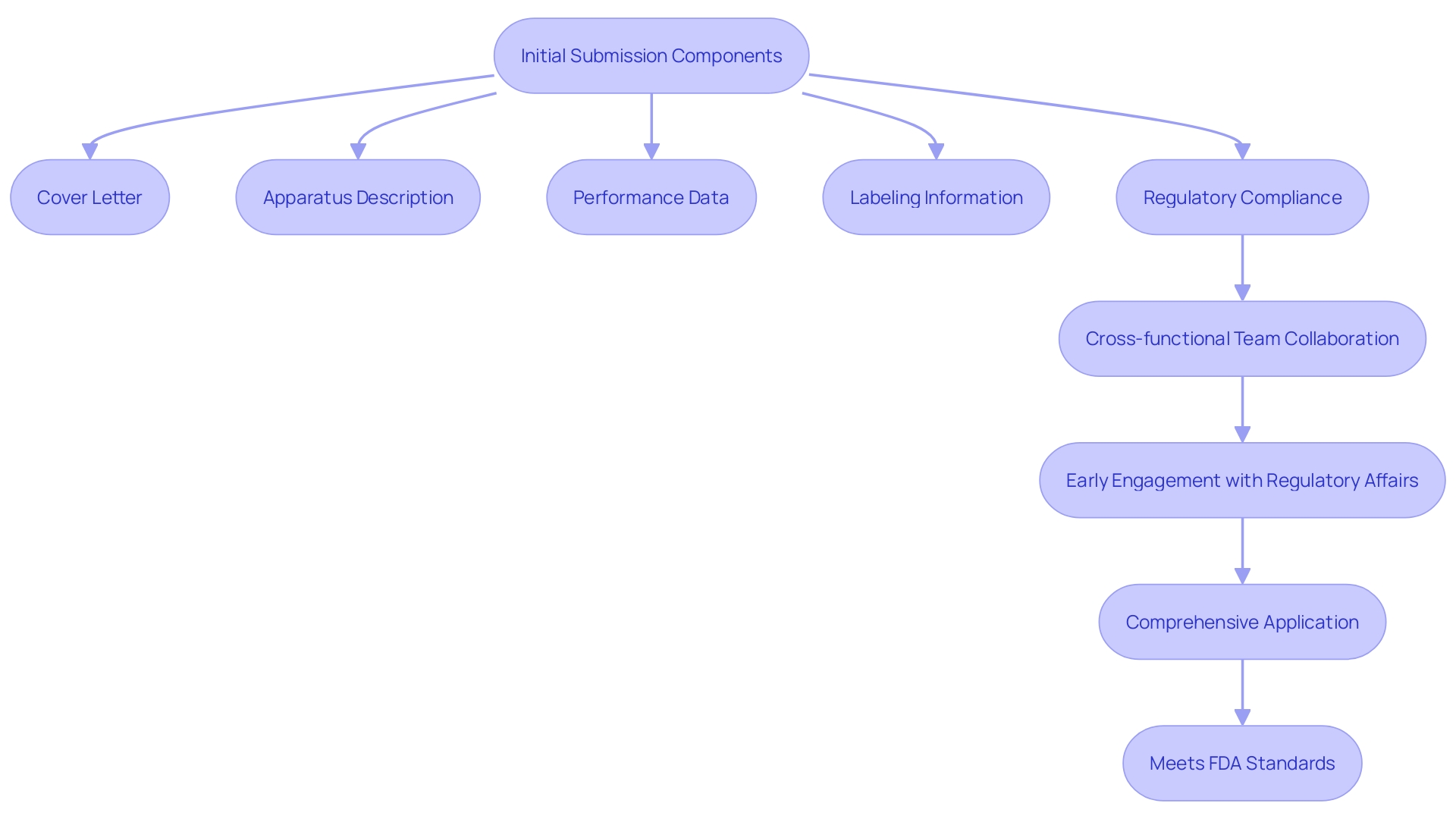
A Special 510(k) application must include several critical components. First, a comprehensive cover letter is essential to introduce the submission. In addition, a detailed description of the apparatus, including its modifications, must be provided to establish a clear understanding of the product. Performance data, crucial for demonstrating the effectiveness and safety of the equipment, should be meticulously documented. Labeling information, including instructions for use, warnings, and cautions, is also a key element of the submission.
To ensure compliance with applicable regulations and standards, substantial evidence must be presented. This includes a thorough comparison table that highlights similarities and differences between the subject item and potential predicates. Differences in intended use, physical and technical characteristics, functionality, and performance should be clearly identified and justified to satisfy FDA's requirements.
Engaging Regulatory Affairs (RA) early in the device development process is crucial. RA provides guidance on recognized standards, special controls, and necessary test plans and protocols. This proactive approach helps ensure that the data generated supports substantial equivalence and meets FDA's expectations.
Moreover, forming a dedicated team of cross-functional members, including experts from Quality, Engineering, Research & Development, Manufacturing, and Marketing, is vital. Collaboration and regular meetings ensure that the 510(k) application is comprehensive and cohesive, reflecting inputs from various subject matter experts. This strategic approach lays a strong foundation for a successful 510(k) filing, ultimately leading to FDA's decision of substantial equivalence.
Preparing the Submission: Essential Documents and Information
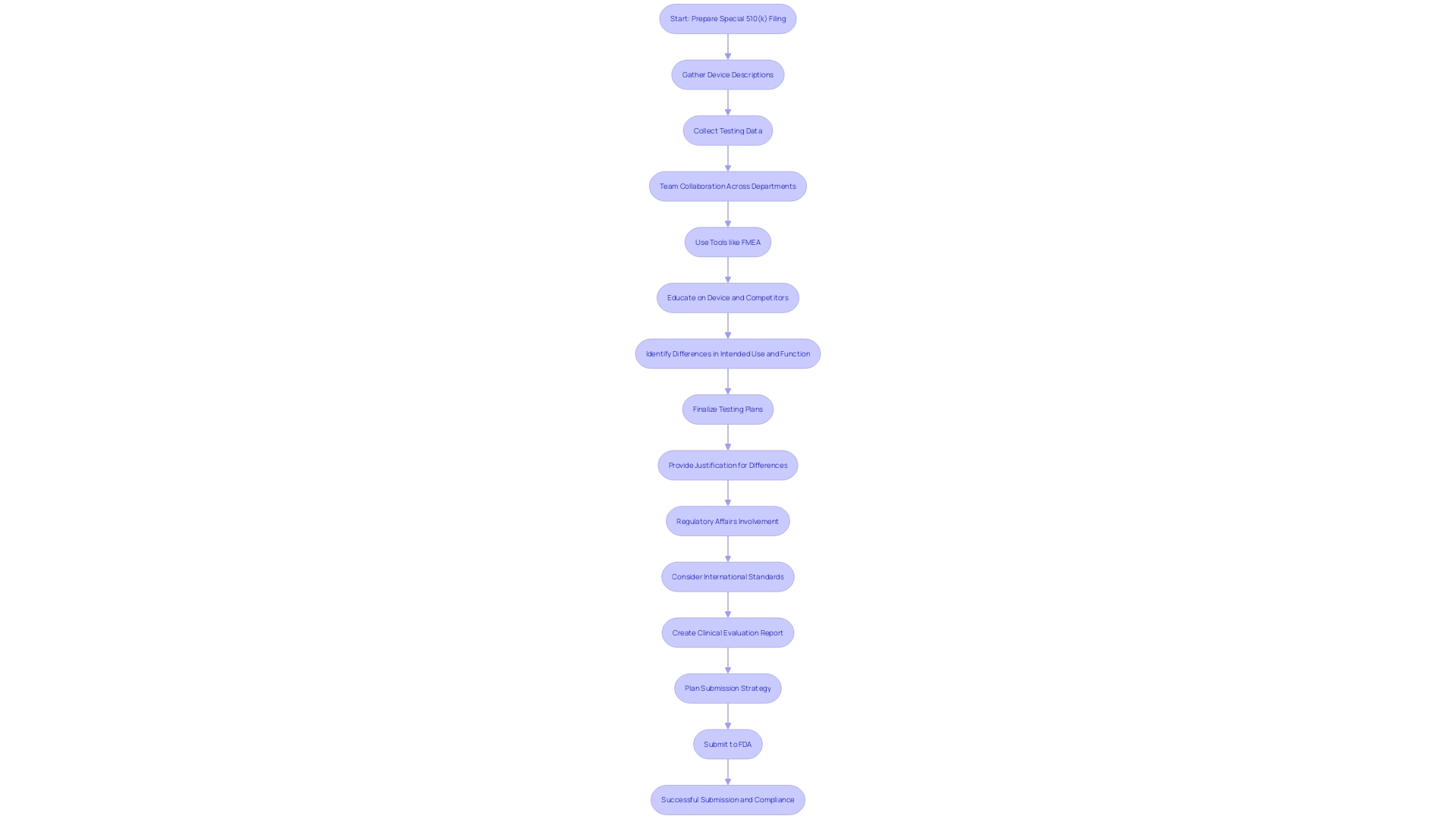
When preparing a Special 510(k) filing, manufacturers must gather essential documents, including the device description, a comparison to the predicate device, and any relevant testing data. Ensuring completeness and clarity in all documentation is critical, as it facilitates the review process and minimizes the chances of delays. It is essential to engage in thorough design and performance testing using tools such as Failure Modes and Effects Analysis (FMEA) to understand clinical use and associated risks better. A dedicated cross-functional team, including members from Quality, Engineering, Manufacturing, and Marketing, should collaborate to provide the necessary inputs. According to an expert, "These tools provide an understanding of what needs to go into a proposal and explain the process to follow to ensure it is accepted by the FDA." Additionally, leveraging international standards and creating Clinical Evaluation Reports early can streamline the process and avoid redundant testing. Regulatory strategy consulting services can also be invaluable in navigating the complexities of FDA 510(k) applications.
Electronic Submission Requirements and eSTAR
The FDA promotes the use of electronic entries for 510(k) applications via the Estar (electronic Template and Resource) system. Created to simplify the application process, Estar enables a uniform method and seeks to enhance communication between producers and the FDA. However, while Estar simplifies certain aspects, it also presents challenges. For instance, the Truth & Accuracy statement, Form 3514, 510(k) Summary, Declaration of Conformity, and Indications for Use Form 3881 do not add significant value and are difficult to use due to rule-based conditional events.
Dr. Roy suggests that regulatory teams can overcome these challenges by creating a structured Estar process. This includes creating a root-level folder for each entry on a shared network drive or cloud platform, with separate folders for each section of the Estar file. Teams can then store the Word files that will be attached to the Estar file in their respective folders and use a separate project management tool to allocate tasks.
Despite its limitations, eSTAR remains a valuable tool for ensuring substantial equIvalence to predIcate devIces, as outlIned In sectIon 513(I)(1)(A) of the FD&C Act. The FDA's goal Is to make InformatIon accessIble to revIewers and submitters, though there is room for improvement in integrating modern technologies like cloud systems and AI to enhance usability and efficiency.
Submission Process and Timeline
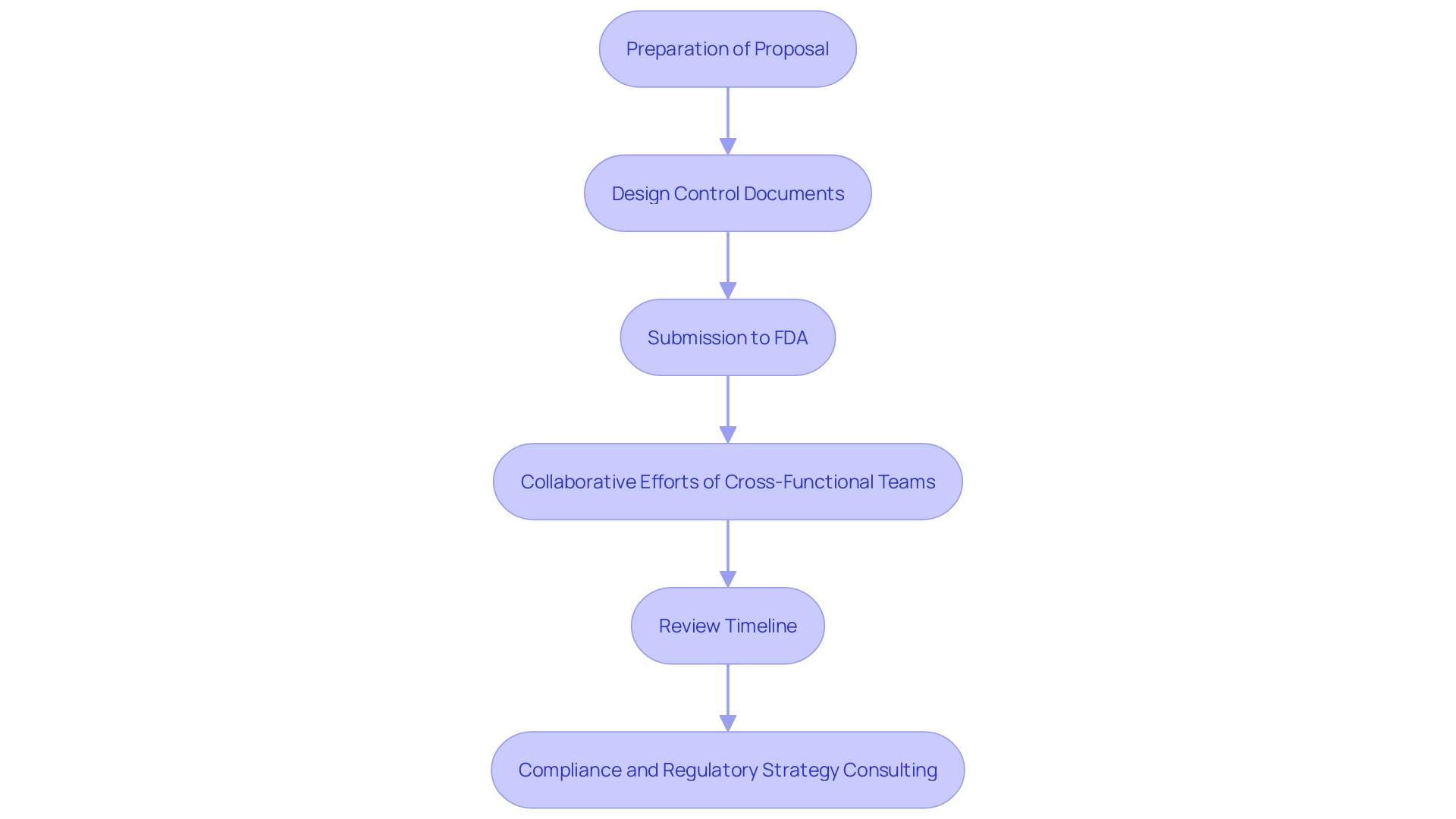
The application process for a Special 510(k) involves several critical steps, each requiring meticulous attention to detail. Initially, preparation of the proposal demands a thorough understanding of design control documents, including user needs, verification, and validation. These elements are essential to inform the contents of the proposal and identify any areas that may need further testing to support substantial equivalence.
Once the document is prepared, it is sent to the FDA, where a dedicated team of cross-functional members, including experts from Quality, Engineering, Research & Development, Manufacturing, and Marketing, plays a pivotal role. Although one individual might compile the contribution, it is a collective effort from many subject matter experts (SMEs).
The FDA aims to complete the review of Special 510(k)s within 30 days. However, the timeline can vary depending on the complexity of the proposal. Recent updates to the FDA's Customer Collaboration Portal have been implemented to enhance user experience and monitor the status of entries more efficiently.
Ensuring compliance with FDA’s expectations is paramount. Therefore, utilizing regulatory strategy consulting services can assist in managing the complexities of FDA 510(k) applications and take advantage of shorter processing times. This approach is fundamental to creating a successful proposal, encompassing an in-depth knowledge of risks and clinical benefits, a cohesive team, and a well-planned strategy.
User Fees and Payment Information
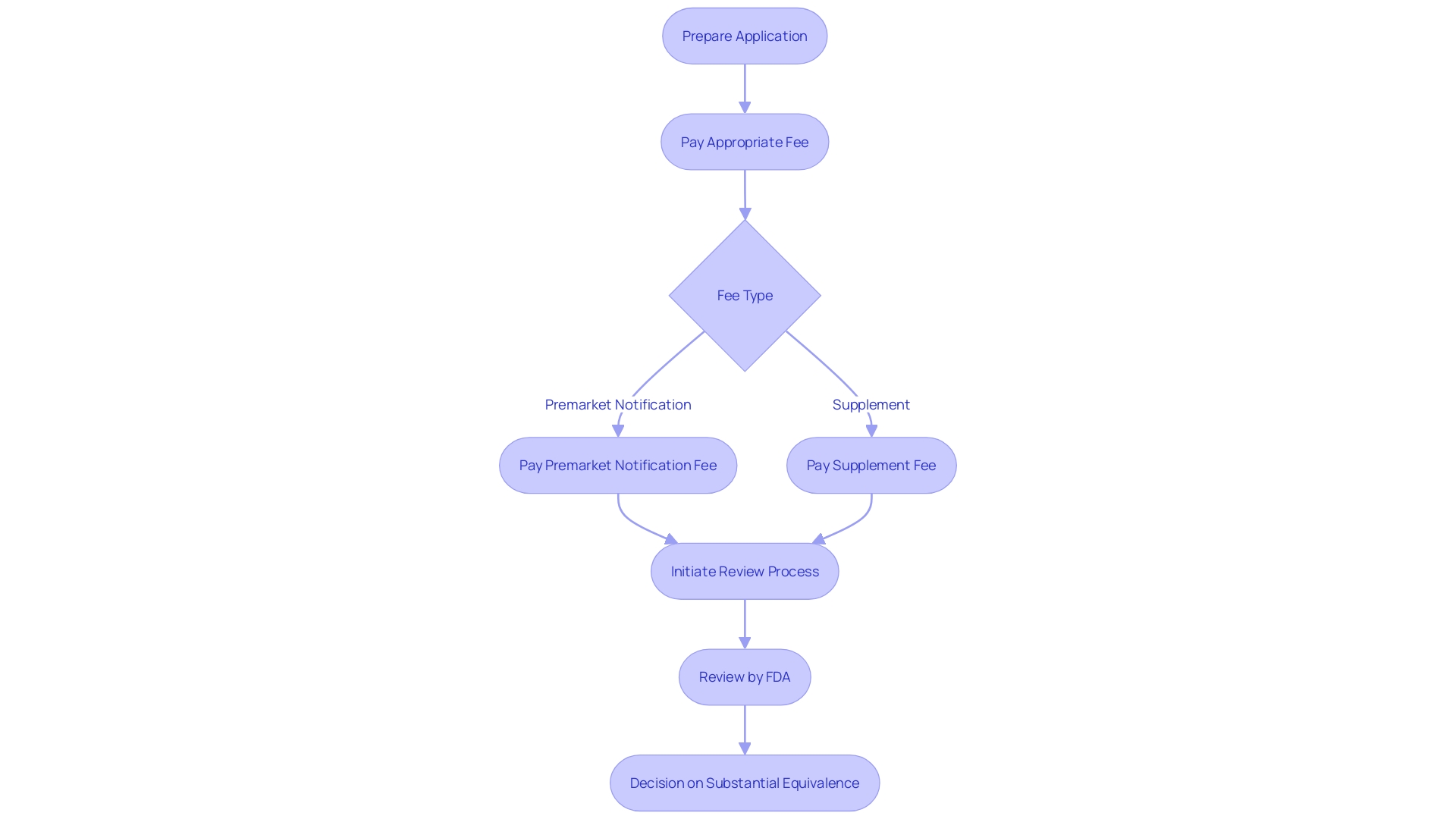
Manufacturers must be mindful of the user fees involved in 510(k) filings, as they are crucial for the timely processing of applications. 'The FDA establishes these fees each year, and they may vary based on the category of application.'. For instance, a 510(k) premarket notification fee is 4.5 percent of the standard fee, while a 180-day supplement incurs 15 percent of the standard fee. It's crucial to recognize that the assessment of an application cannot begin until the necessary fee is paid, which highlights the importance of including the fee during the application process. According to FDA data, manufacturers present an average of 6,182 applications each year, with the hours per response averaging 18 minutes for each application. Ensuring the correct fee is paid upfront helps avoid delays and facilitates a smoother review process.
Tips for a Successful Special 510(k) Submission
To ensure a successful Special 510(k) application, manufacturers should prioritize thorough documentation and clear communication of any modifications. Following FDA guidelines is essential for showing significant similarity to a predicate product. Involving FDA representatives early in the preparation phase can provide valuable insights and clarify uncertainties, enhancing the quality of the application.
In-depth knowledge of the device’s risks, clinical benefits, and comprehensive understanding of FDA expectations are essential. Employing regulatory strategy consulting services can assist in navigating the complexities of the 510(k) application process. By following these steps, manufacturers can significantly improve their chances of a speedier and more successful route to market.
Common Challenges and How to Overcome Them
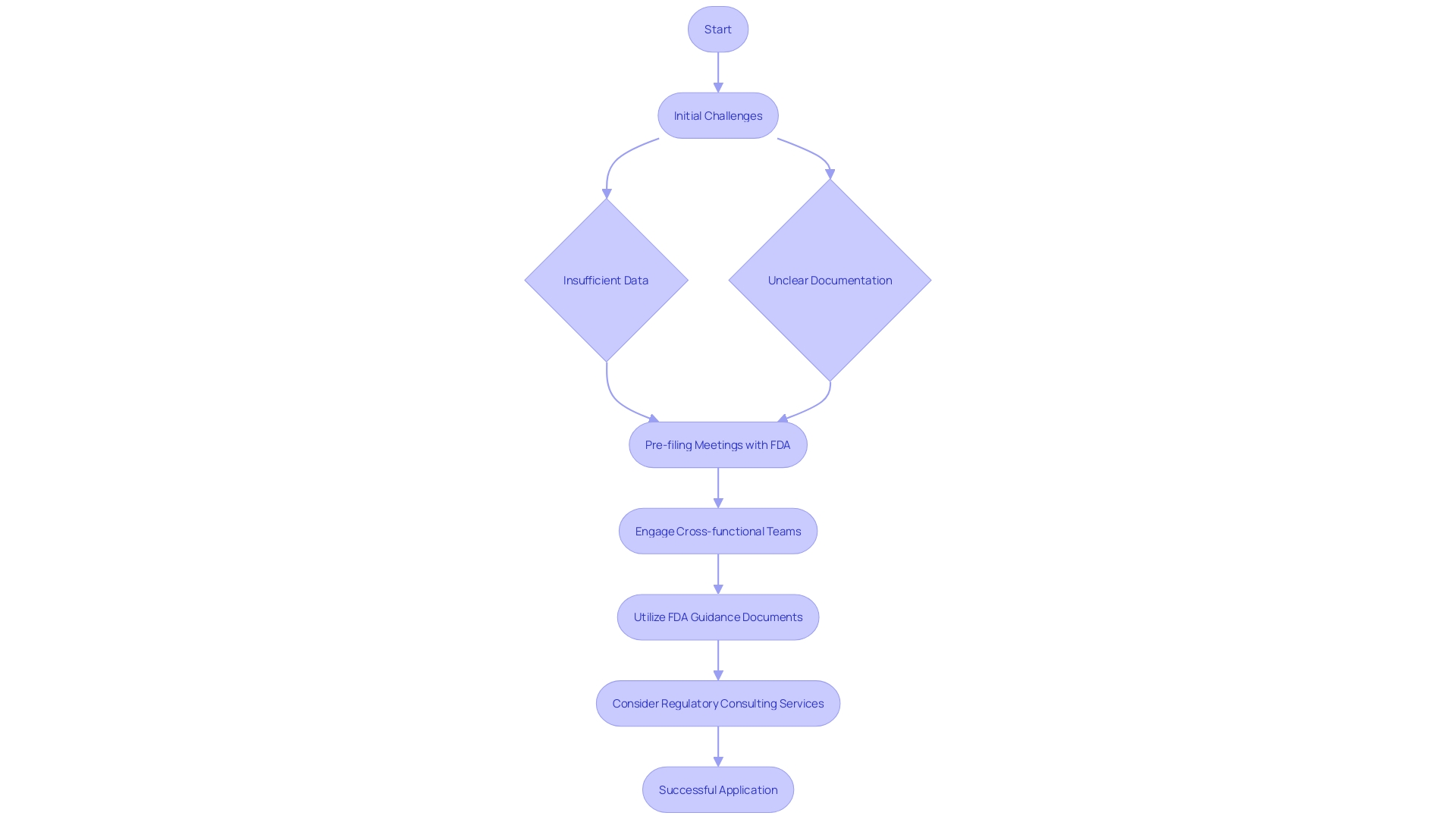
Manufacturers often face several challenges during the Special 510(k) application process, such as insufficient data and unclear documentation. Overcoming these hurdles necessitates meticulous planning and a proactive approach. Pursuing pre-filing meetings with the FDA can offer invaluable feedback and direction, assisting in clarifying expectations and optimizing the filing process.
Engaging a dedicated team of cross-functional members and scheduling regular meetings is essential for success. While the task of writing the document may fall on an individual, it is fundamentally a compilation of inputs from various subject matter experts. This collaborative approach ensures comprehensive and accurate documentation.
Additionally, the FDA's guidance documents and resources on their website are critical tools. They provide a thorough comprehension of the application process, which is essential for fulfilling the regulatory body's expectations.
In light of these complexities, some manufacturers opt for regulatory strategy consulting services. These consultants can navigate the intricacies of FDA 510(k) filings, Premarket Notification (PMA), and De Novo pathways, ensuring that the documentation is robust and meets all regulatory requirements. This strategic planning is supported by the FDA's own experiences and lessons learned from public workshops and industry feedback, which are periodically shared with the public for comment.
Ultimately, understanding the FDA's expectations and engaging in proactive and strategic planning are key to overcoming the challenges of the Special 510(k) submission process.
Conclusion
The FDA clearance process for medical devices is centered on the 510(k) submission types: Traditional, Special, and Abbreviated. Each type serves distinct scenarios, with the Special 510(k) providing a quicker pathway for minor modifications, while the Traditional 510(k) is more detailed.
To qualify for a Special 510(k), modifications must not change the device's intended use or core technology. Early engagement with Regulatory Affairs and collaboration among cross-functional teams are essential for effective documentation and compliance with FDA standards, ensuring substantial equivalence is clearly established.
Successful submissions require detailed device descriptions, performance data, and comparative analyses with predicate devices. While the eSTAR system can streamline the process, it presents some challenges. Understanding user fees and submission timelines is also vital for an efficient review.
Common challenges, such as inadequate data or unclear documentation, can be addressed through proactive planning and pre-submission discussions with the FDA. Utilizing regulatory consulting services can further strengthen the submission.
By focusing on thorough documentation, clear communication, and strategic planning, manufacturers can enhance their chances of timely FDA clearance. A solid understanding of the 510(k) submission process is crucial for ensuring that new medical devices meet essential safety and effectiveness standards.




